Consensus on the digestive endoscopic tunnel technique
2019-02-27NingLiChaiHuiKaiLiEnQiangLinghuZhaoShenLiShuTianZhangYuBaoWeiGangChenPhilipWYChiuTongDangWeiGongShuTangHanJianYuHaoShuiXiangHeBingHuBingHuXiaoJunHuangYongHuiHuangZhenDongJinMouenKhashabJamesLauPengLiR
Ning-Li Chai, Hui-Kai Li, En-Qiang Linghu, Zhao-Shen Li, Shu-Tian Zhang, Yu Bao, Wei-Gang Chen,Philip WY Chiu, Tong Dang, Wei Gong, Shu-Tang Han, Jian-Yu Hao, Shui-Xiang He, Bing Hu, Bing Hu,Xiao-Jun Huang, Yong-Hui Huang, Zhen-Dong Jin, Mouen A Khashab, James Lau, Peng Li, Rui Li,De-Liang Liu, Hai-Feng Liu, Jun Liu, Xiao-Gang Liu, Zhi-Guo Liu, Ying-Cai Ma, Gui-Yong Peng, Long Rong,Wei-Hong Sha, Pateek Sharma, Jian-Qiu Sheng, Shui-Sheng Shi, Dong Wan Seo, Si-Yu Sun, Gui-Qi Wang,Wen Wang, Qi Wu, Hong Xu, Mei-Dong Xu, Ai-Ming Yang, Fang Yao, Hong-Gang Yu, Ping-Hong Zhou,Bin Zhang, Xiao-Feng Zhang, Ya-Qi Zhai
Abstract With the digestive endoscopic tunnel technique (DETT), many diseases that previously would have been treated by surgery are now endoscopically curable by establishing a submucosal tunnel between the mucosa and muscularis propria(MP). Through the tunnel, endoscopic diagnosis or treatment is performed for lesions in the mucosa, in the MP, and even outside the gastrointestinal (GI) tract.At present, the tunnel technique application range covers the following: (1)Treatment of lesions originating from the mucosal layer, e.g., endoscopic submucosal tunnel dissection for oesophageal large or circular early-stage cancer or precancerosis; (2) treatment of lesions from the MP layer, per-oral endoscopic myotomy, submucosal tunnelling endoscopic resection, etc.; and (3) diagnosis and treatment of lesions outside the GI tract, such as resection of lymph nodes and benign tumour excision in the mediastinum or abdominal cavity. With the increasing number of DETTs performed worldwide, endoscopic tunnel therapeutics, which is based on DETT, has been gradually developed and optimized. However, there is not yet an expert consensus on DETT to regulate its indications, contraindications, surgical procedure, and postoperative treatment.The International DETT Alliance signed up this consensus to standardize the procedures of DETT. In this consensus, we describe the definition, mechanism,and significance of DETT, prevention of infection and concepts of DETT-associated complications, methods to establish a submucosal tunnel, and application of DETT for lesions in the mucosa, in the MP and outside the GI tract(indications and contraindications, procedures, pre- and postoperative treatments, effectiveness, complications and treatments, and a comparison between DETT and other operations).
Key words: Digestive endoscopic tunnel technique; Endoscopic submucosal tunnel dissection; Per-oral endoscopic myotomy; Submucosal tunnelling endoscopic resection;Gastrointestinal tract
INTRODUCTION
The digestive endoscopic tunnel technique (DETT) is a new endoscopic treatment technique in which a submucosal tunnel is established, making many diseases that previously would have been treated by surgery become endoscopically curable.Compared with surgery, DETT has certain advantages, such as less trauma and faster recovery. The emergence of DETT is a milestone in the development of endoscopic treatment and significantly broadens the application range of endoscopy. With the increasing number of DETTs performed worldwide, endoscopic tunnel therapeutics,which is based on DETT, has been gradually developed and optimized. However,there is not yet a consensus or guideline on DETT to regulate its indications,contraindications, surgical procedure, and postoperative treatment. Therefore, it is necessary to generate this consensus on the DETT.
The International DETT Alliance (IDETTA) is an un-official technical association composed of 48 endoscopy experts majoring in the DETT method from Chinamainland, China-Hong Kong, United States, and Korea. This consensus was drafted by all the members of the alliance and is the first international consensus on DETT aiming to guide and regulate the performance of DETT by doctors worldwide.Systematic document retrieval was performed by searching the online databases of PubMed, China National Knowledge Internet, and SinoMed for articles published from 2009 to 2018 to generate a better understanding of DETT and to develop key questions regarding pre-operative treatments, indications, techniques, complications,outcomes, and postoperative treatment. The retrieved documents were evaluated, and pertinent documents were adopted. Subsequently, a statement and an explanation were created to construct a questionnaire based on PICO. All 48 members of IDETTA voted on the created statements using the Delphi method. The questionnaire was send by email to each expert. The consensus was assessed at the evidence level and recommendation level according to evidenced-based medicine (Tables 1 and 2).Considering that an increasing number of new evidence-based clinical outcomes may emerge in the future, the association will arrange meetings as necessary to update the consensus.
