Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma
2019-03-28SangMiLeeHongSooKimSangheunLeeJeongWonLee
Sang Mi Lee, Hong Soo Kim, Sangheun Lee, Jeong Won Lee
Abstract Hepatocellular carcinoma (HCC) is one of major causes of cancer mortality worldwide. For decades, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been widely used for staging, predicting prognosis, and detecting cancer recurrence in various types of malignant diseases. Due to low sensitivity of FDG PET for detecting intrahepatic HCC lesions, the clinical value of FDG PET in HCC patients has been limited. However, recent studies with diverse analytic methods have shown that FDG PET has promising role in aiding management of HCC patients. In this review, we will discuss the clinical role of FDG PET for staging, predicting prognosis, and evaluating treatment response in HCC. Further, we will focus on recent clinical studies regarding implication of volumetric FDG PET parameters, the significance of FDG uptake in HCC for selecting treatment and predicting treatment response, and the use of radiomics of FDG PET in HCC.
Key words: Hepatocellular carcinoma; Fluorodeoxyglucose F18; Positron emission tomography; Staging; Prognosis
INTRODUCTION
In 2018, approximately 841000 new cases of liver cancer and 782000 associated deaths occurred worldwide, with liver cancer being the second leading cause of cancer death for males[1]. Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for 75%-85% of cases of liver cancer[1]. HCC is well-known as a highly lethal cancer, showing similar numbers of new cases and cancer deaths[1,2].Hepatitis B virus (HBV), hepatitis C virus (HCV), and alcohol intake are leading causes of HCC. In developed countries, obesity is an emerging risk factor for HCC[2,3].The Barcelona Clinical Liver Cancer (BCLC) staging system, endorsed by the European Association for the Study of the Liver and the European Organization for Research and Treatment of Cancer (EASL-EORTC), has been considered as the standard staging system for HCC in clinical practice because it links tumor stage to treatment strategy and has been validated in various different clinical situations[4]. For HCC patients with stage 0 (single tumor < 2 cm) and stage A (single tumor > 2 cm or 3 tumor nodules < 3 cm), curative treatments including surgical resection, liver transplantation, and local ablation have been indicated[4]. For HCC patients with stage B (multinodular tumors), transarterial chemoembolization (TACE) is primarily recommended[4]. For HCC patients with stage C (tumors with portal vein invasion and/or extrahepatic spread), sorafenib is recommended as the first-line treatment[4].
18F-fluorodeoxyglucose (FDG), a glucose analog, is carried into viable cells by glucose transporter and subsequently phosphorylated by hexokinase, which has the same metabolic pathway as glucose[5]. Therefore, FDG on positron emission tomography (PET) has been widely used as an imaging marker of glucose metabolism of normal organs and cancer tissue[6-8]. Since cancer cells show high rates of glycolysis,FDG uptake is increased in cancer tissue[5]. Thus, FDG PET has shown incremental value for diagnosing, staging, predicting prognosis, and restaging diverse kinds of malignant diseases[5,9-11]. In contrast, FDG uptake in HCC varies according to the differentiation of HCC lesion[12,13]. Earlier studies with FDG PET have shown a low diagnostic ability for detecting intrahepatic HCC lesions[12-14], leading to a preconception that FDG PET has limited clinical value in patients with HCC.Nevertheless, diverse attempts have been performed to establish clinical role of FDG PET in HCC and recent studies have shown encouraging results of FDG PET in aiding management of patients with HCC.
In this review, we first summarized characteristics of HCC cells related with FDG uptake and results of studies regarding diagnostic and prognostic values of FDG PET in HCC. We then overviewed recent studies that dealt with volumetric FDG PET parameters, the significance of FDG uptake in HCC for selecting treatment and predicting treatment response, and radiomics of FDG PET in patients with HCC.
RELATIONSHIP BETWEEN HCC CHARACTERISTICS AND FDG UPTAKE
Because cancer cells have higher glycolytic rates than normal cells, higher amounts of glucose transporter and hexokinase expression are observed in tumor cells, resulting in increased FDG uptake in cancer lesions[15,16]. However, HCC cells show different expression patterns of proteins related to FDG uptake. HCC has lower level of glucose transporter-1 expression than cholangiocarcinoma and hepatic metastatic lesion[17,18].Furthermore, high expression of glucose-6-phosphatase which hydrolyzes FDG-6-phosphate to FDG that can be transported out of the cell has been observed in HCC[19].These different expression patterns contribute to low accumulation of FDG uptake in HCC, thereby reducing sensitivity for detecting HCC lesions[19,20]. Nevertheless,increased glucose transporter expression and hexokinase activity have been demonstrated in high-grade HCC which is positively correlated with FDG uptake[19,21].Therefore, diverse degrees of FDG uptake have been shown according to the histopathological grade of HCC[12,13,20]. Well-differentiated HCC reveals tumor-to-nontumor liver uptake ratio (TLR) of around 1.1, indicating difficulty of differentiating FDG uptake of well-differentiated HCC lesion from that of normal liver tissue[20].Meanwhile, TLR of poorly-differentiated HCC lesion is more than 2.0[20]. A previous study has shown that, by using the degree of FDG accumulation in HCCs, FDG PET could differentiate poorly-differentiated type from well-differentiate type with a sensitivity of 84% and a specificity of 75%[22].
Recent studies have assessed the relationship between HCC characteristics and its FDG uptake at molecular level. Lee et al[18]have compared gene expression profiles between HCCs with low FDG uptake and HCCs with high FDG uptake using surgical specimens of 10 HCC patients. In their study, HCCs with high FDG uptake demonstrated different gene expression profiles compared to those with low FDG uptake, showing increased expression of 11 genes particularly related to tumor cell adhesion, invasion, metastasis, anti-tumoral immunity, and chemotherapeutic response. They suggested that HCCs with high FDG uptake might have a more aggressive nature than those with low FDG uptake and that FDG uptake pattern of HCC could reflect potential of tumor progression and metastasis. Another recent study has evaluated the association between FDG uptake and expression of epithelialmesenchymal transition markers in HCC[21]. Epithelial-mesenchymal transition is the formation process of motile cells from immotile epithelial cells and is known to be involved in the formation of metastatic cancer cells[23]. During epithelial-mesenchymal transition processes, expression levels of mesenchymal markers such as N-cadherin and vimentin increased, while expression of E-cadherin, an epithelial cell junction protein, is lost[21,23]. The recent study demonstrated that, in HCCs with high FDG uptake, expression levels of N-cadherin and vimentin were up-regulated and the expression of E-cadherin was repressed[21]. These significant associations between FDG uptake and expression of epithelial-mesenchymal transition-related proteins in HCC provides a basis for hypothesis that FDG PET might be useful for predicting the risk of extrahepatic metastasis in HCC patients[21].
