Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice
2019-03-28JiangHuaZhouJingJingCaiZhiGangSheHongLiangLi
Jiang-Hua Zhou, Jing-Jing Cai, Zhi-Gang She, Hong-Liang Li
Abstract With the increasing number of individuals with diabetes and obesity,nonalcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent,affecting one-quarter of adults worldwide. The spectrum of NAFLD ranges from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis(NASH). NAFLD, especially NASH, may progress to fibrosis, leading to cirrhosis and hepatocellular carcinoma. NAFLD can impose a severe economic burden,and patients with NAFLD-related terminal or deteriorative liver diseases have become one of the main groups receiving liver transplantation. The increasing prevalence of NAFLD and the severe outcomes of NASH make it necessary to use effective methods to identify NAFLD. Although recognized as the gold standard, biopsy is limited by its sampling bias, poor acceptability, and severe complications, such as mortality, bleeding, and pain. Therefore, noninvasive methods are urgently needed to avoid biopsy for diagnosing NAFLD. This review discusses the current noninvasive methods for assessing NAFLD,including steatosis, NASH, and NAFLD-related fibrosis, and explores the advantages and disadvantages of measurement tools. In addition, we analyze potential noninvasive biomarkers for tracking disease processes and monitoring treatment effects, and explore effective algorithms consisting of imaging and nonimaging biomarkers for diagnosing advanced fibrosis and reducing unnecessary biopsies in clinical practice.
Key words: Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Steatosis;Fibrosis; Noninvasive evaluation
INTRODUCTION
With the increasing number of individuals with diabetes and obesity, nonalcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent, affecting more than one-quarter of adults in the world[1]and 60% of diabetic patients[2]and rising to 90% in the obese people[3,4]. In the United States, the prevalence of NAFLD in adults is 24.13%[1], and it is forecasted to be 33.5% in 2030, and NAFLD cases will reach 100.9 million in the general population[5]. In Asian, the prevalence of NAFLD has reached to 27.37%[1], with 20.09% in China[6]. In some developing countries, such as Sudan,Nigeria, and Iran, the prevalence of NAFLD is about 8.7%-20%[7-9]. The spectrum of NAFLD covers from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). NAFLD, especially NASH, may progress to fibrosis, leading to cirrhosis and hepatocellular carcinoma (HCC)[10]. NAFLD can impose a severe economic burden[11-13], and patients with NAFLD-related terminal or deteriorative liver diseases have become one of the main groups receiving liver transplantation, overtaking hepatitis C patients[14,15]. Based on the double pressure of the increasing prevalence of NAFLD and severe outcomes of NASH, many effective treatments for NAFLD are under development. Lifestyle interventions combined with the loss of 10% of body weight may improve the state of steatosis, inflammation, and even fibrosis[16]. However, the majority of people poorly adhere to long-term, effective lifestyle interventions, which leads to the rapid development of pharmacological treatment. The current therapeutic targets of medicine in clinical trials cover metabolic targets, oxidative stress and inflammation, gut health, and antifibrotics[17-27]. During this period of clinical drug registration, histological biopsy is the key endpoint replacing the long-term main outcomes, such as mortality[28,29]. However, liver biopsy specimens have several limitations, such as representing only approximately 1/50000 of the organ and sampling bias. On the other hand, fibrosis is not uniformly distributed[30], and liver biopsy may cause severe complications, such as mortality,bleeding, and pain. Therefore, it is preferable to use effective noninvasive methods in clinical practice for identifying NAFLD, tracking disease processes, and monitoring treatment effects[31].
DIAGNOSIS OF NAFLD
Normal hepatic fat content is commonly defined when steatosis in liver histology is less than 5% of hepatocytes[32-34]. NAFLD is diagnosed by a histological phenotype of steatosis with the exclusion of other chronic liver diseases in more than 5% of cases[35,36]. However, in clinical practice, noninvasive methods, including assessment of biomarker panels and imaging, are widely applied instead of biopsy for diagnosing NAFLD.
Serum biomarkers and biomarker panels
Fatty liver index (FLI):The FLI is a prevalent biomarker panel consisting of body mass index (BMI), waist circumference, triglycerides, and gamma-glutamyl transferase for identifying NAFLD, with a total score varying between 0 and 100[37].The area under the receiver operating characteristic curve (AUROC) of FLI for identifying NAFLD is 0.84[37], a low cutoff of 30 is used to rule out NAFLD (the negative likelihood ratio 0.2), and a high cutoff of 60 rule is used with a positive likelihood ratio of 4.3. However, the FLI poorly distinguishes moderate-to-severe steatosis from mild steatosis[38].
Hepatic steatosis index (HSI):The HSI is a biomarker panel consisting of BMI,diabetes, and the alanine transaminase (ALT)/ aspartate transaminase (AST) ratio. It had an AUROC of 0.79 and 0.82 in the derivation and validation groups, and the two cutoffs, 30 and 36, achieved a > 90% sensitivity and specificity[39]. However, the HSI accuracy decreases in obese children, with an AUROC of 0.67, sensitivity of 67%, and specificity of 62%[40]. In addition, like the FLI, the HSI poorly distinguishes moderateto-severe steatosis from mild steatosis[38].
SteatoTest:The SteatoTest is a biomarker panel consisting of 10 biochemical tests, age,gender, and BMI. SteatoTest exhibited an AUROC of 0.8 for identifying a > 5% liver fat content in patients with chronic liver diseases[41]. Further studies are needed to validate the SteatoTest for differentiating individuals with NAFLD from healthy people.
NAFL screening score:The NAFL screening score is an easy-to-calculate model for identifying NAFLD with age, fasting blood glucose, BMI, triglyceride, ALT/AST, and uric acid. In a study of 48,489 patients with the gold standard of ultrasound (US), the NAFL screening score had different cutoffs for males and females, with a cutoff of 32 yielding an AUROC of 0.83 for males and a cutoff of 29 yielding an AUROC of 0.86 for females[42]. In recent years, machine learning models based on laboratory parameters have been constructed. Yip et al[42]conducted a study in 922 patients involving 264 NAFLD patients diagnosed by proton-magnetic resonance spectroscopy(1H-MRS). Six biomarkers from 23 routine laboratory tests were included to construct the NAFLD ridge score, with an AUROC of 0.87-0.88. The low cutoff of 0.24 achieved a sensitivity of 92% and negative predictive value (NPV) of 95%, and the high cutoff of 0.44 achieved a 90% specificity with a corresponding positive predictive value(PPV) of 84%[42]. Other biomarker panels, such as the triglyceride and glucose index(TyG) and the FLD index, had a moderate AUROC of 0.78 (0.82-0.87) for identifying NAFLD in Chinese subjects[43-45]. In sum, most studies of biomarker panels for diagnosing NAFLD are based on suboptimal gold standards with US or1H-MRS, and few panels are validated in an independent group. Thus, future studies should not only focus on the gold standard of biopsy but also include a large independent validation group.