We established six categories for evaluation as follows: Preoperative treatment,indications, techniques, complications, treatment outcome (recurrence, metastasis,and prognosis), postoperative follow-up, and pathology. For each clinical question,systematic document retrieval was done by searching PubMed and Igaku Chuo Zasshi for articles.
DEFINITION OF DETT
The concept of DETT was initially put forward by professor Linghu in 2009 after its successful application to a large, circumferential oesophageal lesion[1], and this clinical event marked the birth of DETT. DETT aims to establish a submucosal tunnel between the mucosa and muscularis propria (MP), through which endoscopic diagnosis or treatment is performed for lesions in the mucosa, in the MP, and even outside the gastrointestinal (GI) tract[2].
MECHANISM OF DETT
The key mechanism of DETT is to divide the digestive tract wall into two layers(mucosa and MP) and to maintain the integrity of one layer when the other layer is opened for treatment or diagnosis. Therefore, the intra-luminal and extra-luminal space is isolated, and intra-luminal gas or fluid cannot enter the extra-luminal space[3](Figure 1).
SIGNIFICANCE OF DETT
Based on the practice of original per-oral and per-anal endoscopy, DETT establishes an “artificial tunnel” that is different from the natural GI tract[2]. For a long time, the MP has been considered the boundary between GI internal medicine and surgery; the MP plays an important role in avoiding single-layer perforation and in isolating intraluminal chemical liquids, gases, or bacteria from the normal tissue in the extraluminal space. Development of the tunnel technique has enabled digestive endoscopy to treat lesions outside the GI tract, thus converting certain digestive surgeries to super-minimally invasive endoscopic operations[4]. DETT bridges digestive internal medicine and surgery and is considered a milestone of digestive endoscopic techniques.
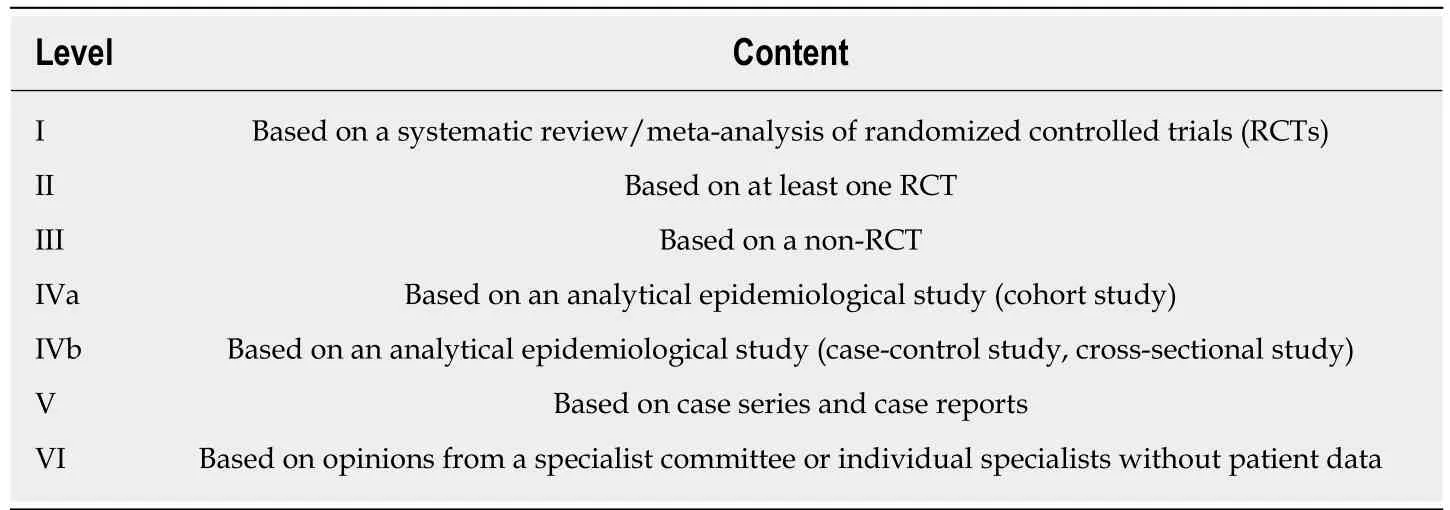
Table 1 Evidence-level classification
At present, the tunnel technique application range covers the following[5]: (1)Treatment of lesions originating from the mucosal layer, e.g., endoscopic submucosal tunnel dissection (ESTD) for oesophageal large or circular early-stage cancer or precancerosis; (2) treatment of lesions from the MP layer, per-oral endoscopic myotomy (POEM), submucosal tunnelling endoscopic resection (STER), etc.; and (3)diagnosis and treatment of lesions outside the GI tract, such as resection of lymph nodes and benign tumour excision in the mediastinum or abdominal cavity.
