吡嗪酰腙配体CuⅡ/NiⅡ配合物的合成、晶体结构及DNA结合性质
2019-03-13王碗碗宋雨飞吴伟娜
王碗碗 王 元*, 张 玲 宋雨飞 吴伟娜*, 陈 忠
(1河南理工大学化学化工学院,河南省煤炭绿色转化重点实验室,焦作 454000)
(2江西科技师范大学材料与机电学院,南昌 330013)
Schiff bases and their metal complexes have been widely used in medical fields such as anticancer,antibacterial,and antiviral drugs[1-2].Generally,the presence of a heterocyclic ring in the synthesized Schiff bases plays a major role in extending their pharmacological properties[3].Particularly,pyrazinecontaining thiosemicarbazone has been extensively investigated as potential anticancer agents[4],because pyrazine is a vital nitrogen heterocyclic compound with various biological activities and a key structural unit for the drug design[4-6].However,acylhydrazones bearing pyrazine unit,as their structural analogs,received much less attention[4,7-8].
Our previous work has showed that 2-acetopyrazine benzoylacylhydrazone could form stable complexes with transition metal ions,such as CuⅡ,NiⅡ and ZnⅡ[5].It is noted that the biological activities of acylhydrazones often show a high dependence on their substituent[9].In this regard,we chose 2-aceto-3-methylpyrazine as the starting material for the formation of benzoylacylhydrazone ligand HL(Scheme 1).In this paper,the structures and DNA-binding properties of its NiⅡ/CuⅡ complexes have been discussed in detail.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received.Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1)were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.1H NMR spectra of HL was acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMS as internal standard.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.The interactions between the complexes and ct-DNA are measured by using literature method[5]via emission spectra on a Varian CARY Eclipse spectrophotometer.
1.2 Preparations of HL,complexes 1 and 2
As shown in Scheme 1,the ligand HL was produced by condensation of 2-aceto-3-methylpyrazine(1.36 g,0.01 mol)and benzoylhydrazide(1.36 g,0.01 mol)in ethanol solution (30 mL)with continuous stirring at room temperature for 3 h.Yield:2.08 g(82%).Elemental analysis Calcd.for C14H14N4O(%):C:66.13;H:5.55;N:22.03.Found(%):C:66.28;H:5.44;N:21.99.FT-IR(cm-1): ν(C=O)1 685, ν(C=N)imine1 582,ν(C=N)pyrazine1 542.1H NMR(400 MHz,DMSO-d6):δ 10.97(1H,s,NH),8.52(2H,s,pyrazine-H),7.91(2H)and 7.53~7.60(3H)for phenyl-H,2.80(3H,s,CH3),2.43(3H,s,CH3).
The complexes 1 and 2 were generated by reaction of the ligand HL (5 mmol)with equimolar of NiSO4or CuSO4in methanol solution(10 mL)at room temperature for 1 h.Crystals suitable for X-ray diffraction analysis were obtained by evaporating the corresponding reaction solutions at room temperature.
1:Brown blocks.Anal.Calcd.for C31H39N8O7S0.5Ni(%):C:52.41;H:5.53;N:15.77.Found(%):C:52.30;H:5.64;N:15.54.FT-IR(cm-1):ν(C=O)1 654,ν(C=N)1 562,ν(C=N)pyrazine1 532.
2:Green blocks.Anal.Calcd.for C29.5H32N8O7.5SCu2(%):C:45.55;H:4.15;N:14.41.Found(%):C:45.41;H:4.28;N:14.32.FT-IR(cm-1):ν(C=O)1 652,ν(C=N)1 560,ν(C=N)pyrazine1 535.
1.3 X-ray crystallography
The X-ray diffraction measurement for 1(size:0.20 mm×0.20 mm×0.20 mm)and 2(size:0.15 mm×0.14 mm×0.12 mm)were performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation (λ =0.071 073 nm)using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[10].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[11].All non-hydrogen atoms were refined anisotropically.The H atomsofdisordered methanolmolecule(occupancy value of each atom being 0.5)in 2 were not added.All the other H atoms were positioned geometrically and refined using a riding model.Details of the crystal parameters,data collection and refinements for 1 and 2 are summarized in Table 1.
CCDC 1876785:1;1876789:2.

Table 1 Crystal data and structure refinement for complexes 1 and 2
2 Results and discussion
2.1 Crystal structure description
Diamond drawings of complexes 1 and 2 are shown in Fig.1.Selected bond distances and angles are listed in Table 2.As shown in Fig.1a,the asymmetric unit of 1 contains one discrete cationic NiⅡion,a half of sulfate anions for charge balance,and three lattice methanol molecules.The center NiⅡion with a distorted octahedron geometry is coordinated by two acylhydrazones with [ONN]donor set.It should be noted that one of the acylhydrazone ligands is neutral,since the bond length of C8-O1(0.123 3(3)nm)is clearly shorter than that of C22-O2(0.126 5(4)nm).In the solid state,intermolecular N-H…O(N4-H4…O4,with D…A distance being 0.270 0(3)nm,D-H…A angle being 139.6°)and O-H…O (O7-H7…O6,with D…A distance being 0.278 0(12)nm,D-H…A angle being 159.6°;O5-H5…O3,with D…A distance being 0.296 1(8)nm,D-H…A angle being 118.8°;O6-H6…O4,with D…A distance being 0.281 5(7)nm,D-H…A angle being 125.0°)hydrogen bonds are helpful to stabilize the crystal structure(Fig.1a).