FDG PET IN STAGING HCC
Due to reduced FDG uptake in low-grade HCCs, previous studies have consistently reported a low sensitivity of FDG PET for detecting primary HCC lesions, ranging from 36% to 70%[12-14,20,24-26]. A previous study by Teefey et al[27]even reported that FDG PET detected none of cancer lesions in nine patients with HCC who underwent imaging examinations for work-up of liver transplantation. Based on results of these studies, EASL-EORTC guidelines has mentioned that FDG PET scan is not accurate for early diagnosis of HCC[4]. In contrast, FDG PET has shown promising results for detecting extrahepatic metastasis. Because poorly-differentiated HCCs tend to metastasize more frequently, a positive statistical correlation between FDG avidity of primary HCCs and tendency of extrahepatic metastasis has been shown, suggesting that metastatic HCC lesions would also have increased FDG uptake[28]. Extrahepatic metastasis is not an unusual finding in HCC. During staging work-up of HCC,extrahepatic metastases have been found in up to 37% of patients, with the most frequent site of metastasis being the lung, followed by lymph node, bone, and adrenal gland[29,30]. In previous studies, FDG PET has demonstrated high sensitivities of 77%-100% for detecting extrahepatic metastasis[28,31-34]. FDG PET has also shown superior diagnostic ability for detecting bone metastasis compared to bone scintigraphy and incomparable diagnostic accuracy for detecting lymph node metastasis compared to conventional computed tomography (CT) scan[31,32]. However, FDG PET has a low sensitivity of 20% for detecting pulmonary metastases of less than 1 cm[31]. Therefore,the diagnostic accuracy of chest CT was superior to that of FDG PET for detecting pulmonary metastasis[31].
Clinical utility of imaging modality in staging malignant disease depends on whether the imaging examination can make further shift in cancer staging compared to conventional examinations, thereby, changing treatment modality[35]. In this respect,high diagnostic ability of FDG PET for detecting extrahepatic metastasis might not justify the clinical use of FDG PET in staging HCC. Delineation of additional extrahepatic metastatic lesion by FDG PET might have no significant effect on staging and selecting treatment in HCC patients whose extrahepatic metastases are already found by conventional imaging modalities[35]. The clinical role of FDG PET in staging HCC should be evaluated in terms of ability to change cancer stage and treatment.However, only a small number of studies have assessed this ability of FDG PET in HCC patients, which are summarized in Table 1. In previous studies, FDG PET changed stage and treatment modalities in 1.5%-25% of HCC patients, mainly due to additional detection of extrahepatic metastases[26,34,35]. A recent study by Cho et al[35]has enrolled the largest number of patients (457 consecutive patients with HCC) among the studies. In their study, 5.0% of patients with BCLC stage A (6 out of 119 patients)and 1.4% of patients with BCLC stage B (1 out of 71 patients) were upstaged to stage C by FDG PET while none of patients with BCLC stage 0, C, or D had a shift in stage by FDG PET. Furthermore, additional extrahepatic metastases detected by FDG PET were found only in patients with T2 (3 out of 111 patients, 2.7%) and T3 (4 out of 78 patients, 5.1%) classifications of HCC. They suggested that routine staging FDG PET could have the clinical utility in patients with BCLC stage A and B or with T2 and T3 classifications.
FDG UPTAKE OF HCC AS A PREDICTOR FOR PROGNOSIS
Because FDG uptake of HCC is associated with tumor differentiation and aggressiveness, it is reasonable to assume that FDG uptake of HCC might have significant association with prognosis[18,19,21]. Therefore, most studies on FDG PET in HCC patients have assessed the prognostic value of FDG PET for predicting clinical outcomes. These studies are summarized in Tables 2-4. In previous studies, visual analysis, maximum FDG uptake of tumor expressed as standardized uptake value(SUV), and TLR have been the most commonly used FDG PET parameters for evaluating FDG uptake of HCCs. Visual assessment and SUV are also commonly used as PET parameters in studies on other malignant diseases[36,37]. TLR has been preferred in studies with HCC patients because TLR is known to correlate more closely with HCC doubling time and represent metabolic activities of HCCs more precisely than SUV[38-40].
In previous studies on HCC patients who underwent curative surgical resection(Table 2), FDG uptake of HCC showed significant association with tumor recurrence,especially early recurrence after surgery, and overall survival, demonstrating worse survival in patients with high FDG uptake[41-46]. However, several studies have failed to show the relationship between FDG PET findings and clinical outcomes on multivariate analysis[47-49]. A recent study by Kim et al[49]has retrospectively enrolled 226 patients with HBV-related HCC and evaluated the prognostic value of FDG PET findings. Results of that study revealed that, although positive FDG uptake of HCCs was significantly associated with overall survival, there was no significant difference in disease-free survival according to findings of FDG PET, suggesting that FDG PET could not predict the exact prognosis in patients with HBV-related HCC, because recurrence of HBV-related HCC also included intrahepatic metastasis or de novo recurrence. A retrospective multicenter study including 526 patients from nine Korean institutions has made a prognostic prediction model by combining alphafetoprotein (AFP)-des-gamma-carboxy prothrombin-tumor volume (ADV) score and FDG PET findings that are all available on staging work-up before surgical resection[50]. The prognostic prediction model exhibited significant differences in tumor recurrence rates and overall survival rates according to ADV scores and PET findings, showing recurrence rate of 67.9% and survival rate of 70.6% in patients with high ADV score and hypermetabolic HCCs while the recurrence rate and survival rate in patients with low ADV score and isometabolic HCCs were 21.1% and 96.6%,respectively. The authors of that study suggested that, by using a combination of ADV scores and FDG PET findings, the risk of HCC recurrence could be reliably predicted.
In previous studies on HCC patients who underwent liver transplantation (Table 3), FDG PET findings consistently showed significant associations with recurrencefree survival and overall survival, demonstrating high recurrence rates after liver transplantation in patients with high FDG uptake[39,51-61]. To select candidates for liver transplantation, the Milan criteria (a solitary tumor no more than 5 cm in diameter or 2 to 3 tumors no more than 3 cm in diameter) and the University of California San Francisco (UCSF) criteria (a solitary tumor up to 6.5 cm in diameter or up to 3 tumorsno more than 4.5 cm with a total diameter up to 8 cm) have been commonly used[62,63].Therefore, most studies have compared the prognostic value of FDG PET with the conventional criteria or combined FDG PET findings with the conventional criteria to further stratify recurrence risk after liver transplantation[51-58,61]. In previous studies,patients beyond the Milan criteria, but, showing negative finding on FDG PET had clinical outcomes comparable to those within the Milan Criteria[52,53,55,61]. Furthermore,even if patients met the Milan criteria, higher recurrence rate was found in those with high FDG uptake of HCCs than that in those with low FDG uptake[39,51]. A previous study by Lee et al[58]has proposed new selection criteria with FDG PET finding and total tumor size (10 cm). The new criteria had similar area under the receiver operating characteristic curve value for predicting disease-free survival compared to the Milan criteria or the UCSF criteria[58]. Takada et al[60]have performed a retrospective multicenter study with 182 HCC patients who underwent living donor liver transplantation from 16 Japanese medical centers. In that study, FDG PET finding was found to be an independent predictive factor for tumor recurrence along with the Milan criteria and serum AFP level. Patients beyond the Milan criteria but with low serum AFP level and negative FDG PET finding (19%) had similar 5-year recurrence rate to those within the Milan criteria (6%). They also had significantly lower 5-year recurrence rate than those beyond Milan criteria with high serum AFP level and positive PET finding (53%). They suggested that FDG PET could provide additional information for making decisions regarding liver transplantation for HCC patients[60].