PREVENTION OF INFECTION AND CONCEPTS OF DETTASSOCIATED COMPLICATIONS
Although the GI tract is not a germfree environment, an intact mucosa plays an important role in preventing infection. However, the occurrence rate of infection can be increased when the mucosa is incised and an endoscope colonized with bacteria enters the submucosal tunnel during DETT. The origins of post-DETT infection mainly include: (1) Bacteria colonized in the oral cavity and oesophagus: Streptococcus viridans, Staphylococcus aureus, and enteric bacilli (Escherichia coli, Acinetobacter,Pseudomonas aeruginosa, etc.); (2) food and liquid retention in the oesophagus; (3)operation-related factors, such as intra- and postoperative bleeding, accidental injury of the mediastinum or lungs, and incomplete closure of the incision.
As reported in previous studies, preoperative use of antibiotics to prevent infections is recommended in DETT for the treatment of achalasia cardia (AC) and tumours of the oesophageal or cardia MP, with specific steps such as administering prophylactic antibiotics intravenously half an hour before surgery, with the infusion completed within 30 min. A single dose is enough if the surgery is performed within 60 min. If the surgery lasts for more than 1-2 half-life periods of the antibiotics,another dose is administered intravenously. The application of postoperative prophylactic antibiotics should not exceed 48 h[6,7].
According to relevant studies, the bacteria associated with DETT are mainly gramnegative bacteria, including Pseudomonas aeruginosa and Acinetobacter. Thus, secondand third-generation cephalosporins, such as cefuroxime and ceftriaxone, are the first choice for antibiotic prophylaxis. For patients allergic to penicillin, aztreonam combined with clindamycin or third-generation quinolones can be a substitution[8,9]. In addition, there is an opinion that acidic electrolysed oxidizing water not only has an obvious bactericidal effect on Staphylococcus aureus, Enterococcus faecalis, and Pseudomonas aeruginosa but is also harmless and free of residual toxicity. Therefore, it is suggested to use this liquid to flush the tunnel. However, no multicentre largesample controlled study has verified this method.
In conclusion, we recommend the intravenous administration of prophylactic antibiotics to prevent infection between 30 min pre-surgery and 48 h postsurgery.Other measures to prevent infection[10,11]include: (1) Fasting for 48-72 h before surgery and flushing the oesophagus and stomach with sterile water under endoscopy to reduce the number of bacteria; (2) gargling with sterile water or 0.9% saline repeatedly before the operation; (3) strictly sterilizing the endoscope and requiring the use of disposable sterile devices and sterile water during the operation; (4)coagulating exposed vessels in a timely manner to prevent haemorrhage and avoiding accidental injuries of normal tissues and organs outside the oesophageal wall; and (5)washing the tunnel and aspirating the liquid thoroughly as well as tightly closing the incision with clips (level of evidence: I; strength of recommendation: B).
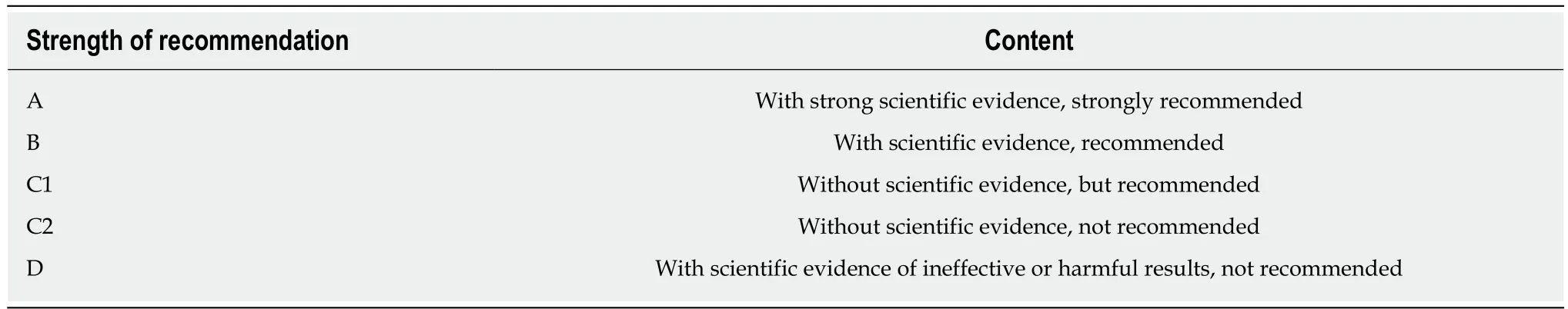
Table 2 Recommendation level
In contrast with surgery, in endoscopic procedures, the amount of bleeding is difficult to determine and is also related to the experience level of the operators. Thus,it is difficult and unreliable to evaluate bleeding according to the amount and duration. We suggest evaluating intraoperative bleeding by using endoscopic resection bleeding (ERB) three-level and five-grade methods, which include the following five grades: ERB-0: No bleeding during the whole operation; ERB-c(controlled): Endoscopic controllable bleeding (divided into three grades, namely,ERB-c1: Easily controlled bleeding under endoscopy with stable vital signs and no need for blood transfusion; ERB-c2: Bleeding degree between c1 and c3; and ERB-c3:Endoscopically controllable bleeding with intra- or postoperative blood transfusion);and ERB-unc (uncontrolled): Uncontrollable bleeding under endoscopy that needs to be handled by surgery or vessel embolotherapy.