Complex 2 crystallizes in the triclinic space group P1 with one discrete dimeric CuⅡunit in the unit cell.As illustrated in Fig.1b,two CuⅡions of the dimer are separated by 0.317 4 nm and doubly bridged by two O atoms from two acylhydrazone ligands to form a nearly planar four-membered Cu2O2core (r.m.s.deviation:0.030 15 nm).Each of the CuⅡions is also coordinated by two N atoms from one L-ligand (bond length of C8-O1 and C22-O2 are 0.128 8(5)and 0.129 2(5)nm,respectively)and one O atom from the η2-SO42-at the outer axial sites,giving a distorted square pyramid coordination geometry(τ=0.185 and 0.262 for Cu1 and Cu2,respectively)[12].The O-H…O (O7-H7…O5,with D…A distance being 0.272 4(7)nm,D-H…A angle being 168.3°;O8…O7,with D…A distance being 0.257 4 nm)hydrogen bonds are also present in the crystal(Fig.1b).

Fig.1 Diamond drawings of 1(a)and 2(b)with 10%thermal ellipsoids
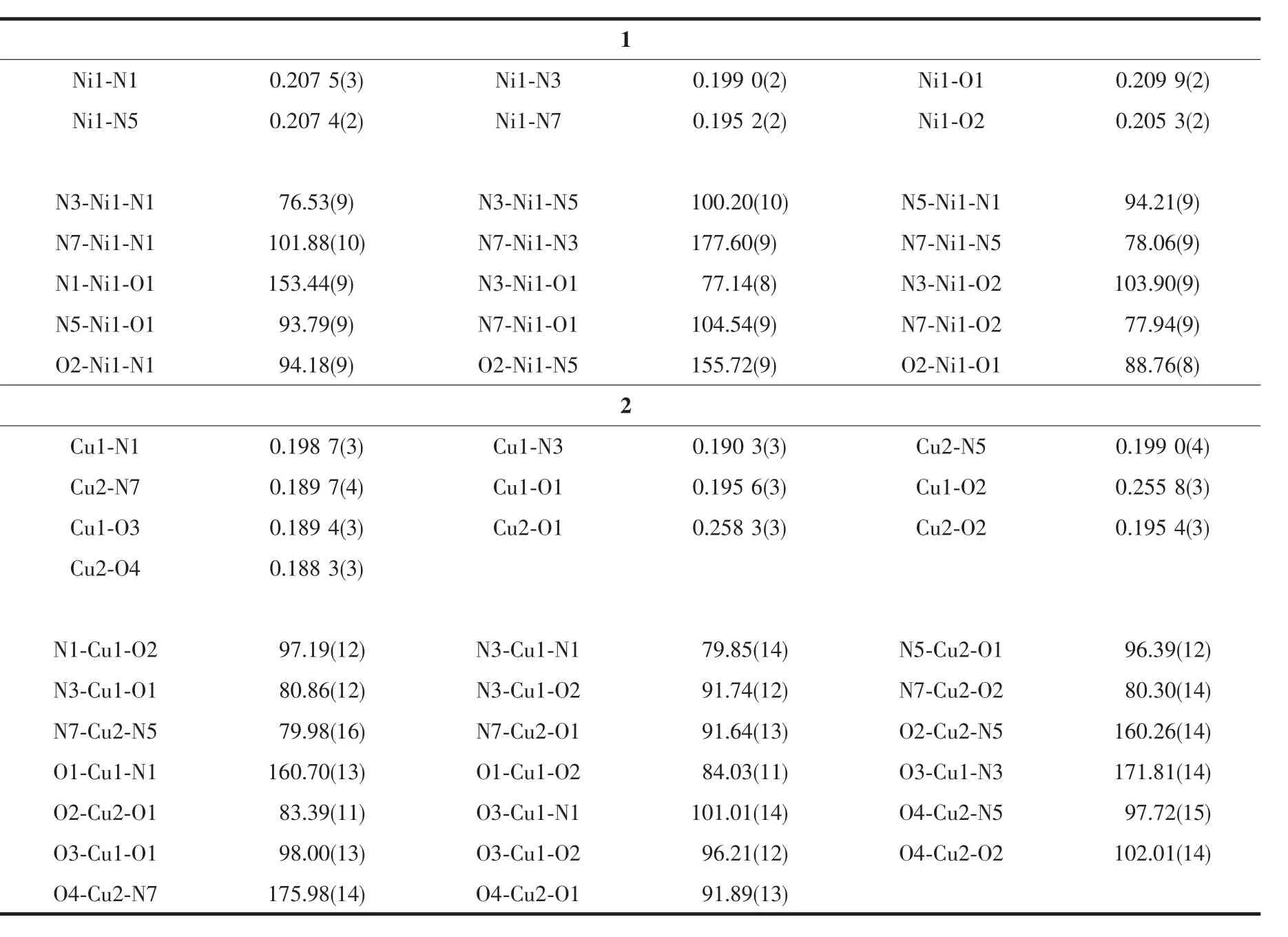
Table 2 Selected bond lengths(nm)and angles(°)in complexes 1 and 2
2.2 IR spectra
The FT-IR spectral regions for both complexes were more or less similar due to the similar coordination modes of the ligands.The ν(C=O),ν(C=N)imineand ν(C=N)pyrazinebands were at 1 663,1 578 and 1 559 cm-1, respectively.They shifted to lower frequency values in the IR spectra of complexes,indicating that the carbonyl O,imine N and pyrazine N atoms take part in the coordination[5].It is in accordance with the crystal structure study.