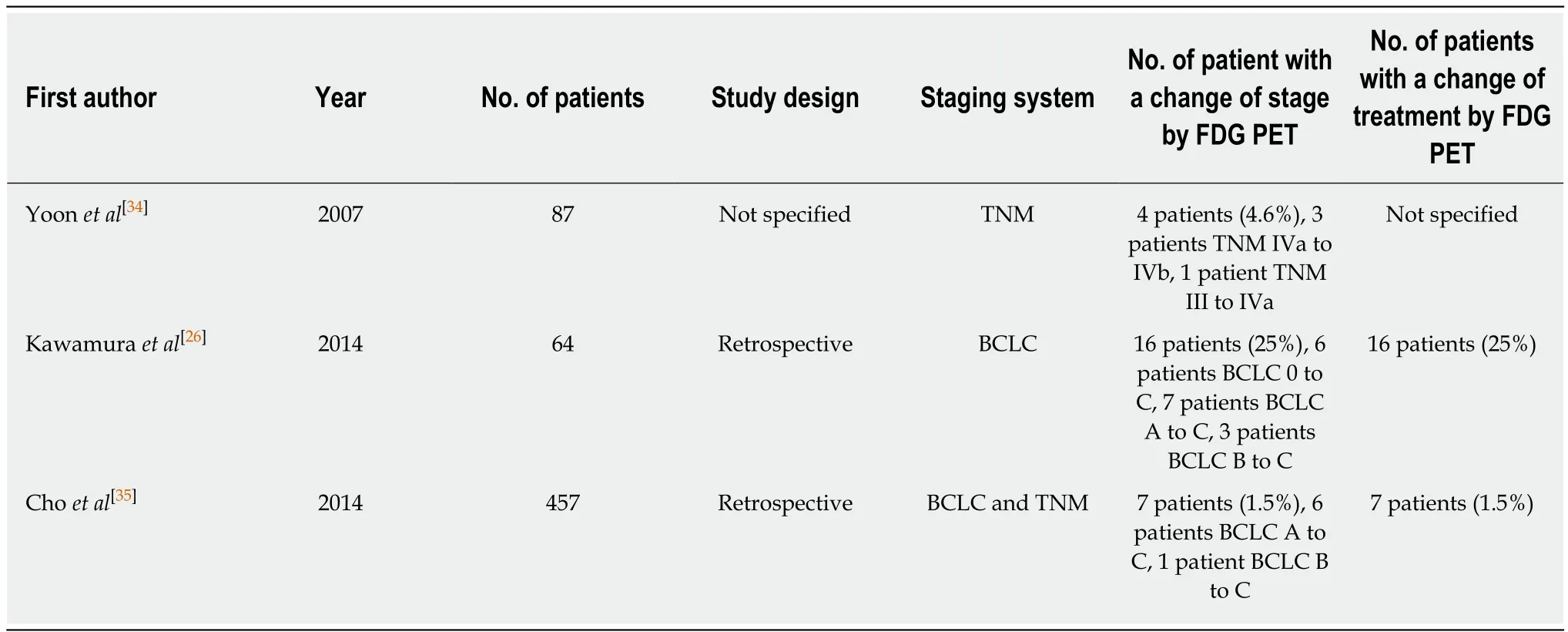
Table 1 Current literature evaluating the role of 18F-fluorodexoyglucose positron emission tomography in staging hepatocellular carcinoma
The prognostic value of FDG PET has also been assessed in HCC patients treated with palliative treatments including TACE, concurrent chemoradiotherapy (CCRT),radiotherapy, transarterial radioemolization (TARE), and sorafenib (Table 4)[40,64-76]. For patients treated with TACE, CCRT, or radiotherapy, previous studies showed longer progression-free survival and overall survival in patients with low FDG uptake of HCCs, indicating significant associations between FDG avidity of HCCs and clinical outcomes[64-70]. For patients treated with TARE using yttrium-90 (90Y), contradictory results have been shown between studies. Previous studies have revealed longer progression-free survival and overall survival in patients with low FDG uptake of HCCs[72,74], including a recent prospective study with uniform patient cohort[75]. In contrast, one study showed no significant association between FDG uptake of HCCs and survival[73]and another study even showed better progression-free survival in patients with high FDG uptake[71]. This controversy could be due to the small number of enrolled patients with heterogeneous clinical conditions among studies and further larger studies are warranted. Only two studies have evaluated the prognostic value of FDG PET in patients treated with sorafenib monotherapy[40,76]. Both studies showed significantly better survival in patients with low FDG uptake of HCCs[40,76]. However,only a small number of patients are enrolled in both studies and concomitant local therapies are also commonly performed in patients with BCLC stage C. These might have limited analyses in these studies.
Recently, the Korean Society of Nuclear Medicine Clinical Trial Network (KSNMCTN) working group has performed a retrospective multicenter study to assess clinical role of FDG PET in HCC patients[77-80]. They retrospectively recruited 847patients with newly diagnosed HCC who underwent pretreatment FDG PET/CT from seven university hospitals at Korea and published several studies regarding the prognostic value of FDG PET[77,78]. One of their studies included 317 patients with BCLC stage 0 or A from the cohort and evaluated the predictive value of FDG PET for recurrence-free survival and overall survival[77]. They classified the 317 patients into two groups, a curative therapy cohort (patients who underwent surgical resection,liver transplantation, and local ablation) and a TACE cohort, and assessed the relationship between FDG PET findings and survival in each group. TLR was an independent predictor for both recurrence-free survival and overall survival in the curative therapy cohort. However, TLR failed to show association with survival in the TACE cohort. In the TACE cohort, only the Model for End-Stage Liver Disease score was an independent prognostic factor for overall survival. Considering that only patients who could not undergo curative therapy due to unsuitable HCC location or impaired liver function were included in the TACE cohort, underlying liver function rather than FDG uptake of HCC might have a significant association with survival[77].Another study by KSNM CTN has evaluated prognostic value of FDG PET in 291 patients with BCLC stage C[78]. They classified patients into two groups; patients with intrahepatic metastasis and patients with extrahepatic metastasis. They showed that higher TLR was associated with extrahepatic metastasis and was an independent predictor for overall survival in both groups. Furthermore, patients with intrahepatic metastases but high TLR had a poor prognosis comparable to patients with extrahepatic metastases and low TLR, suggesting the prognostic significance of primary HCCs uptake irrespective of the extent of metastasis.