Compared with the non-tunnel technique, DETT has the superiority of maintaining the mucosal integrity, while it may create defects in the MP during incisions or resections. MP defect (MPD) grading is suggested to classify the defection. It includes three grades: MPD-0: No defection on the MP; MPD-pt (partial thickness): Defection on the MP without full perforation; MPD-ft (full thickness): Full perforation on the MP.
METHODS FOR ESTABLISHING A SUBMUCOSAL TUNNEL
Patient position
The positions commonly used in the operation include the left lateral position, supine position, and supine position with the right shoulder raised. Anatomically, the oesophagus is located behind the trachea and heart and in front of the spine. It is relatively safe to establish a submucosal tunnel at the proximal posterior wall of the oesophagus. A proper position may contribute to the safety and simplicity of the operation. The different positions are described as follows:
Left lateral position: This is the routine position for endoscopic examinations, which helps the endoscopist to identify the anatomic orientation of the oesophageal wall[12-14].Because the common direction to operate a device under endoscopy is the six o'clock position, it is necessary to rotate the endoscope to adjust the fit direction for a tunnel to be established in the right rear oesophageal wall.
Supine position:This position facilitates the selection of the proximal posterior oesophageal wall for the operation; however, it might be difficult to advance the endoscope, given the degree of twisting of the patient's head[10,15,16]. In addition, fluid will remain in the rear oesophageal cavity due to gravity; such fluid may soak the tunnel incision during the operation, affecting the endoscopic view.
Supine position with the right shoulder raised:This position falls in between the left lateral and supine positions, with the patient's head twisting less, and the device can be withdrawn in a naturally relaxed condition to the proximal rear oesophageal wall under endoscopy, facilitating the approach and withdrawal of the endoscope as well as the whole operation. As reported in the relevant literature, this position is mainly advantageous with respect to no fluid retention at the right rear oesophageal wall(because it is not the lowest point in this position), with no effect on the operation field[10,17].
Consequently, during both ESTD and STER, operators can select the optimal position according to the lesion site before the operation. For POEM, the supine position with the right shoulder raised is recommended (level of evidence: II; strength of recommendation: B).
Tunnel incision and closure
Figure 1 Mechanism of digestive endoscopic tunnel technique, demonstrating a tunnel that is created between the mucosal and muscularis propria layers.
Tunnel incision creates an entryway to introduce the endoscope into the tunnel, which affects the convenience of going into or out of the tunnel, the tunnel's internal pressure, and the difficulty of incision closure. Incisions may vary for different sites and even for the same site, and different choices can be made on the basis of the operators' preferences and the anatomical demands. There are three methods to create a tunnel incision(Figure 2):
Longitudinal incision:The oesophageal mucosa is incised longitudinally at a length of approximately 1.8-2.0 cm, with metal clips used to close such incisions from distally to proximally after the operation[18], thus facilitating wound closure. However, more metal clips are required, and it is relatively challenging to introduce an endoscope into the tunnel; moreover, the endoscope is closely encased by the tunnel incision,with a higher gas pressure inside the tunnel (Figure 2A-D).
Transverse incision:The oesophageal mucosa is incised horizontally at a length of approximately 1.2 cm[18-20]; the entrance is wider than that of the longitudinal incision,facilitating endoscope entry into the tunnel and gas escape out of the tunnel. This incision is mainly disadvantaged by the difficulty of its closure, which entails suturing of the first clip in the middle of the incision's anal side; such a clip can be used as an“anchor”, based on which the suturing is performed longitudinally[20](Figure 2E-H).
Inverted T incision:This inverted T entry incision is combined with a 0.8-cm transverse incision and a 1.0-cm longitudinal incision[21]. Such a tunnel incision resembles an inverted “T”. By virtue of the wide tunnel space, it can facilitate the entry of the endoscope and the postoperative closure of the incision, as well as exhaust and drainage outwards, thus reducing the incidence of gas-related complications incurred by the tunnel technique (Figure 2I-L).
All three entry methods of tunnel incision are commonly used in clinical endoscopic practice. Comparing the incision area, gas pressure inside the tunnel, and difficulty of incision closure, we recommend the inverted T mucosal incision (level of evidence: III; strength of recommendation: B), given the following advantages: A larger area of the incision, ease of introducing an endoscope into the submucosa, a low gas pressure less likely to cause intraoperative gas-related complications, and fewer clips (only 4-5) required to close the incision after the procedure[22,23].
APPLICATION OF DETT FOR MUCOSAL LESIONS: ESTD IN OESOPHAGEAL MUCOSAL LESIONS
In 2009, a large circular oesophageal case successfully resected by a tunnel technique was initially reported by the team of Linghu et al[1]. Later, in 2013, the team published their research results from using the tunnel technique to treat large circular early oesophageal cancer in endoscopy, and defined the English name as ESTD[24].Compared with the traditional endoscopic submucosal dissection (ESD), ESTD has certain advantages such as a shorter operation time, faster dissection, high complete resection rate, and low complication rate, which have caused this technique to be rapidly spread and widely used around the world, especially in the endoscopic treatment of large or circular early oesophageal cancer and precancerosis. For lesions of different sizes, single-tunnel or multi-tunnel procedures are optional.