Imaging
US:US is the first-line imaging test used in clinical practice in individuals with suspected NAFLD[35], with a typical appearance of a hyperechogenic liver. One recent meta-analysis demonstrated that compared with histology, US had a pooled sensitivity of 85% and specificity of 94% for moderate-to-severe steatosis[46]. In contrast, US was incapable of detecting steatosis of less than 20%[36,47]or steatosis in individuals with morbid obesity[38]. In addition, the accuracy of US for hepatic steatosis assessment is affected by the presence of severe fibrosis[48]and intra- and inter-observer variability. To detect NAFLD at early stage, the computed-assisted US hepatic/renal ratio (H/R) and US hepatic attenuation rate are used to assess steatosis quantitatively[47,48]. Both measurements exhibit a slightly better performance than conventional US for assessing hepatic steatosis with an excellent performance with a sensitivity of 95% and specificity of 100%, but the NPV is still low (72% for US H/R ratio and 67% for US hepatic attenuation rate)[48,49]. In addition, this quantitative US model could improve the reliability and reproducibility in comparison with conventional US, when it is standardized by a tissue-mimicking phantom, while these findings are needed to verify in further studies[49]. Above all, US is still recommended for diagnosing moderate and severe steatosis in current guideline[44].
Computed tomography (CT):Nonenhanced CT has been used in clinics to evaluate the severity of fatty liver since 1970, based on the fact that hepatic attenuation is inversely associated with the hepatic fat content. Normal liver has an attenuation value of 50-65 HU, and 8-10 HU higher than that of the spleen. However, the attenuation value of the liver may decrease to less than 40 HU when fatty infiltration occurs. Nonenhanced CT outperforms US in evaluating the severity of fatty liver,achieving a specificity of 100% and sensitivity of 82% for diagnosing higher (>30%)degrees of hepatic steatosis[50]. Contrast-enhanced CT images are another CT model that can reduce the radiation exposure of nonenhanced CT[51]. However, contrastenhanced CT may be more suitable for severe hepatic steatosis using paraspinal or intercostal muscle as the standard reference[52]because its sensitivity for mildmoderate hepatic steatosis is only 25%[53]. CT may also be used for hepatic fat quantification, such as dual-energy CT and hepatic attenuation measurement, but these methods for assessing fatty liver should be sufficiently validated in future clinical studies[54]. Although CT is more effective for evaluating hepatic steatosis, it is also limited by insufficient accuracy for mild-to-moderate hepatic steatosis and radiation exposure, especially in children[52].
Controlled attenuation parameter (CAP):CAP, a parameter based on ultrasonic signals, is measured by the FibroScan® with an M probe (3.5 MHz), with a result of 100-400 dB/m. CAP with an M probe is reported to have an AUROC of 0.82 for differentiating any degree of steatosis vs no steatosis[55]. In addition, the cutoff of 248 dB/m yields a sensitivity of 69% and specificity of 82%[55]. In addition, the study suggests deducting 10 dB/m from the optimal cutoff of the CAP value for individuals with NAFLD or NASH. However, the M probe is less accurate in differentiating hepatic steatosis in obese people[56]. Therefore, the XL probe was devised to overcome these limitations of the M probe with a lower failure rate and low reliability for measuring liver stiffness in patients with a BMI ≥ 28kg/m2[57]. The XL probe has a higher AUROC than the M probe for distinguishing any degree of steatosis and no steatosis[58]. Even so, CAP is limited by a low sensitivity for mild steatosis and operator dependency. Few studies have compared CAP with1H-MRS for measuring steatosis, and more studies in the future are required to further explore the role of CAP for steatosis assessment.
Magnetic resonance based techniques:Magnetic resonance imaging (MRI)determines steatosis by signal intensity differences on opposed-phase or fat saturation MRI[59]. MRI-derived proton density fat fraction (MRI-PDFF) is a robust, noninvasive MRI-based methods for assessing hepatic steatosis[60]. It uses MRI-visible protons that combine with fat in the liver to quantify steatosis by dividing all protons in the liver.Tang et al[60]found that MRI-PDFF was significantly associated with the histological steatosis grade according to the NASH-CRN grade (ρ = 0.69, P < 0.001), independent of age, sex, other NASH parameters, and NASH diagnosis. The robust correlation was confirmed in several studies[61-63]. Tang et al[60]also reported an AUROC value of 0.99 for any grade of steatosis vs grade 0, 0.83 for grade 2 or higher vs grade 1 or lower,and 0.89 for grade 3 vs grade 2 or lower. In addition, MRI-PDFF is superior to other imaging tools for the assessment of hepatic steatosis[64,65], and its performance is not affected by obesity. MRI-PDFF is also regarded as a robust noninvasive method to monitor the treatment effect[66]; this aspect will be described in detail below.1H-MRS is another MR-based technique that directly measures the chemical compositions of the liver[67]. It is usually used in clinical studies of NAFLD representing biopsy for measurement of intrahepatocellular lipid (IHCL) through calculating PDFF[6,52].1HMRS was reported to have a high correlation with biopsy in steatosis assessment[69]and a sensitivity of 80% for diagnosis of liver fat content ≥ 5%[70].1H-MRS was reported to have a good accuracy to detect small amounts of liver fat. Nasr et al[6]found that1H-MRS had a specificity of 100% and sensitivity of 79% with a PDFF cutoff value of 3%, a specificity of 94% and sensitivity of 87% with a PDFF cut-off value of 2%. Although recognized as the most accurate noninvasive tool to assess PDFF quantitatively, MRS is limited to its device- and operator-dependency, complexity,and potentially errors[71]. Complex-based chemical shift imaging-based MRI (CSEMRI)is regarded as a promising method to quantify PDFF, which could quantitatively assess liver fat content with a refined pulse sequence[72-74]. It exhibits a high correction with MRSPDFF (r2 = 0.985 for 1.5 T MR systems, r2 = 0.991 for 3.0 T MR systems)[71].MR diffusion weighted imaging (DWI) measures motion of water protons diffusing and tissue perfusing[75,76]and is regarded another promising tool for assessing liver fat content[77], while it exerts poor performance for detecting steatosis in comparison with MRS and dual echo in phase and out of phase imaging[78]. Therefore, more studies are needed to evaluate the performance of DWI in the future.
Clinical implication
US is recommended as the first-line diagnostic method in assessing steatosis, while serum biomarkers and biomarker panels are alternative tools when imaging tools are not available in larger scale screening studies (Table 1)[35]. An increasing number of biomarker panels are used in clinical and research applications, while most are validated in studies with relatively small populations, in individuals at their health checkup, or in studies with suboptimal gold criteria. Therefore, future well-designed studies are needed to develop a more effective noninvasive biomarker panel for identifying NAFLD. MRI-PDFF not only exerts an excellent performance for diagnosing NAFLD but also accurately detects changes in fat content during disease progression[79]; however, MRI-PDFF is costly, time-consuming, and device dependent,which makes it difficult for wide application. More effective, feasible, and easily operated tools are needed for diagnosing NAFLD, especially for early steatosis.