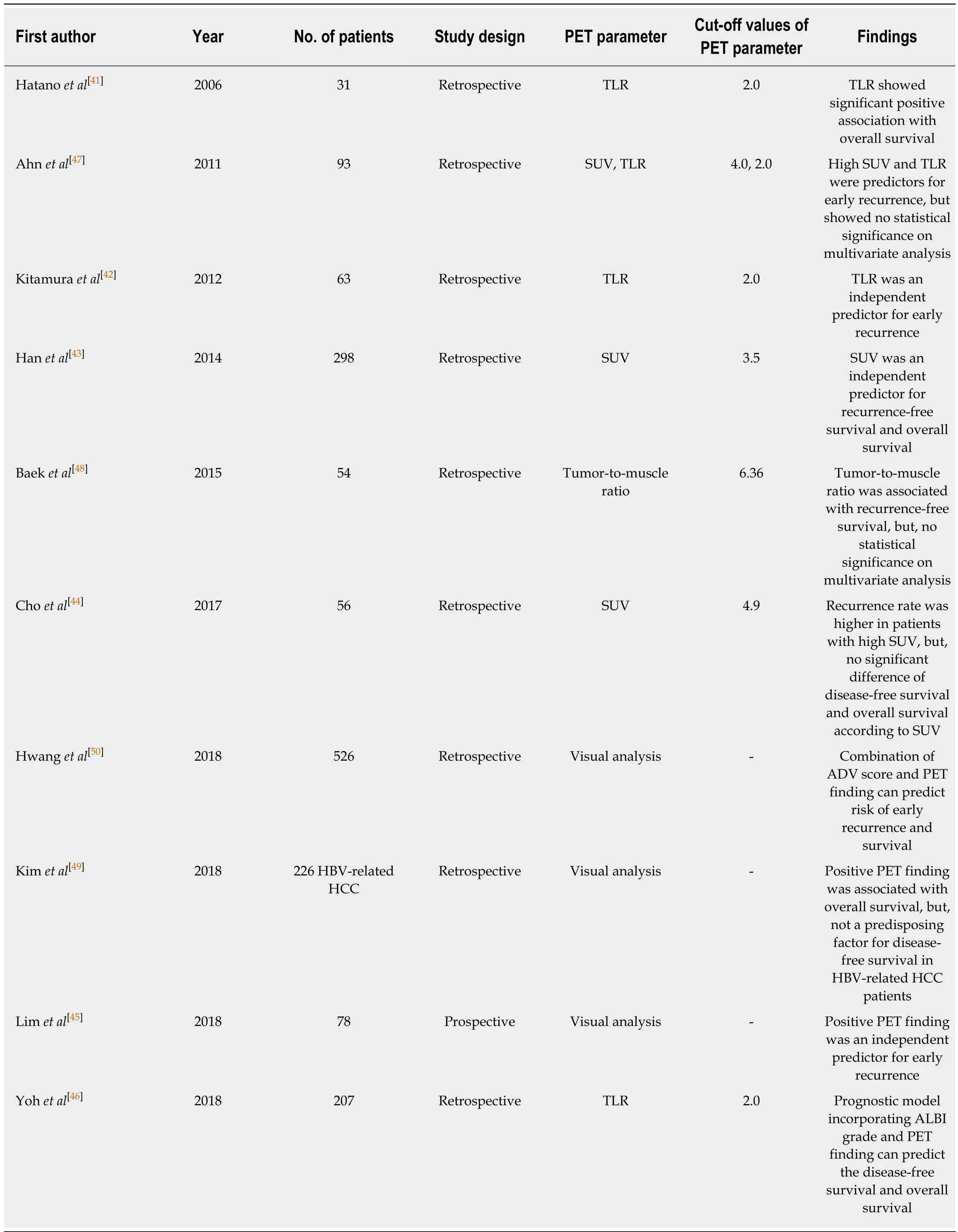
Table 2 Current literature evaluating the prognostic value of 18F-fluorodexoyglucose positron emission tomography in hepatocellular carcinoma patients with surgical resection
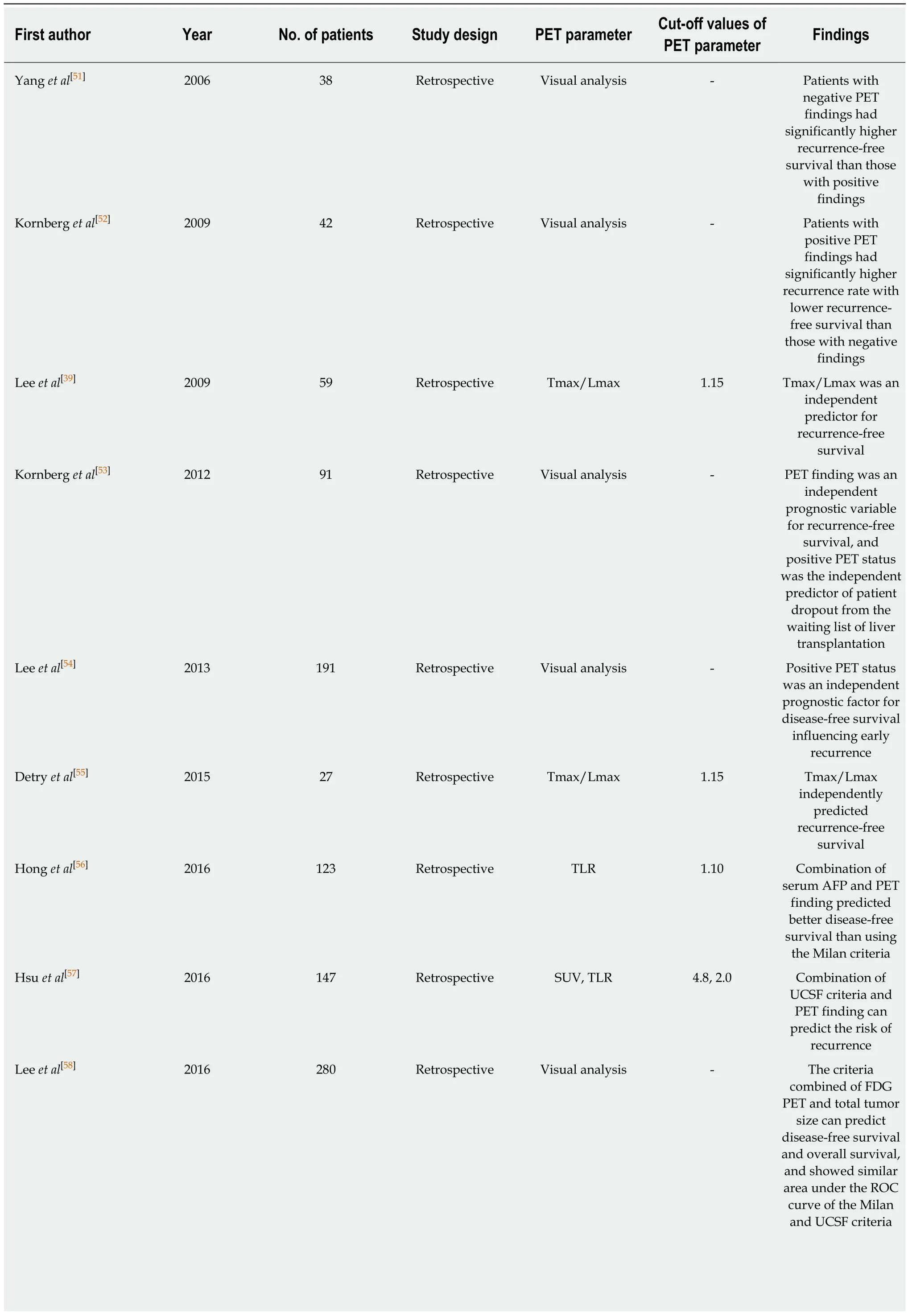
Table 3 Current literature evaluating the prognostic value of 18F-fluorodexoyglucose positron emission tomography in hepatocellular carcinoma patients with liver transplantation
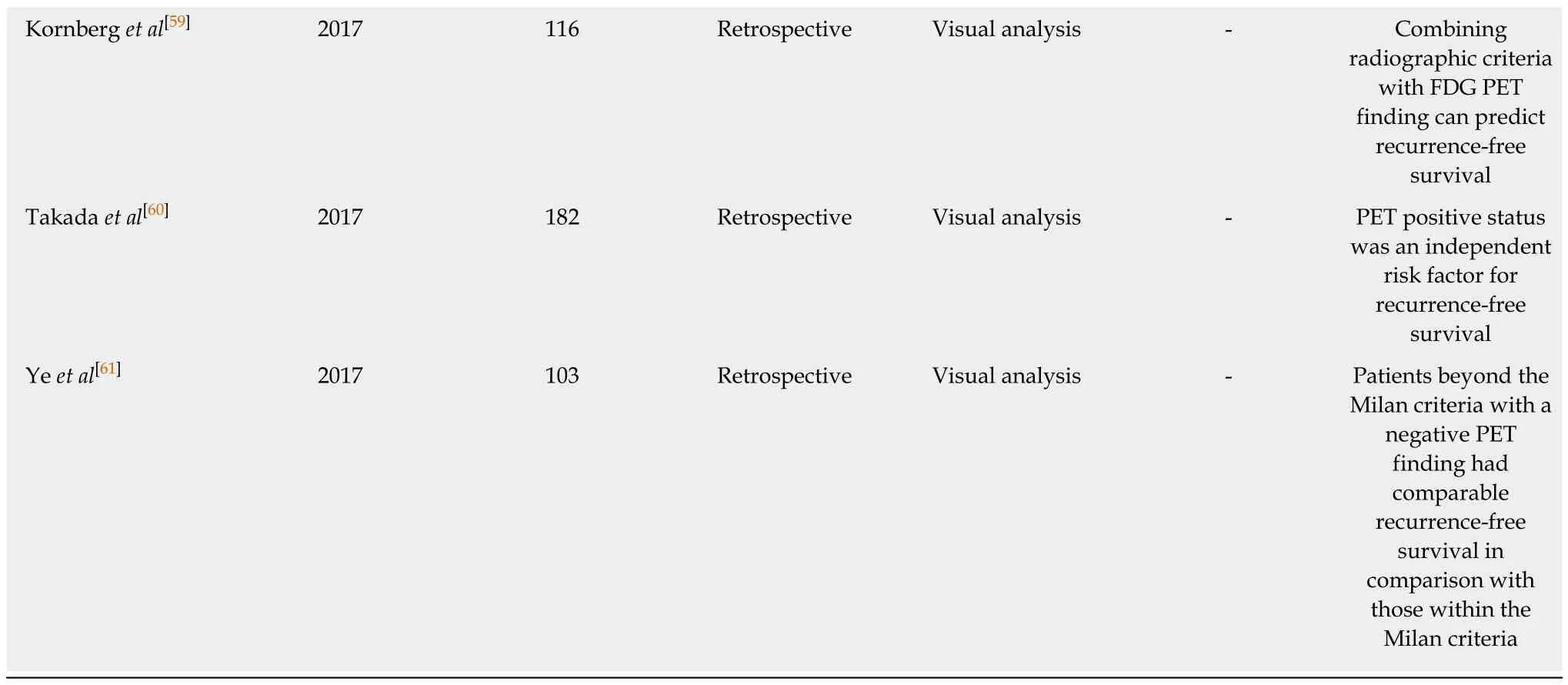
FDG: 18F-fluorodexoyglucose; PET: Positron emission tomography; HCC: Hepatocellular carcinoma; Tmax/Lmax: Maximum FDG uptake of tumor-tomaximum FDG uptake of the normal liver ratio; TLR: Tumor-to-non-tumor liver uptake ratio; SUV: Standardized uptake value; AFP: Alpha-fetoprotein;UCSF: University of California San Francisco; ROC: Receiver operating characteristic.
In addition to FDG uptake of primary HCC tumors, a recent study by Lee et al[81]has evaluated the prognostic value of FDG uptake of portal vein tumor thrombosis.HCC cells can spread to other segments of the liver and distant organs via portal vein;therefore, portal vein tumor thrombosis has a significant impact on the prognosis of HCC patients[81-83]. Lee et al[81]have enrolled 166 HCC patients with portal vein tumor thrombosis but no extrahepatic metastasis and compared the prognostic value of FDG uptake of portal vein tumor thrombosis with FDG uptake of primary tumor. Their results revealed that only FDG uptake of portal vein tumor thrombosis was an independent predictor for both progression-free survival and overall survival. They also found that patients with high FDG uptake of portal vein tumor thrombosis had worse survival than those with low FDG uptake, irrespective of the degree of FDG uptake of primary HCCs. Based on these results, they concluded that FDG uptake of portal vein tumor thrombosis rather than FDG uptake of primary tumor should be used to predict clinical outcomes in locally advanced HCC.