Indications and contraindications
Indications: Early cancer and precancerous lesions of the oesophagus, stomach, colon,and rectum with a transverse diameter over 2 cm[24-37].
Contraindications: Lesions deeper than the submucosa found by endoscopic ultrasound (EUS); lymphatic or distant metastasis found on EUS, computed tomography (CT), or positron emission tomography-CT; Progressive stage cancer or undifferentiated tumour; Coagulation, cardiopulmonary dysfunctions, or other endoscopic, anaesthetic contraindications.
Procedures for ESTD
The main procedures for ESTD are as follows:
Lesion evaluation:Use a magnifying endoscope, Lugol's solution stain, and indicarmine stain to evaluate the character, depth, and extent of the lesion.
Circular marking:The circumferential markings are made at least 5 mm outside the margin of the lesion by argon plasma coagulation (APC) or an electric knife. For circular lesions, circumferential markings are made outside the oral and anal margins.
Mucosal incision:After sufficient lifting by submucosal injection, two transversal or cambered incisions are successively made by an electric knife in the anal and oral mucosa.
Establishment of a submucosal tunnel:One submucosal tunnel is created from the oral incision to the anal incision with a submucosal injection and dissection. The anal incision not only indicates the terminus of the tunnel but is also conducive to lowering the intra-tunnel pressure. The endoscope should be drawn out of the tunnel repeatedly during the dissection process to ensure that the direction of the tunnel conforms to the axis of the lesion and to avoid too much normal tissue being resected.
Bilateral resection:The remaining bilateral mucosa is resected by an electric knife 5 mm outside the marking points to completely dissect the whole lesion.
Treatment of the wound surface:Visible vessels are treated by haemostatic forceps or APC with fibrin glue sprayed on the ulcer when necessary. For lesions over three quarters of the whole circle, we suggest placing a fully covered retrievable metal stent for 4 to 8 wk to prevent postoperative stricture. The schema chart of ESTD is shown in Figure 3.
Pre- and postoperative treatments
Routine preoperative tests, including coagulation function, CT, EUS, and endoscopic examinations, are suggested to be accomplished and the general physical condition should be evaluated to exclude patients with anaesthetic or endoscopic contraindications. For those who take anticoagulant drugs, antiplatelet drugs, or other coagulation-influencing drugs, a 5-7 d withdrawal period is required before the operation[38]. The duration of the preoperative fast is related to the location of the lesion, which means a 12 h fast and 4 h water fast for upper GI tract lesions without outflow tract obstruction, a longer fast time according to the food emptying time for the upper GI tract lesions with outflow tract obstruction, and a 3-d non- or lowresidue diet with bowel preparation for colonic and rectal lesions. For upper GI lesions, defoaming and anti-mucus agents are orally taken 30 min before the operation. For the lower GI tract, a thorough bowel preparation is required.Postoperative treatments are as follows:
Diet:Without complications, water intake is permitted after 48-72 h, and the diet is changed gradually from a clear liquid diet to a semi-liquid diet in 2 weeks.
Antacids:Proton pump inhibitors (PPIs) should be administered intravenously for 3 d and then taken orally for 4 wk.
Anti-infection:Antibiotics are routinely used for 2-3 d; when there are signs of infection, the antibiotic period should be prolonged or higher-level antibiotics should be used.
Follow-up:An endoscopic examination is suggested to be performed 3, 6, and 12 mo after the operation with endoscopic biopsy if necessary. Then, a routine endoscopic examination is performed every year to find recurrence or residual lesions in a timely manner.
Single-tunnel ESTD and multi-tunnel ESTD
ESTDs can be divided into single-tunnel and multi-tunnel ESTDs. To establish a submucosal tunnel conveniently and quickly, an approximately 2 cm-wide tunnel is generally preferred. Single-tunnel ESTD is recommended for lesions less than 1/2 of the oesophageal circumference, while multi-tunnel ESTD is recommended for lesions larger than 1/2 of the oesophageal circumference (level of evidence: II; strength of recommendation: A)[39-43]. Excess tunnels have no significant advantage compared with double tunnels.
Figure 3 Schema chart of endoscopic submucosal tunnel dissection, demonstrating a tunnel that is created to resect mucosal lesions.
Complications and treatments
Similar to ESD, the main complications of ESTD are bleeding, perforation, and oesophageal stenosis[24,25,27,34,41,44]. The bleeding rate ranges from 0% to 5.9%, and the perforation rate is 0%-4%; the rate of occurrence of stenosis, which is related to the circular extent and depth of the lesions, is not certain. Ono S performed a study on 84 oesophageal ESD patients, and the stenosis rates of < 1/2, < 3/4, and ≥ 3/4 circular lesions were 2% (1/49), 20% (5/25), and 90% (9/10), respectively[45]. Treatments for complications are as follows:
Bleeding:Most intraoperative bleeding can be stopped successfully under endoscopy.When patients have haematemesis, melena, or a significant decrease in haemoglobin,postoperative bleeding should be considered, and thrombotic drugs should be given immediately with a blood transfusion when necessary. If the bleeding amount is large and incurable by conservative treatment, endoscopic haemostasis may help. Vessel interventional embolization and surgery are required when all of the above therapies are performed in vain.