DIAGNOSIS OF NASH
NASH is characterized by steatosis, ballooning, and inflammation, with/without fibrosis, which accelerates disease progression. Early detection of NASH is conducive to the prevention of NASH-related fibrosis. Noninvasive biomarkers for NASH include simple serum biomarkers, biomarker panels, and imaging.
Serum biomarkers
Cytokeratin-18 (CK18):CK18, an intermediate filament protein, is one of the most studied biomarkers for the diagnosis of NASH. It is cleaved during the period of cell death, containing CK18 M30 and CK18 M65[80]. A meta-analysis of 25 studies reported that M30 and M65 had pooled AUROCs of 0.82 and 0.80, while the pooled sensitivity and specificity were 75% and 77%, and 71% and 77%, respectively[81]. Therefore, CK18 is commonly used with other serum biomarkers to diagnose NASH. Anty et al[82]found that combining metabolic syndrome, ALT, and CK18 in a morbidly obese population could achieve an AUROC of 0.88 compared with CK18 alone, with an AUROC of 0.74. Grigorescu et al[83]reported that the triple combination of adiponectin,CK18, and interleukin (IL)-6 achieved an AUROC of 0.90, a specificity of 85.7%, and a sensitivity of 84.5%. However, the results should be further verified in future studies.In addition, some studies have examined the difference in the accuracy of CK18 in assessing NASH with different stages of fibrosis. Huang et al[84]found an AUROC of 0.93 for NASH with fibrosis stages 3-4 and 0.63-0.78 for NASH with fibrosis stages 0-2,which may indicate that CK18 can predict the disease severity in NASH patients.
Inflammatory markers:CXCL10 is a proinflammatory cytokine involved in diabetes and obesity[85]. In a previous study, CXCL10 exhibited a moderate accuracy for differentiating NASH from simple steatosis (AUROC, 0.68) and non-NASH (AUROC,0.77)[86]. Tumor necrosis factor-α (TNF-α) and IL-8 are common inflammatory markers,which also exhibit a moderate performance with a sensitivity and specificity of 72%and 76%, and 65% and 68%, respectively[87]. However, when combining these two markers with pyroglutamate, the panel could achieve a sensitivity of 91% and specificity of 87%[87].
Adipocytokines and hormones:Fibroblast growth factor 21 (FGF21) secreted by the liver is another potential biomarker for NASH. One study reported that FGF21 had an AUROC of 0.62, and the two cutoffs of 126 and 578 pg/mL had a > 90% sensitivity and specificity for diagnosing NASH, but the PPV and NPV of FGF21 were moderate(0.59-0.78) and low (0.49-0.60), respectively[88]. To improve the PPV and NPV, FGF21 was combined with CK18, which improved the PPV to 82% and the NPV to 74%.Adiponectin was reported to be decrease in NASH patients[89], which had an AUROC of 0.71 for diagnosing NASH[83]. However, the AUROC could reach to 0.90 when adiponectin was combined with CK18 M65 and IL-8[83]. Other adipocytokines, such as leptin and resistin, may be potentially markers for diagnosing NASH, while they are needed to be further validated in more groups[29].
Other serum biomarkers:Serum iron is a common protein associated with oxygen radicals, which contribute to necroinflammation and fibrosis, two important parameters of NAFLD[90,91]. Serum iron was higher in individuals with NASH than in those with simple steatosis[92,93]. In a Japanese study, serum ferritin exhibited a moderate performance for diagnosing NASH (AUROC, 0.73)[94]. Another study of 619 biopsy-proven NAFLD patients constructed a scoring system that combined serum ferritin with type IV collagen 7S and fasting insulin, which could be used to predict NASH with an AUROC of 0.78-0.85[95].
Biomarker panel
NASHTest:The NASHTest combines 13 parameters to diagnose NASH in three categories, namely, NASH, Borderline NASH, and No-NASH, according to Kleiner's criteria[96,97]. A study with 257 people found that the NASHTest achieved an AUROC of 0.79 for NASH, 0.69 for borderline NASH, and 0.77-0.83 for no-NASH[98].
NASH ClinLipMet score:The NASH Clin score is a biomarker panel combining AST,fasting insulin, and the PNPLA3 genotype at rs738409, which achieved an AUROC of 0.78 for diagnosing NASH in 384 patients with a histological diagnosis[98,99]. To improve the accuracy, Zhou et al[98]added metabolic syndrome-based factors to the NASH Clin score, which was named the ‘NASH ClinLipMet score‘. This latter score can improve the AUROC to 0.87 and the sensitivity to 75%. However, it is moresuitable for research because the measurement of fasting insulin and PNPLA3 genotype is costly and complex in clinical practice.
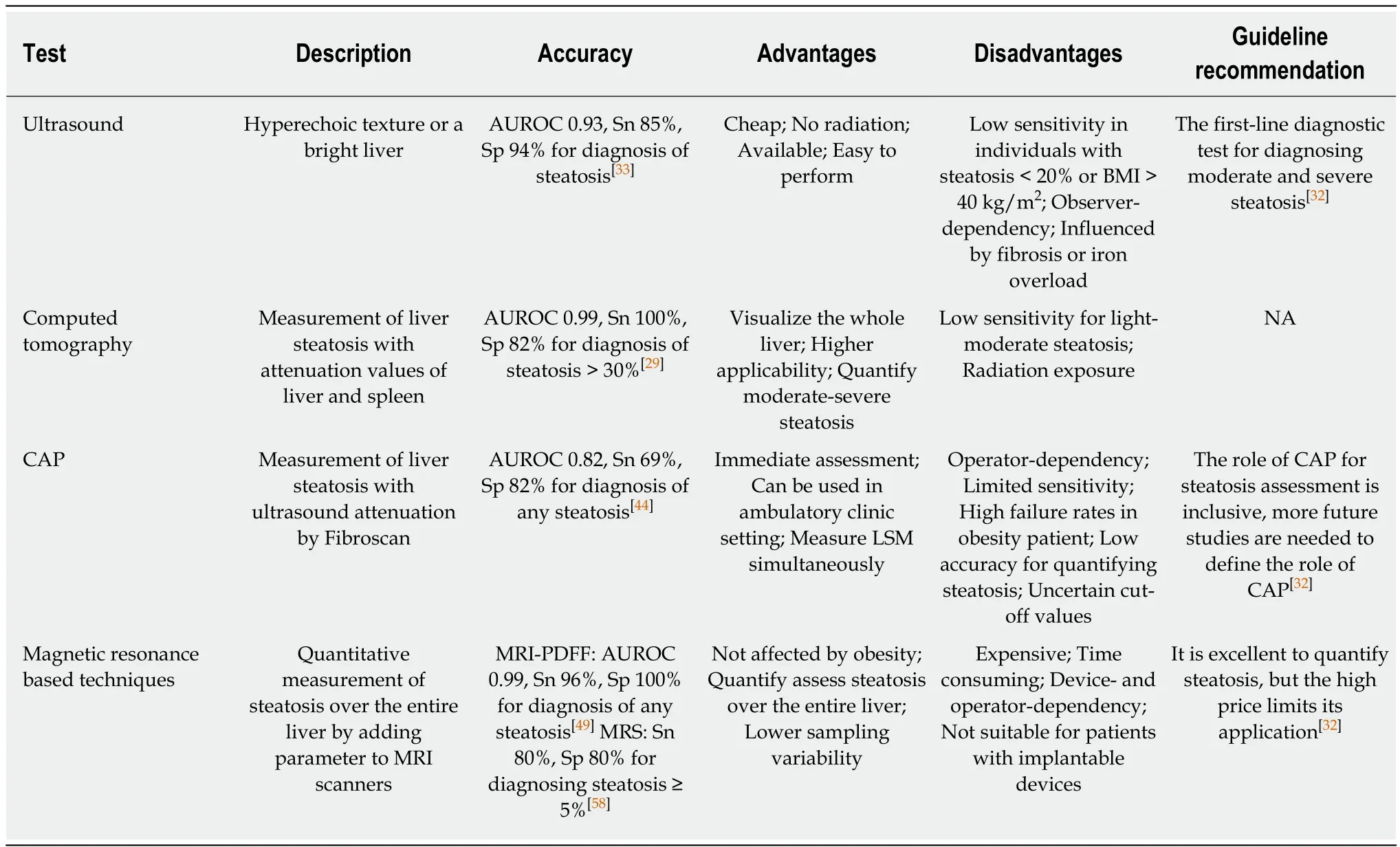
Table 1 Imaging modalities for diagnosing nonalcoholic fatty liver disease