VOLUMETRIC PARAMETERS OF PET AND RECURRENCE PATTERN OF HCC
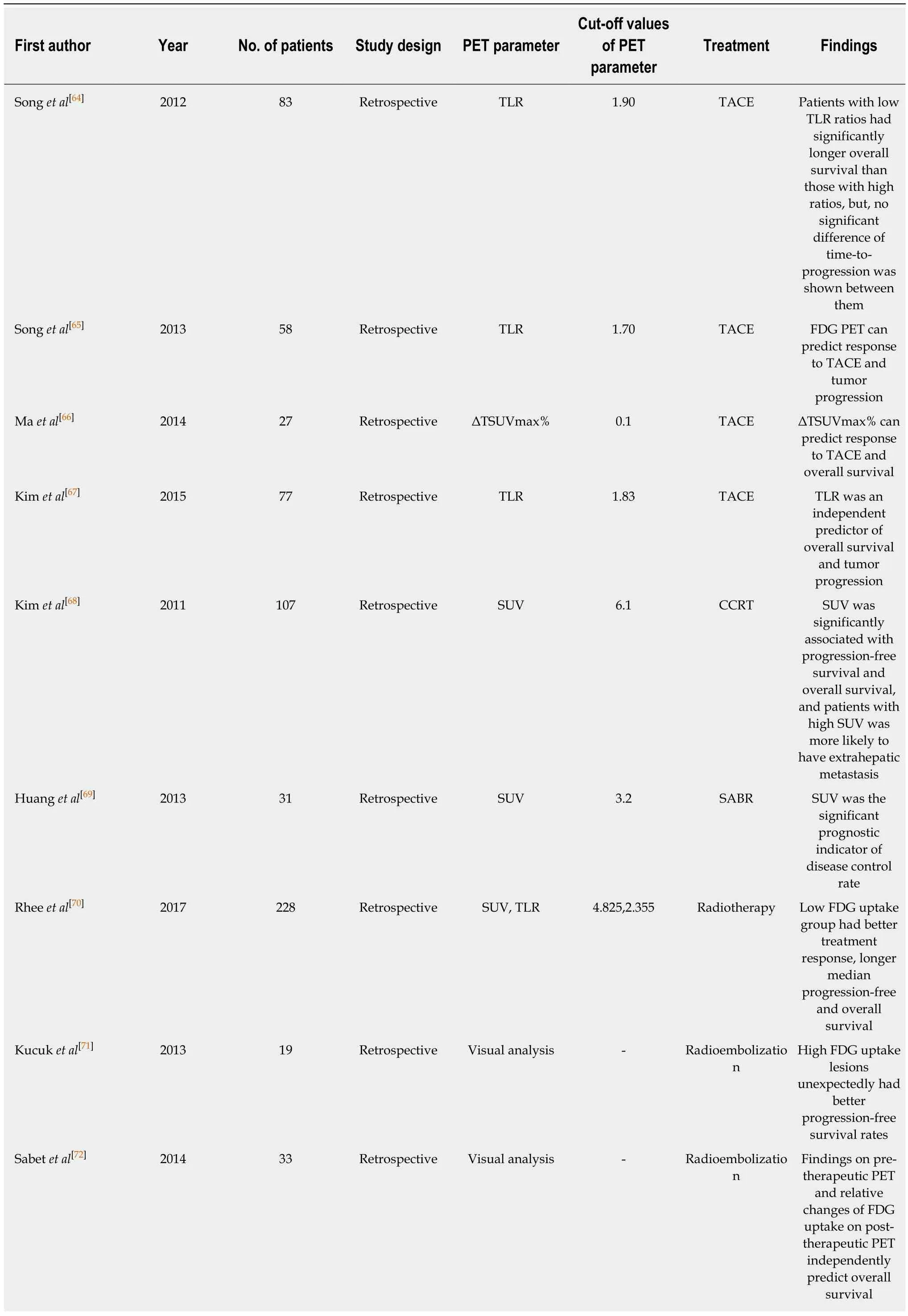
Table 4 Current literature evaluating the prognostic value of 18F-fluorodexoyglucose positron emission tomography in hepatocellular carcinoma patients with treatments other than surgical resection and liver transplantation
FDG: 18F-fluorodexoyglucose; PET: Positron emission tomography; HCC: Hepatocellular carcinoma; TLR: Tumor-to-non-tumor liver uptake ratio;ΔTSUVmax%: Relative changes of maximum FDG uptake of tumor between pre-therapeutic and post-therapeutic FDG PET scans; SUV: Standardized uptake value; TACE: Transarterial chemoembolization; CCRT: Concurrent chemoradiotherapy; SABR: Streotactic ablative radiotherapy; AFP: Alphafetoprotein.
In recent decades, volumetric PET indices such as metabolic tumor volume (MTV)and total lesion glycolysis (TLG) have been considered as promising PET parameters that can accurately reflect the metabolic burden of malignant lesion[84-86]. SUV and TLR represent only the highest metabolic activity of cancer lesion. On the other hand, MTV is defined as tumor tissue volume that has FDG uptake beyond the intensity of FDG uptake of normal tissue; thus, it can reflect tumor extent[85-87]. TLG is the product of MTV and mean FDG uptake of tumor, which combines both metabolic and volumetric information of the tumor[84,86]. A number of studies have demonstrated that MTV and TLG have higher predictive values for survival than SUV in various malignant diseases during the last two decades[84,86-89]. However, in HCC patients,clinical study that calculated MTV and assessed the prognostic value of MTV was first published in 2015[90]. Since then, only a few studies have evaluated the clinical implication of volumetric PET parameters[91-93].
To measure MTV of cancer lesion, two processes should be performed: delineating tumor lesion from surrounding normal tissue and determining threshold SUV to identify metabolically active tumor volume[90]. Due to heterogeneous and diverse degrees of FDG uptake in HCC and relatively high FDG uptake in normal liver tissue,it is difficult to perform both processes in HCC, thus hindering attempts to measure volumetric PET parameters[90]. Lee et al[90]have proposed a novel method using intensity-volume histogram to measure MTV of HCCs that can surpass these limitations. They drew regions of interest over HCC lesion and normal liver tissue and prepared intensity-volume histogram (a plot of volume of a given structure as a function of the SUV) of the HCC lesion and normal liver tissue (Figure 1). Using such intensity-volume histograms of HCC and normal liver tissue, the sum of tumor voxels with higher FDG uptake than normal liver tissue could be calculated for each patient.They calculated MTV2SD(defined as the sum of the tumor voxels over the SUV of the 97.5thpercentile of the normal liver tissue voxels) from 59 HCC patients without extrahepatic metastasis. On survival analysis of their study, MTV2SDhas more significant prognostic value for predicting progression-free survival and overall survival than TLR.