Perforation:Perforations during the operation are mostly successfully closed under endoscopy with common methods, including closure by metal clips, an over the scope clip (OTSC), blocking by fibrin glue, a fully covered retrievable metal stent and so forth. Common mental clips can completely close perforations smaller than 10 cm,while the new metal clip-the OTSC-can close perforations as long as 2 cm with a larger occlusal force. For large perforations and those incisions that are difficult to close by routine endoscopes, fully covered retrievable metal stents can effectively block the wound. Early finding of tardive perforation is very important for treatment and prognosis. Generally, tardive perforations within 12 h can be successfully treated by endoscopic closure and conservative treatments because of the mild inflammatory response and exudation. Perforations showing a failure of endoscopic closure, with severe mediastinal infection or unstable haemodynamics are suggested to be treated by surgery.
Stenosis:There is no satisfying treatment for stenosis yet, and common therapies in clinical practice include hormone injection or oral administration, balloon dilatation,fully covered retrievable metal stent placement, endoscopic radical incision, auto balloon dilatation, and autoplastic flap transplantation[46]. Other complications include pain, infection, transient bacteraemia, aspiration pneumonia, and gas-related complications.
In general, for large and circumferential oesophageal lesions, ESTD can achieve a faster dissection rate, reduced operative duration, and decreased incidence of intraoperative complications than regular ESD[47-51](level of evidence: I; strength of recommendation: A).
DETT FOR MP LESIONS: POEM
POEM is mainly indicated for patients with AC. In 2007, Pasricha et al[52]first conducted an animal experimental study on the feasibility of POEM in treating AC. In 2010, Inoue et al[53]reported the first use of POEM for the clinical treatment of AC and achieved good clinical efficacy. Since then, POEM has been widely used worldwide and has become one of the most effective methods to treat AC. The main characteristics of AC patients are weakened oesophageal peristalsis, decreased clearance function, and a high lower oesophageal sphincter (LES) pressure, leading to dysphagia[54-56]. The main clinical symptoms include dysphagia, reflux, retrosternal pain, and weight loss, which may affect the patient's quality of life. POEM can relieve the LES pressure to the greatest extent by establishing an oesophageal submucosal tunnel and dissecting the LES circular muscle. At the same time, it can prevent perforation by maintaining an intact submucosal tunnel. Currently, POEM is relatively safe and effective as a minimally invasive therapy for AC by endoscopy[57-59].
Classification of AC
Morphological classification of AC (barium oesophagram classification):AC on barium oesophagram shows decreased oesophageal peristalsis and a narrow distal oesophagus with a “bird beak” appearance. The mucosa of the stenosis is smooth, and oesophageal dilatation can be seen above the stenosis. According to the extent of oesophageal dilatation on barium oesophagram, AC can be divided into three grades:Grade I: Oesophageal diameter < 4 cm (mild); Grade II: 4 cm ≤ oesophageal diameter≤ 6 cm (moderate); and Grade III: Oesophageal diameter > 6 cm or even curved to an S-type (sigmoid-like appearance) (severe). Although the oesophageal morphology can be visually displayed on barium oesophagram, the shape of the oesophageal cavity cannot be displayed.
Pressure classification of AC (high-resolution manometry [HRM] classification[60,61]):HRM is considered to be the gold standard for the diagnosis of AC, which is characterized by the disappearance of oesophageal smooth muscle peristalsis and incomplete LES relaxation, often associated with LES hypertension. According to the HRM results, AC can be divided into three types: Type I is classic AC, which shows that the oesophageal peristalsis is significantly weakened, while the oesophageal pressure is not high, that is, the oesophageal motility is ineffective; type II manifests as the disappearance of oesophageal peristalsis and an obvious elevation of the total oesophageal pressure; type III manifests as an oesophageal fistula that causes blockage of the lumen. Manometry can show changes in the oesophageal pressure,which has certain significance for evaluating the therapeutic effect of surgical treatment, but its effectiveness for guiding the choice of surgical methods is not clear.
Endoscopic classification of AC (Ling classification):In 2011, Professor Linghu et al[62]proposed an endoscopic morphological classification for AC, which divides the oesophageal lumen into three categories: Straight, curved, and diverticulum, and named it the Ling classification. The details are presented in Table 3 and Figures 4 and 5. Because barium oesophagram and HRM cannot visually show the shape of the oesophageal lumen and the shape of the oesophageal cavity is closely related to the choice of surgical methods, the Ling classification has obvious advantages in guiding POEM operations. The preoperative classification of the oesophageal morphology of AC patients helps the operator select the appropriate surgical method to effectively reduce the occurrence of complications[63-65](level of evidence, II; strength of recommendation: B).