Other biomarker panels:Tai et al[100]constructed a simple biomarker panel with the parameters of BMI, ALT, and triglycerides. It achieved an AUROC of 0.80-0.82 in the training and validation cohorts and only included 180 morbidly obese patients after bariatric surgery. Li et al[101]developed a clinical score with ALT, gamma-glutamyl transpeptidase, C-reactive protein, and ApoB/ApoA1 ratios. The cutoff of 3.8 gave a sensitivity of 90% and a specificity of 87% for distinguishing NASH from NAFLD, but the panel is limited to a small sample and lacks validation in an independent group.
Imaging for NASH
NASH consists of various parameters; thus, it is difficult to use routine imaging techniques (ultrasonography, CT, or MRI) to distinguish between NASH and simple steatosis. Elastography was investigated to distinguish NASH and simple steatosis.Chen et al[102]found that the cutoff of 2.74 kPa of magnetic resonance elastography(MRE) had an AUROC of 0.93, but the study had several limitations, such as a small sample and a clear histological definition. Vibration-controlled transient elastography(VCTE) was performed in South Korean patients with an AUROC of 0.75 and a sensitivity of 86% for diagnosing NASH, but the specificity was only 58%[103]. Another biomarker, liver iron accumulation (LIC), measured by the MR signal decay values, is reported to be significantly related to NAFLD disease severity or fibrosis progression.The MRI-based technology assessing LIC was found to have an AUROC of 0.91 for assessing NASH, with a sensitivity of 83% and specificity of 80%[103]. Multiparametric MRI technology was used to quantify hepatic steatosis, iron accumulation and fibrosis by 1H-MRS, a T2* map and a T1 relaxation time map, respectively[104-107]. The technology is regarded as a promising imaging biomarker in small studies[108]but awaits independent confirmation from larger trials.
New biomarkers
Many potential biomarkers involving NASH are under study[109-114]. Circulating microRNAs are potentially regarded as attractive biomarkers for NAFLD disease severity due to their stability. A meta-analysis found that miR-34a was reported to have a moderate AUROC of 0.78[115]. MiR-122 had a pooled AUROC of 0.64-0.70 for differentiating NASH and simple steatosis[116,117]. The combination of miR-122, -192,and -21 with CK18-Asp396 achieved an AUROC of 0.83 for diagnosing NASH, while the optimal cutoff gave a moderate sensitivity and specificity[118]. Other new methods have been investigated, such as breath volatile organic compounds (VOCs). Breath VOCs are closely related to oxidative stress, inflammation, and liver diseases[119-121].Froukje et al[122]found that a panel consisting of three exhaled compounds, 1-propanol,3-methyl-butanonitrile, and n-tridecane, had an AUROC of 0.77, PPV of 81%, and NPV of 82% for differentiating NASH and non-NASH. In addition, some studies have focused on omic markers. The production of lipidomic, proteomic, metabolomics, and microbiome markers was elevated in NASH patients[123-131], but more studies with larger validation groups in the future are needed to confirm these findings.
Clinical implication
Noninvasive biomarkers for NASH are an attractive field. CK18 is regarded as a popular biomarker for NASH, but the accuracy varies in current studies. Biomarker panels perform well in diagnosing NASH, but most of them are not validated in an independent group. Although other noninvasive biomarkers, such as imaging and gene biomarkers, are reported to be relatively high in accuracy, effective methods should be available, simple, inexpensive, and accurate in the clinic. In addition, serum biomarkers (e.g., CK18) are less accurate for diagnosing NASH with mild fibrosis,which could lead to higher rates of misdiagnosis. To improve the diagnosis of early NASH, biomarker panels or the combination of serum biomarkers with imaging may contribute to ruling in or ruling out NASH with early fibrosis, but this prospect should be verified in future studies.
DIAGNOSIS OF NAFLD RELATED FIBROSIS
According to the recommendation of the NASH-CRN, fibrosis is categorized into nonfibrosis or mild fibrosis (Metavir = F0-1), significant fibrosis (SF, Metavir ≥ F2),advanced fibrosis (AF, Metavir ≥ F3), and cirrhosis (Metavir = F4)[88]. The fibrosis stage is reported to increase the overall mortality in individuals with NAFLD, but not NASH[127]. Furthermore, SF, AF, and cirrhosis increased the hazard ratios by 1.6-, 3.04-,and 6.53-fold for overall mortality in comparison with that of F0-F1[127]. Therefore, it is urgent to identify early fibrosis through effective noninvasive methods.