2.3 UV spectra
The UV spectra of the ligand HL,1 and 2 in CH3OH solution(10 μmol·L-1)were measured at room temperature (Fig.2).The spectra of HL featured two main band at around 230 nm (ε=10 950 L·mol-1·cm-1)and 290 nm(ε=15 423 L·mol-1·cm-1),which could be assigned to characteristic π-π*transition of pyrazine and imine units,respectively[13].In the spectrum of complex 1,these two bonds red-shifted to 256 nm(ε=12 832 L·mol-1·cm-1)and 306 nm(ε=15 191 L·mol-1·cm-1),respectively.By contrast,two bands of the ligand were merged into one at 271 nm(ε=17 968 L·mol-1·cm-1)in the spectrum of complex 2.Moreover,new peaks at 400 nm(ε=15 314 L·mol-1·cm-1)and 401 nm (ε=12 955 L·mol-1·cm-1)were observed in the spectra of complexes 1 and 2,respectively,primarily due to the ligand-to-metal charge transfer(LMCT)[14].This indicates that an extended conjugation forms in anionic ligand after complexation.
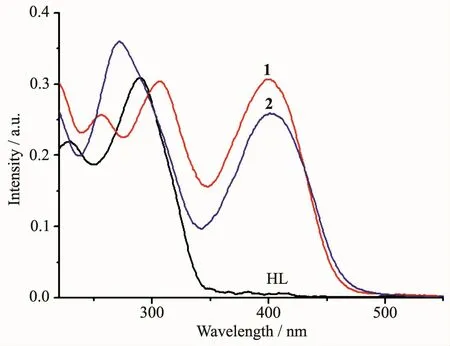
Fig.2 UV spectra of the ligand HL,complexes 1 and 2 in CH3OH solution at room temperature
2.4 EB-DNA binding study by fluorescence spectrum

Fig.3 Emission spectra of EB-DNA system in the absence and presence of ligand HL(a),complexes 1(b)and 2(c)
Itis wellknown thatEB can intercalate nonspecifically into DNA,which causes it to fluoresce strongly.Competitive binding of other drugs to DNA and EB will result in displacement of bound EB and a decrease in the fluorescence intensity[14-15].The effects of the ligand and complexes on the fluorescence spectra of EB-DNA system are presented in Fig.3.The fluorescence intensities of EB bound to ct-DNA at about 600 nm showed remarkable decreasing trends with the increasing concentration ofthe tested compounds,indicating that some EB molecules are released into solution after the exchange with the compounds.The quenching of EB bound to DNA by the compounds is in agreement with the linear Stern-Volmer equation:I0/I=1+Ksqr[14-15],where I0and I represent the fluorescence intensities in the absence and presence of quencher,respectively,Ksqis the linear Stern-Volmer quenching constant,r is the ratio of the concentration of quencher and DNA.In the quenching plots of I0/I versus r,Ksqvalues are given by the slopes.The Ksqvalues were 0.545,1.823 and 1.236 for the ligand HL,complexes 1 and 2,respectively.The results indicate that interaction of the complexes to DNA is stronger than that of the ligand HL,because the complexes have higher rigidity to bind the base pairs along DNA,thus increasing their binding abilities.
3 Conclusions
Two complexes with a pyrazine-containing benzoylhydrazone ligand were prepared and characterized by single-crystal X-ray crystallography.In complex 1,the center NiⅡion is surrounded by two acylhydrazones with [ONN]donorset,givinga distorted octahedron geometry.By contrast,complex 2 contains one discrete dimeric CuⅡunit in the unit cell.Moreover,the fluorescence spectra indicated that the interaction of the complexes to DNA is stronger than that of the ligand HL.Further research is needed to better determine the relationship between structures and activities.