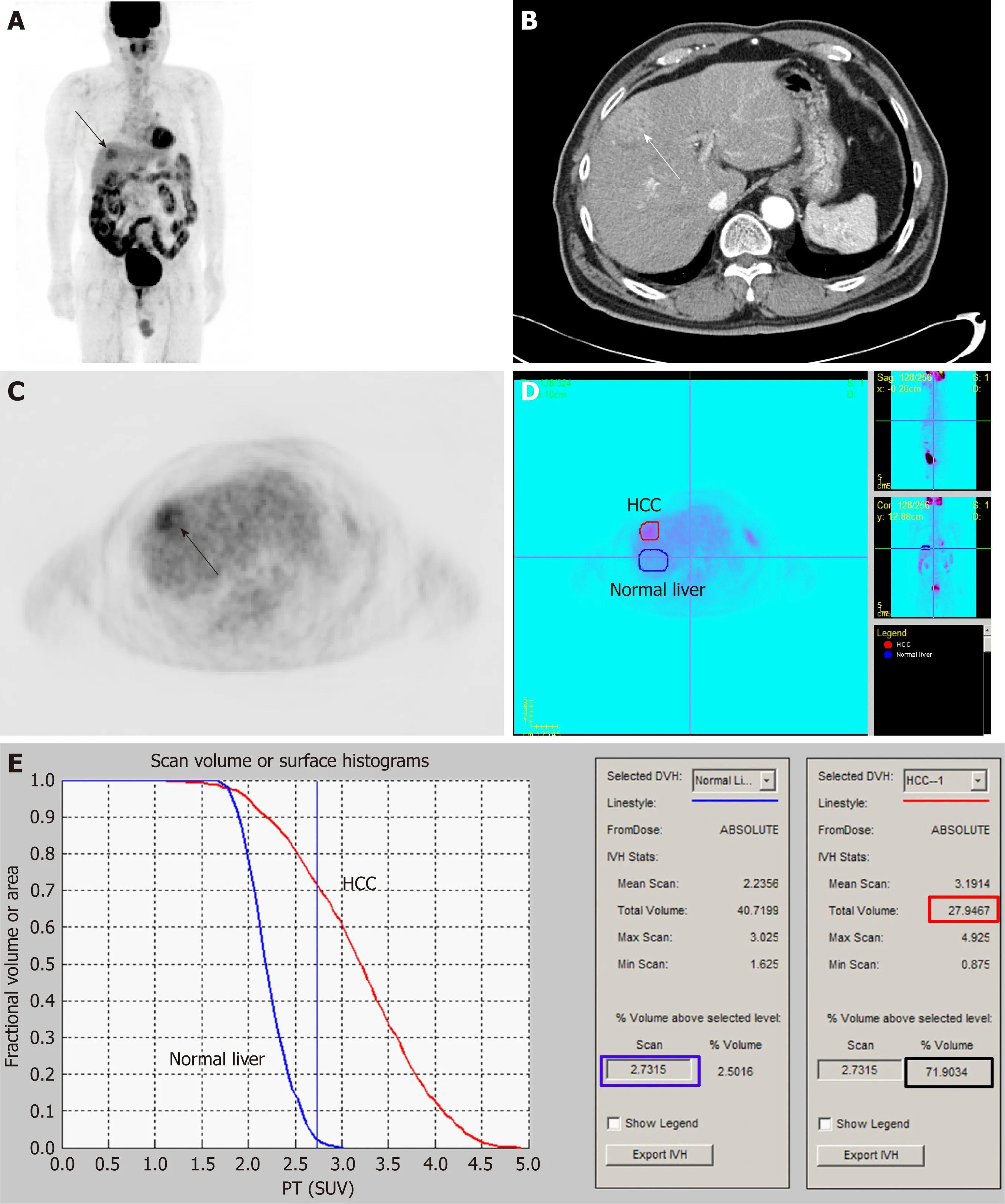
Figure 1 An example of the method for measuring metabolic tumor volume based on intensity-volume histogram from reference[88] with permission.
After publishing the first study with MTV in HCC, authors of the study have tried to assess the clinical value of volumetric PET parameters for predicting recurrence pattern of HCC[92,93]. In HCC patients, extrahepatic metastasis after curative surgical resection is known to be associated with poor prognosis due to limited therapeutic option[42,92]. Furthermore, early recurrence with an interval of less than one year after operation is also known to be a significant indictor for worse survival which is comparable to the survival of patients with extrahepatic metastasis[47,93,94]. In previous studies, tumor factors associated with tumor aggressiveness including tumor stage,size, and grade have been found to be significant predictors for both extrahepatic and early recurrences[47,93,95,96]. FDG uptake of HCC is also related to tumor grade and aggressiveness, therefore, SUV and TLR of HCCs have also been shown to be associated with the risk of extrahepatic and early recurrences[42,45,47,97]. Considering that volumetric PET parameters can more precisely reflect metabolic characteristics and burden of cancer lesions than FDG uptake intensity[86,89], volumetric PET parameters of HCC can have more significant association with recurrence patterns of HCC than SUV or TLR. In two recent studies on the relationship between volumetric PET parameters and recurrence pattern of HCC, the authors have measured MTV and TLG of HCCs using SUV of the 97.5thpercentile of the normal liver tissue as threshold SUV and demonstrated that both MTV and TLG have superior prognostic value for predicting both extrahepatic recurrence and early intrahepatic recurrence than TLR in HCC patients after curative surgical resection[92,93]. On the other hand, late intrahepatic recurrence with an interval of more than 1 year after surgery was associated with HCV positivity and serum albumin level, while none of volumetric PET parameters could predict the risk of late intrahepatic recurrence[93]. These results indicate that the risk of both extrahepatic and early intrahepatic recurrences is associated with metabolic tumor burden, while the risk of late intrahepatic recurrence is related to underlying liver function and multicentric tumor formation tendency[93]. Authors of these studies have suggested that volumetric PET parameters of HCC could be used to predict the recurrence pattern and to select patients who might show poor survival after curative surgical resection[92,93].
Kim et al[91]have calculated volumetric PET indices and evaluated their prognostic values in 110 HCC patients who underwent liver transplantation. Different from the method used in the study by Lee et al[90], they calculated uptake ratio between the maximum SUV of HCCs and background tissue, inferior vena cava (TBRIVC) or normal liver tissue (TBRNL), and used TBRIVCof 2.0 and TBRNLof 1.5 as threshold values for measuring MTV. MTVIVCand MTVNL(defined as the sum of the tumor voxels which had TBRIVCof more than 2.0 and TBRNLof more than 1.5) were measured. With MTV and mean value of TBR, uptake-volume product (UVP) was calculated for each background tissue. Results of their study revealed that both TBRIVCand UVPIVCwere independent predictors for recurrence-free survival. The authors suggested that inferior vena cava might be a more reliable background tissue than the normal liver in measuring MTV. They have also suggested that volumetric parameters as well as metabolic activity of HCCs are effective predictors of recurrence after liver transplantation[91].