Proprietary biomarkers of fibrosis
The proprietary biomarkers of fibrosis include the procollagen of type III collagen(PIIINP), precursor C3-protein (PRO-C3), hyaluronic acid (HA), and TIMP1. Serum PIIINP is a common fibrosis marker during fibrogenesis. It has a good performance for diagnosing SF (AUROC, 0.81)[128]. Another PRO-C3 is a marker of the N-terminal propeptide of type III collagen. Several studies have demonstrated that PRO-C3 has an AUROC of 0.75-0.83 for diagnosing AF and 0.76 for cirrhosis[129,130]. HA is an important element of the extracellular matrix, and it has AUROCs of 0.87, 0.89, and 0.92 for SF, AF, and cirrhosis, respectively[131]. TIMP1 is a fibrosis biomarker reflecting tissue matrix remodeling, while TIMP1 shows a moderate performance for diagnosing SF (AUROC, 0.74)[128]. To improve the accuracy, some models were constructed by combining several specific fibrosis biomarkers or combinations of these fibrosis biomarkers with other variables. The enhanced liver fibrosis (ELF) test is a commercial tool that combines three circulating matrix turnover components,including HA, PIIINP, and TIMP-1, with age[128]. Using a cutoff of 9.8, the ELF test identified AF with a PPV of 72% and NPV of 97%[132]. Another model consisting of PRO-C3, age, platelets, and the presence of diabetes can achieve an AUROC of 0.86-0.87 and an NPV of 0.97 for identifying AF[129]. However, further studies validating these biomarkers in a large independent group are needed in the future.
Nonproprietary biomarkers of fibrosis or biomarker panels
AST-to-platelet ratio index (APRI):The APRI was originally a simpler calculation for diagnosing fibrosis severity in chronic hepatitis C[133]. A recent meta-analysis reported that the APRI had an AUROC of 0.70 for SF, 0.75 for AF, and 0.75 for cirrhosis[49].Additionally, the pooled sensitivity of the APRI was relatively low, with a range of 0.33-0.73 for different cutoffs.
FIB-4:FIB-4 is a common biomarker panel used for assessing fibrosis severity and includes age, platelet count, AST, and ALT. FIB-4 was primarily devised to assess the liver fibrosis severity in hepatitis C patients who were also infected with human immunodeficiency virus[134]. An AUROC value of 0.75 for SF, 0.80 for AF, and 0.85 for cirrhosis was reported in NAFLD patients[49]. Two cutoffs were used for a higher PPV and NPV. For instance, using a cutoff of 1.3 for FIB-4, the panel predicted AF with an 85% sensitivity, 65% specificity, 36% PPV, and 95% NPV. On the other hand, using a cutoff of 3.25, FIB-4 predicted AF with a 26% sensitivity, 98% specificity, 75% PPV,and 85% NPV[135]. The two cutoffs may improve the PPV and NPV, avoiding unnecessary biopsy, while the specificity of FIB-4 was 0.35 for assessing AF in elderly individuals ≥ 65 years of age, which contributed to a high false positive rate[136].Therefore, this study recommended a low cutoff of 2 for elderly patients > 65 years of age, with a 77% sensitivity and 70% specificity. In addition, a recent Japanese study of 1050 biopsy-confirmed NAFLD patients recommended cutoffs of 1.88 and 2.67 for 60-69 years of age and 1.95 and 2.67 for ≥ 70 years of age[137].
NAFLD fibrosis score (NFS):The NFS is the most common noninvasive biomarker panel for assessing fibrosis severity; the panel consists of age, BMI, hyperglycemia,AST/ALT ratio, platelets, and albumin. A multicenter study of 733 people reported a low cutoff of -1.455 for AF with a PPV of 51%-56% and NPV of 88%-93%, and a high cutoff of 0.676 yielded a PPV of 82%-90% and NPV of 80%-85%[138]. Using this model,75% of biopsies could be spared with 90% correct prediction. In addition, Xiao et al[49]demonstrated that the NFS had an AUROC of 0.72 for SF, 0.73 for AF, and 0.83 for cirrhosis. The NFS was widely validated in different races, with a high AUROC and NPV[135,137,138]. However, a low cutoff of 0.12 for NFS assessing fibrosis is recommended for the elderly due to a high false positive rate[136]. The NFS and FIB-4 are recommended to identify those at low or high risk for AF or cirrhosis in clinical guidelines.
BARD score:The BARD score was an easily calculated score system for assessing fibrosis severity, containing the parameters of BMI, aldosterone renin activity ratio,and the presence of type 2 diabetes mellitus. A score of 2-4 increased the risk of AF by 17-fold, with an AUROC of 0.81 and NPV of 96%, but a low PPV of 43%[139]. However,a subsequent study validated that the tool in the Japanese group could not achieve a similar performance with an AUROC of 0.73 and NPV of 77% for AF[140]. In addition, a meta-analysis reported that the BARD score had a pooled AUROC of 0.64 for SF, 0.73 for AF, and 0.70 for cirrhosis in NAFLD patients[49]. Even so, the BARD score was a valuable model for predicting SF due to its ease and lack of indeterminate results in clinical application.
Imaging
VCTE:VCTE is the first Food and Drug Administration (FDA)-approved elastographic modality performed by FibroScan employing US-based technology.This technology measures the velocity of a 50 MHz shear wave that is emitted by a probe in the intercostal space into the liver. The velocity is positively related to liver stiffness with a range of 1.5 to 75 kPa. A higher shear wave value indicates higher liver stiffness. However, technical failure was found to be a common phenomenon during the operation, ranging from 6.7% to 27.0%, and was primarily reported to be related to a high BMI[141,142]. The “M” probe was the most prevalent probe measuring shear wave velocity, with an AUROC of 0.83 for SF, 0.87 for AF, and 0.92 for cirrhosis[49]. Although the “XL” probe was usually used for fibrosis in obese people to reduce the failure rate, this rate was still 35% in patients with a BMI over 30 kg/m2[143].Even so, the FibroScan XL probe yields an AUROC of 0.82 for SF, 0.86 for AF, and 0.94 for cirrhosis. One study investigating the suitable cutoffs indicated that 5.8 and 9.0, 7.9 and 9.6, and 10.3 and 11.5 had a > 90% sensitivity and specificity for SF, AF, and cirrhosis, respectively[141]. However, the PPV was low for diagnosing fibrosis, and transient elastography easily misclassifies AF as mild. One study comparing transient elastography with the NFS and FIB-4 found that transient elastography was better for AF and cirrhosis but less accurate for diagnosing fibrosis vs nonfibrosis and significant fibrosis[70]. Therefore, some studies have used VCTE along with a serum biomarker. Thomson et al[144]combined VCTE with a FibroMeter and achieved a PPV of 84% for SF and PPV of 89% for AF.
Shear wave elastography (SWE):SWE is a new method integrated into conventional US for assessing fibrosis. It can measure the shear wave velocity and provide a 2-D,real-time, color map of liver elasticity, but it should be conducted under apnea, and the region of the color map should be large vessel-free and at least 15 mm below the capsule. SWE reportedly has a high diagnostic performance for fibrosis assessment in chronic hepatitis patients[145,146]. In NAFLD patients, SWE yielded an AUROC value of 0.86 for SF, 0.89 for AF, and 0.88 for cirrhosis, respectively[147]. The results also demonstrated that SWE was better than FibroScan and acoustic radiation force impulse (ARFI). No specific regulations are recommended by the manufacturer for assessing the quality of measurement; thus, some studies assessed the failure rate of SWE with reliability criteria of FibroScan[147]. In addition, as with the ARFI, the accuracy of SWE is affected by interobserver variation and food intake[148]. Therefore,these measurements are recommended to be performed by very experienced radiologists in patients with fasting for at least 2 h[148].