Grading of the degree of oesophageal submucosal adhesion
Due to the need to establish a tunnel under the mucosa during the POEM operation,extensive submucosal adhesion will affect the tunnel establishment and muscle incision process. In 2018, Professor Linghu published a study on the classification of AC submucosal adhesions[66]. According to the degree of inflammation of the oesophageal mucosa, AC can be classified into six grades, that is, levels A-F (Table 4).The specific grading is as follows: Grade A: Normal mucosa, with a clear vascular texture; Grade B: Rough mucosa with a vague vascular texture; Grade C: White granular mucosa without an obvious vascular texture; Grade D: Pachyntic, striated, or sulcus-shaped mucosa without an obvious vascular texture; Grade E: Ulcer in the mucosa; Grade F: Mucosal scarring. Grades E and F are further classified into four subtypes according to the involvement of the oesophageal lumen: (1) Ulcer/scar ≤1/4; (2) 1/4 < ulcer/scar ≤ 1/2; (3) 1/2 < ulcer/scar ≤ 3/4; (4) ulcer/scar > 3/4.Grading of the oesophageal mucosal inflammation is somewhat correlated with the grading of the oesophageal submucosal adhesion. Generally, a submucosa with Grades A and B mucosal inflammation shows mostly mild adhesion (Figure 6). A mucosal submucosa with Grade C mucosal inflammation shows mostly moderate adhesion (Figure 7). A mucosal submucosa with Grades D, E, and F mucosal inflammation shows mostly severe adhesion (Figure 8).
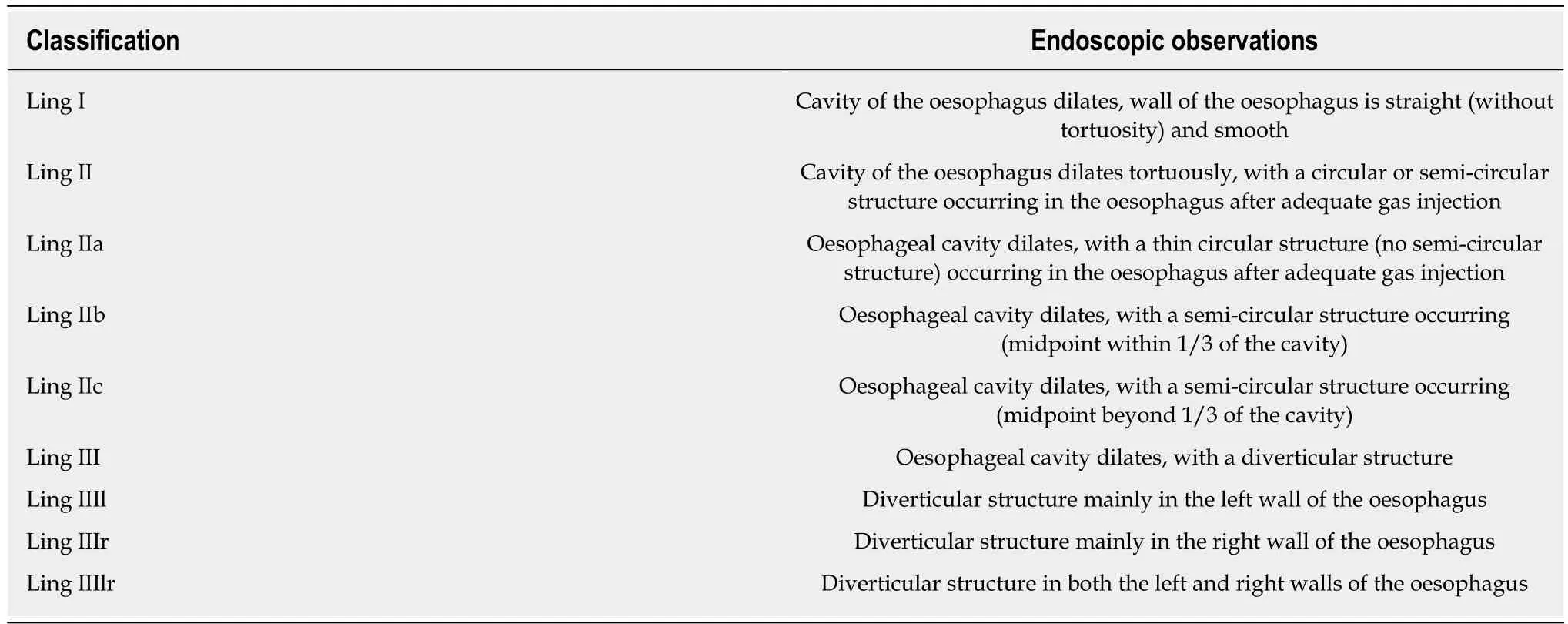
Table 3 Endoscopic morphological classification of achalasia cardia
Tunnel anatomy
Vessels from the lower oesophagus to the cardia are in a grid pattern (Figure 9A); the crescent-like structure is visible when approaching the cardia (Figure 9B). Then, the ampulla-like structure appears after entering the crescent-like structure (Figure 9C),where there are branching vessels mainly characterized by bulky vascular roots(Figure 9D). The tunnel below the cardia changes and shows a steep downward form(Figure 9E); the vessels are stubby and multi-branched in shape (Figure 9F), and the beadlike vessels are visible (Figure 9G).