USING FDG PET FOR SELECTING TREATMENT OF HCC
Increased FDG uptake in HCCs reflects aggressive biological activity of tumor and is associated with poor survival[18,19,78]. Therefore, patients with high FDG uptake of HCCs might have poor response to treatment. Previous studies on patients treated with TACE have shown that patients with high FDG uptake have poor response to treatment[65,98]. Furthermore, in HCC patients with high FDG uptake, major hepatectomy that can minimize the possibility of residual tumor rather than minor hepatectomy should be selected to obtain survival benefit[99]. However, in studies on HCC patients treated with external beam radiotherapy, paradoxical relationship between FDG uptake of HCC and treatment response has been shown[100-102]. Kim et al[100]have retrospectively enrolled 35 HCC patients with TNM stage III-IV who underwent FDG PET and subsequent radiotherapy with concurrent chemotherapy or TACE. They showed that patients having HCCs of SUV ≥ 2.5 (80%; 16 out of 20 patients) had significantly higher objective response rate to radiotherapy than those with HCCs of SUV < 2.5 (40%; 6 out of 15 patients). Choi et al[101]have retrospectively reviewed 45 metastatic bone lesions in 22 HCC patients treated with radiotherapy.They also revealed significantly better infield progression-free survival and infield event-free survival in tumors with SUV of ≥ 3.0 compared to those in tumors with SUV of < 3.0 (1-year progression-free survival, 88% vs 34%; 1-year event-free survival,82% vs 52%). Another retrospective study by Jo et al[102]has investigated the predictive value of FDG uptake of HCC in 36 HCC patients treated with radiotherapy. In that study, patient group with high SUV (≥ 5.1) showed significantly higher objective tumor response (63.6% vs 36.4%) than patient group with low SUV (< 5.1). In spite of high tumor response, patient group with high SUV had worse overall survival mainly due to the occurrence of distant metastasis. Authors of these studies have explained this paradoxical relationship by radiosensitivity of highly proliferating tumor[100,102]. In a previous study, FDG uptake of HCCs has shown a positive association with tumor doubling time[38]. Because rapidly proliferating tumors are radiosensitive, HCCs with high FDG uptake are considered to have better response to radiotherapy than those with low uptake[100,102]. However, HCCs with high FDG uptake also have high risk for early recurrence and distant metastasis[92,93]. Therefore, viable tumor cells in residual lesions after radiotherapy could spread more rapidly and more frequently to extrahepatic organs, resulting in worse overall survival[102].
Results of these studies on radiotherapy have indicated that HCCs might show different responses to treatment according to the degree of FDG uptake of HCC. In this respect, a recent study reported by KSNM CTN working group has demonstrated interesting results[79]. The study retrospectively enrolled 214 intermediate-to-advanced stage patients without extrahepatic metastasis who underwent CCRT or TACE as an initial treatment from a cohort of 847 HCC patients from seven hospitals. Authors of the study have classified these enrolled patients into two patient groups according to TLR of HCCs (patient groups with TLR > 2.0 and ≤ 2.0) and compared clinical outcomes between patients treated with CCRT and TACE for each patient group. In patient group with TLR > 2.0, patients treated with CCRT demonstrated significantly longer progression-free survival and overall survival than those treated with TACE.Meanwhile, for patient group with TLR ≤ 2.0, there were no significant differences in progression-free survival or overall survival between patients treated with CCRT and those treated with TACE. The authors suggested that, for patients with high FDG uptake, multimodality treatment including radiotherapy could be more effective in tumor control while HCCs with low FDG uptake seemed to be less affected by the treatment modality. Considering that HCCs with low and high FDG uptake have different tumor characteristics, genetic disposition, and recurrence pattern[18,19,21,93],different treatment strategy might be needed according to findings of FDG PET in HCC patients. However, as the study by KSNM CTN working group[79]was retrospectively performed, further prospective study is needed to validate the role of FDG PET in selecting treatment modality.
RADIOMICS OF FDG PET IN HCC
Currently, the concept of radiomics has been widespread in the field of oncology[103].Radiomics is defined as high-throughput extraction of a large number of imaging features that can comprehensively quantify tumor phenotypes[104,105]. Textural features of cancer tissue on medical images are associated with genomic and proteomic expression patterns of cancer cells and many studies have revealed that radiomics signature made of textural features can independently predict prognosis in diverse cancers including head and neck cancer, lung cancer, breast cancer, and esophageal cancer[103,105,106]. However, in HCC, only a single study by Blanc-Durand et al[107]has evaluated the prognostic value of textural features of HCC. In radiomics study of FDG PET, image preprocessing including the process of delineation and segmentation of tumor lesion is essential for textural analysis of PET images[105,108]. Considering that HCC lesions have heterogeneous and variable FDG uptake, it is difficult to delineate HCC lesions from normal liver tissue accurately as shown in aforementioned section with volumetric PET parameters[90]. This might act as the main hurdle to perform textural analysis in FDG PET images of HCC. Blanc-Durand et al[107]have retrospectively enrolled 47 HCC patients who underwent pretreatment FDG PET and subsequent transarterial radioembolization using90Y. Using PET images of whole liver including both tumor and non-tumoral liver for textural analysis, they extracted 108 textural features from these images. They claimed that, by introducing wholeliver in the radiomics model, both hepatic function and HCC biology could be integrated into one system. With mainly using two textural features, strength (a textural feature describing pattern perceivability, its value is high when intensity pattern is easily defined and visible) and variance (a textural feature describing a deviation from the mean), predictive radiomics scoring systems for progression-free survival and overall survival were generated. On multivariate survival analysis, these radiomics scoring systems turned out to be independent negative predictors for both progression-free survival and overall survival. Moreover, prognostic values of radiomics scoring systems did not differ even after stratification by BCLC staging and tumor size. The authors suggested that whole-liver radiomics approach representing a balance between normal liver tissue and tumor burden could provide prognostic information for HCC patients. However, further studies with more patients are warranted to validate the methodology and results of their study.
CONCLUSION
FDG PET is a non-invasive imaging method that can evaluate biological activity of HCC. Although FDG PET has been considered to have low sensitivity for detecting HCCs, it can detect unexpected extrahepatic metastasis with incremental prognostic value for predicting survival. Furthermore, recent studies have demonstrated encouraging results of FDG PET for predicting recurrence pattern and aiding the selection of treatment for HCC. With development of new analytic methods of FDG PET images such as volumetric and textural analyses, clinical use of FDG PET in HCC patients would be continuously evolving. FDG PET should be considered as an imaging biomarker that can provide information for selecting management strategies in HCC patients rather than a simple diagnostic imaging modality with a limited sensitivity.
杂志排行
World Journal of Gastroenterology的其它文章
- Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice
- Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals
- Clinical assessment and identification of immuno-oncology markers concerning the 19-gene based risk classifier in stage lV colorectal cancer
- Hemodynamic changes in hepatic sinusoids of hepatic steatosis mice
- Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus
- Early gastric cancer diagnostic ability of ultrathin endoscope loaded with laser light source