ARFI:ARFI elastography is an alternative tool for fibrosis assessment integrated into conventional US. It uses short-term acoustic pulses to produce shear waves[149], with the results expressed in m/s. ARFI should be operated under apnea, and the region of interest should be a vessel-free region. ARFI had an AUROC of 0.77 for SF, 0.84 for AF, and 0.84 for cirrhosis[147]. Another meta-analysis reported that the pooled sensitivity and specificity were 80.2% and 85.2%, respectively, for detecting SF[150].However, its accuracy was affected by the presence of severe steatosis[151,152]. Further studies are needed to explore the optimal cutoffs of ARFI at different levels of steatosis.
MRE:MRE is a noninvasive MRI-based method measuring liver stiffness by using a modified phase-contrast method[153-156]. MRE can assess the entire liver with a high success rate[157]. It is not affected by steatosis and may be applied in patients with obesity, ascites, or bowel interposition between the liver and anterior abdominal wall[158]. The available MRE model contains 2D-MRE (shear wave frequency 60Hz)and 3D-MRE (shear wave frequency 40Hz). 2D-MRE is more frequently used for assessing liver fibrosis in NAFLD patients. A meta-analysis reported that the pooled AUROCs of 2D MRE for diagnosing SF, AF, and cirrhosis were 0.87, 0.90 and 0.91,respectively[159]. 3D-MRE had a better performance (AUROC, 0.98) for detecting AF than 2D-MRE (AUROC, 0.92)[160], and the NPVs of 2D-MRE and 3D-MRE were 0.98 and 1.0, respectively[160]. Compared to other noninvasive fibrosis biomarkers, MRE was superior to FibroScan, ARFI, and common biomarker panels for discriminating dichotomized fibrosis stages in NAFLD patients[65,161]. Xiao et al[42]found that MRE had an AUROC of 0.96, sensitivity of 0.84, and specificity of 0.90 for detecting AF, which was better than BARD score, NFS, and FibroScan. Considering the higher accuracy of MRE in diagnosing fibrosis, it is increasingly regarded as a promising surrogate biomarker for monitoring fibrosis progression and endpoints of fibrosis therapy[60].However, MRE has several limitations. It cannot be applied to individuals with hepatic iron overload due to the interfering signal intensity. On the other hand, the cost of MRE and its dependence on MRI facilities limit its wide application.
New biomarkers
Serum DNA methylation has been investigated as a potential biomarker for assessing fibrosis. The plasma DNA methylation of PPARγ promoter was reported to have a good performance for diagnosing AF (AUROC, 0.91), and the cutoff of 0.81 gave a PPV of 91% and NPV of 87%[162]. In addition, the DNA methylation at the PPARγ promoter is superior to the NFS in diagnostic performance and avoids using two cutoffs, but it should be validated in more independent groups.
Clinical implication
Biomarker panels are cheap, feasible, reproducible, and have a good NPV for fibrosis,but they are limited by its low PPV (Table 2). MRE shows excellent accuracy for fibrosis severity but may only be used in some drug studies due to its high cost and unavailability (Table 3). Transient elastography together with biomarker panels would be widely used for assessing fibrosis, but the efficiency should be evaluated in more independent groups. Above all, it is recommended to combine serum biomarkers or clinical rules with imaging tools to diagnose fibrosis, which could reduce unnecessary diagnostic liver biopsies.
NONINVASIVE BIOMARKERS FOR DISEASE PROGRESSION AND THERAPY
Tracking disease progression
NAFLD significantly increases the risk of liver disease-related morbidity, mortality,and liver transplantation[163,164]. Fibrosis, but not simple steatosis and NASH, increased the risk of mortality in NAFLD patients in a retrospective study with a mean followup period of 20 years[119]. Singh et al[165]found that one stage of fibrosis progression takes 14.3 years and 7.1 years in individuals with simple steatosis and NASH patients,respectively. In addition, most NAFLD cases are asymptomatic until the disease has progressed to cirrhosis, and repeated biopsy is impractical. Therefore, there is a need to apply useful noninvasive biomarkers to monitor disease progression. A prospective study with a median of seven follow-ups found that the ELF test had an AUROC of 0.87 for predicting liver-related clinical outcomes, which was higher than that ofbiopsy (AUROC, 0.82)[166]. Sebastiani et al[167]found that baseline liver histology, APRI,FIB-4, and NFS for predicting clinical outcomes had AUROCs of 0.85, 0.89, 0.89 and 0.79, respectively. Another study reported that FibroScan had an accuracy of 0.73 for predicting all-course mortality[168]. Further studies are needed to determine more effective noninvasive biomarkers for the progression of NASH to NASH-related fibrosis and the progression of NASH-related fibrosis to adverse clinical outcomes.
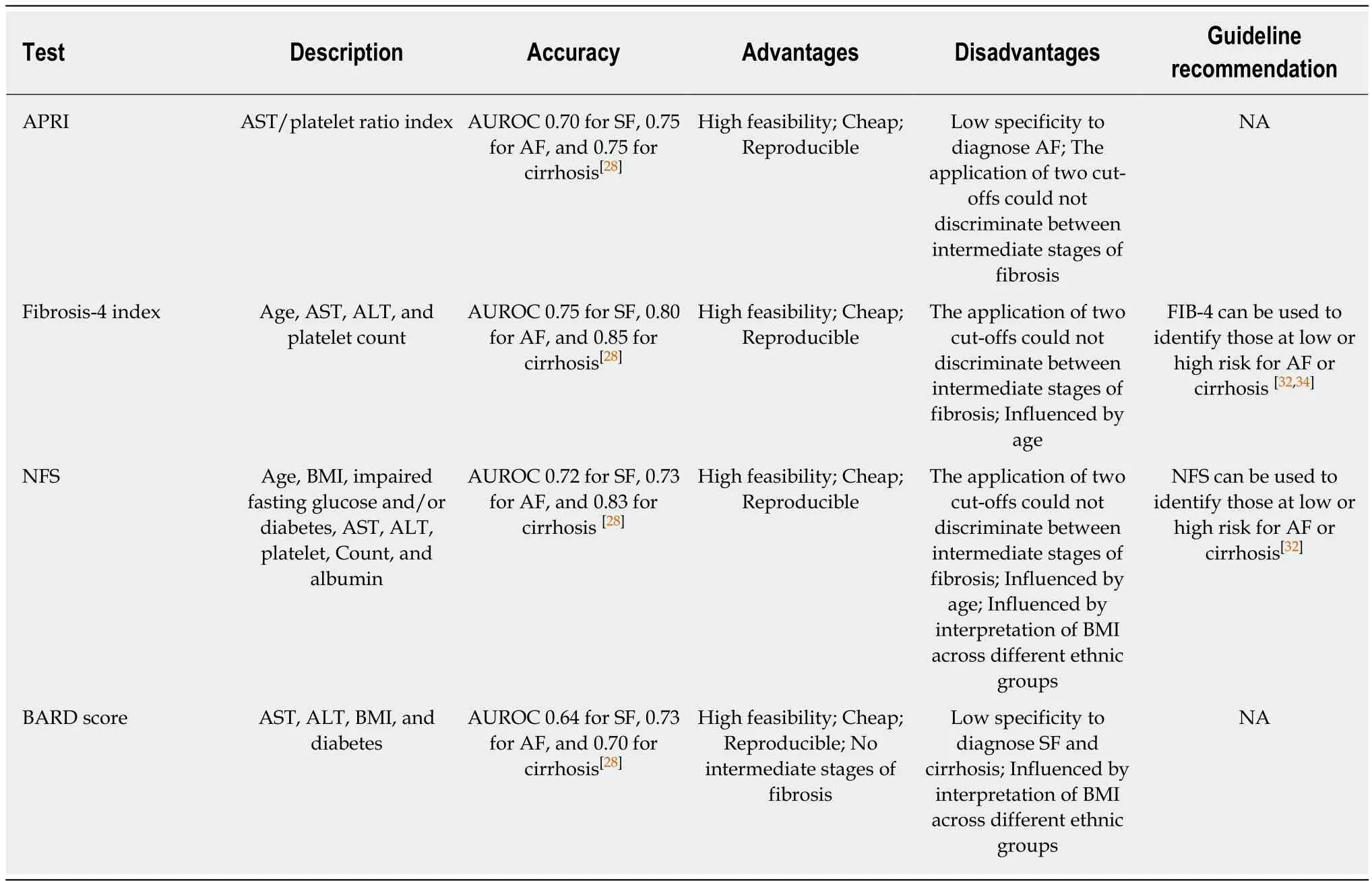
Table 2 Biomarker panels for diagnosing nonalcoholic fatty liver disease related fibrosis
Monitoring responses to therapies
In terms of NAFLD treatment, it is impractical to observe the primary endpoint of mortality due to long-term follow-up[28,169,170]. Therefore, the FDA recommends that histological improvement be confirmed when the resolution of NASH is obtained without the worsening of fibrosis or when fibrosis is improved without the worsening of NASH[171]. However, repeated biopsy hinders the development of drugs; thus, there is a need to investigate noninvasive surrogates replacing biopsy. MRI-PDFF was usually employed to evaluate the liver fat content change in clinical trials of NASH patients[66]. A study of 113 NASH patients treated with obeticholic acid found that MRI-PDFF had an AUROC of 0.81 for reduced histological steatosis grade[172]. In contrast, a recent phase II trial of selonsertib found that MRI-PDFF had an AUROC of 0.70 for reduced histological steatosis grade, and the optimal cutoff was 0% with a PPV of 39% and NPV of 92%[173]. Therefore, whether the change in MRI-PDFF could be regarded as an effective surrogate endpoint for NASH treatment should be further evaluated. Liver function has been regularly regarded as a noninvasive biomarker for assessing the monitoring treatment effect, while ALT concentrations in about twothirds of patients is normal[174], and NASH patients usually exhibit spontaneous changes in liver function. Therefore, the ALT change is usually accompanied by a steatosis change, which is regarded as an effective noninvasive endpoint substituting the histological changes in NASH[171]. The change in liver stiffness measurement(LSM) measured by MRE was evaluated to investigate the antifibrosis effect in NAFLD. Jayakumar et al[173]showed that the MRE had an AUROC of 0.62, PPV of 39%,and NPV of 92% for fibrosis improvement. The biomarker panel has also been investigated for predicting fibrosis improvement in intervention studies of NASH patients. Vilar et al[175]constructed a model consisting of three variables, glycatedhemoglobin, platelets, and ALT, which demonstrated an AUROC of 0.96 for fibrosis improvement, which is higher than the change in platelet count (AUROC, 0.80), APRI(AUROC, 0.50), FIB-4 index (AUROC, 0.63), and NFS (AUROC, 0.77). The biomarker panels may be the ideal noninvasive tools for assessing the response during the process of therapy, but they should be accurate, available, inexpensive, and simple.
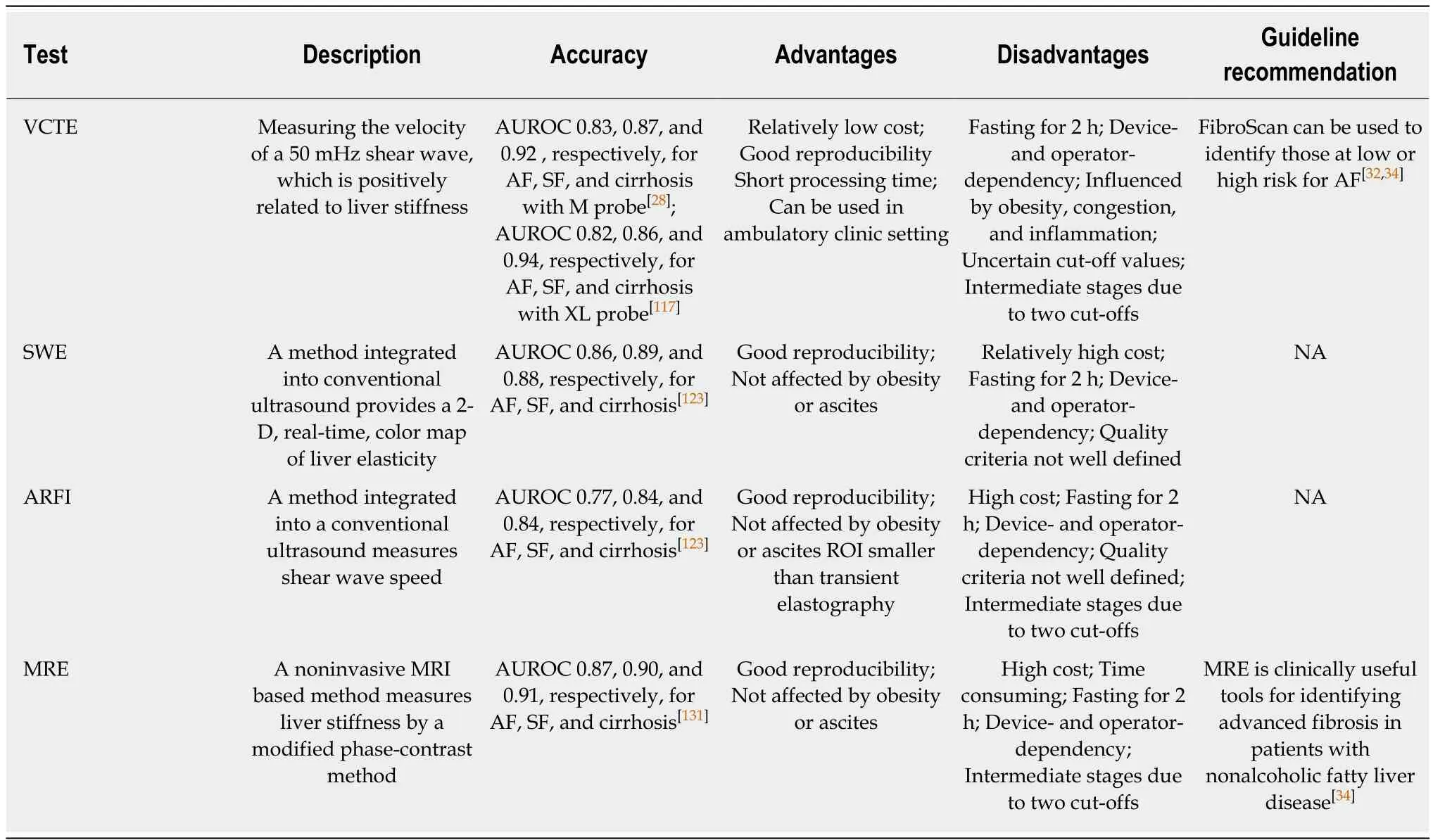
Table 3 Imaging modalities for diagnosing nonalcoholic fatty liver disease related fibrosis
CONCLUSION
The past several years have witnessed the extensive development of noninvasive methods in the NAFLD field, from serum biomarkers and imaging to omics. US and H-MRI have a relatively high accuracy for diagnosing NAFLD, and US is prevalently used in clinical practice and research due to its availability and low cost. There are currently no effective noninvasive biomarkers recommended for diagnosing NASH.Future studies are needed to investigate more efficient noninvasive biomarkers for distinguishing NASH from simple steatosis. VCTE is the FDA-approved elastographic model for assessing fibrosis severity, and it could further improve the diagnostic performance when combined with biomarker panels. Furthermore, effective algorithms consisting of imaging and nonimaging biomarkers should be applied to clinical practice to reduce unnecessary biopsies (Figure 1). In addition, there is a need to investigate the cost-effectiveness of noninvasive evaluations in diagnosing NAFLD,tracking disease progression, and monitoring responses to the therapies.
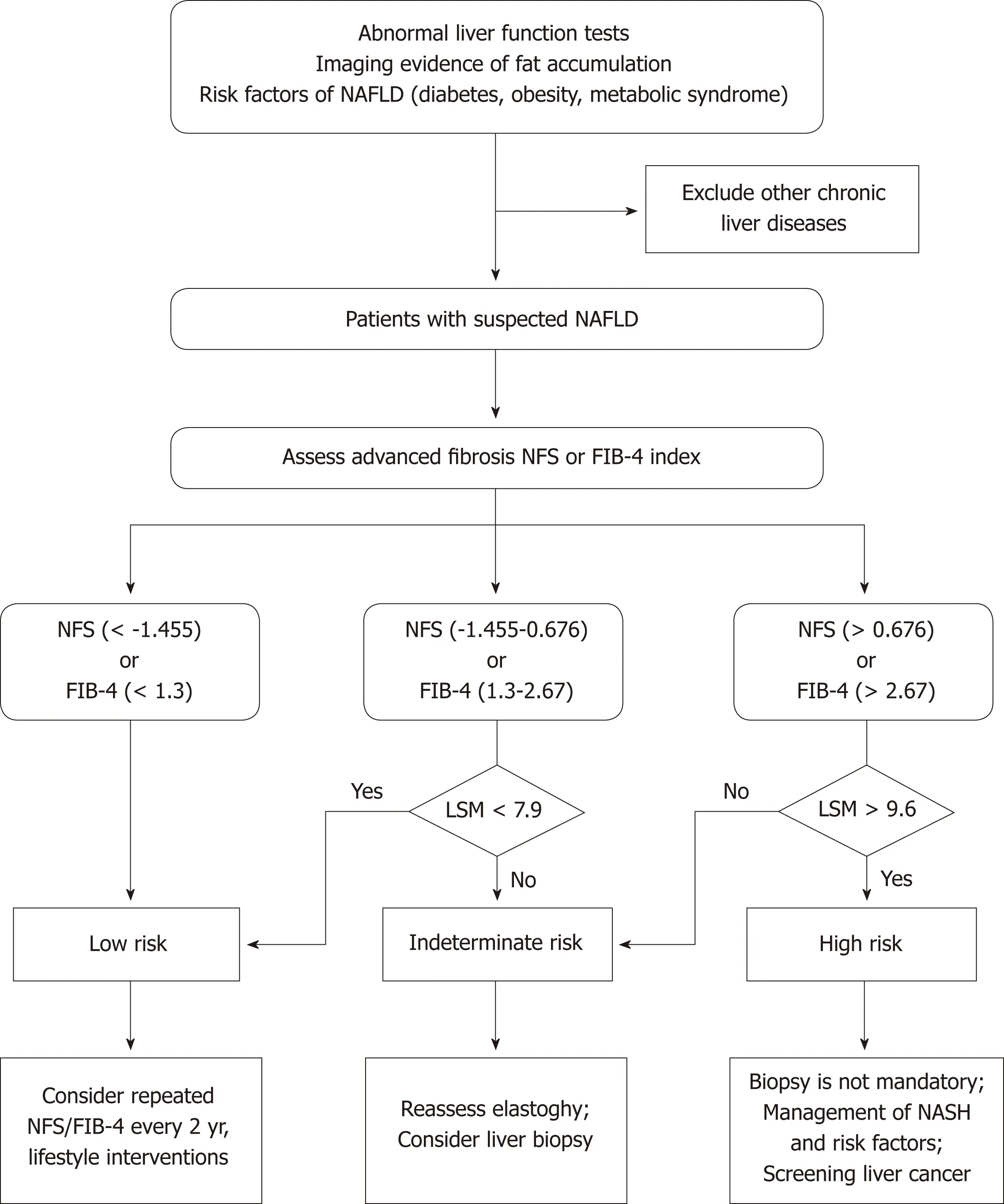
Figure 1 Clinical algorithm with noninvasive testing and liver content measurement by Fibroscan for detecting advanced fibrosis in nonalcoholic fatty liver disease patients.
ACKNOWLEDGEMENTS
The authors thank the staff at Institute of Model Animal of Wuhan University and Department of Cardiology, Renmin Hospital of Wuhan University.
杂志排行
World Journal of Gastroenterology的其它文章
- Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma
- Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals
- Clinical assessment and identification of immuno-oncology markers concerning the 19-gene based risk classifier in stage lV colorectal cancer
- Hemodynamic changes in hepatic sinusoids of hepatic steatosis mice
- Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus
- Early gastric cancer diagnostic ability of ultrathin endoscope loaded with laser light source