Standard operating procedure of POEM and identification of the tunnel's terminus
We usually choose the posterior wall of the oesophagus to establish a tunnel that better follows a path that is flat and straight. The length is up to 10-15 cm for a standard tunnel, and the standard operating procedure is as follows:
Submucosal injection and tunnel entry creation:The fluid applied in the ESD (such as saline) is injected to form a liquid mat 8-10 cm above the oesophagus sphincter. The pressure of the LES with severe stenosis should be controlled, and thus a volume of 4-5 mL is adequate in case of tearing of the cardia mucosa. The mucosa is incised when it is fully elevated. Three different incision methods exist for tunnel entry: The longitudinal incision, transverse incision, and inverted “T” incision. The advantage of the inverted “T” incision is that it facilitates the introduction of the endoscope, and the operator can make a much shorter incision. Moreover, it is convenient for fluid and air to flow from the tunnel cavity, reducing the pressure of the tunnel and lowering the risk of pneumothorax, pneumoperitoneum, and postoperative infection.
Establishment of the tunnel:A submucosal dissection is performed through the entry incision gradually to create a tunnel between the mucosa and the MP. The end point of the tunnel should be 2-3 cm distal to the cardia.
MP incision:The MP is incised 5-7 cm from the oral side of the gastro-oesophageal junction (GEJ) to a position 2 cm below the cardia.
Tunnel entry incision closure:Metal clips are used to close the incision. The location of the first clip is crucial, and the distal side of the entry incision may be a good choice because it narrows the incision, reduces the pressure, and is easily caught. The following metal claps should be fixed in sequence every 0.2-0.3 cm from the distal to proximal direction until the entry incision is fully closed. The three incisions all employ the longitudinal sealing method. The schema chart of POEM is shown in Figure 10.
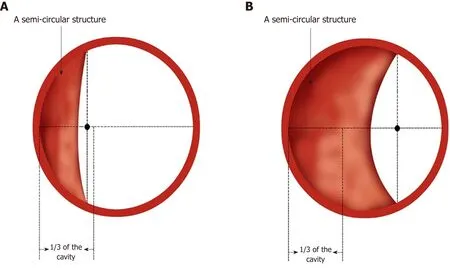
Figure 4 Simulated diagram of endoscopic observations in Ling IIb and Ling IIc. A: Ling IIb. The arrows indicate 1/3 of the oesophageal cavity, and the semiannular structure's midpoint remains within this range; B: Ling IIc. The arrows indicate 1/3 of the oesophageal cavity, and the crescent-like structure's midpoint goes beyond this range.
The end point of the tunnel lies 2-3 cm to the anal side of the GEJ. Correctly determining the anatomic location of the GEJ during POEM is of great importance.The following methods of affirming the end point are recommended: (1) The distance from the incisor could be used to help determine the location of the GEJ; (2) the cardia is the narrowest site in the tunnel, and a portion of it may form an ampulla-like structure with serious adhesion. The myotomy should be performed until 2 cm below the cardia; (3) the location of the GEJ may be confirmed by the pattern and path of the submucosal blood vessels and the oesophageal lumen in the tunnel. The end point crosses over 2-3 cm below the region with a paliform pattern of the lower part of the cardia; and (4) another method to confirm the terminus of the tunnel is to observe whether the whitish region of the mucosa on the anal side reaches a location 2 cm below the cardia when retrieving the endoscope from the tunnel and reversing it at the gastric fundus.
Tunnel establishment methods
There are three types of tunnel establishment methods as follows: Standard-tunnel POEM, short-tunnel POEM, and simultaneous submucosal and muscle dissection POEM (POEM-SSMD).
Standard-tunnel POEM: The standard tunnel is up to 10-12 cm long, extending from a position approximately 8-10 cm on the oral side of GEJ to 2-3 cm on the anal side.This technique is applicable for types Ling I, Ling II, and Ling IIb.
Short-tunnel POEM: The short tunnel is established from a location 5 cm on the oral side to 2 cm on the anal side of the GEJ. The length of the entire tunnel is approximately 6-8 cm. This technique is applicable for types Ling IIc and Ling III. The main operation procedure is as follows: (1) Submucosal injection and tunnel entry creation: The fluid applied in the ESD (such as saline) is injected to form a liquid mat 5 cm above the oesophageal sphincter. The mucosa is incised when it is fully elevated. It is not advised to adopt a longitudinal incision in short-tunnel POEM because there exists enough valid oesophagus; (2) establishment of the tunnel: A tunnel is established in the same way as the standard-tunnel POEM. The difference is that the tunnel is much shorter when performing short-tunnel POEM; (3) incision of the MP:The starting point of the myotomy is recommended to occur 2 cm on the oral side of the GEJ, and the end point of the myotomy is at 2 cm below the cardia. The length of the myotomy ranges from 3 to 5 cm; and (4) entry incision sealing: This step is performed in the same way as the standard-tunnel POEM.
POEM-SSMD: The MP is incised directly after establishing a super-short tunnel without damaging the mucosa and muscularis mucosa when the muscularis mucosa is completely adhesive to the MP. POEM-SSMD is applied in AC patients when a severe adhesion exists in the submucosa of the cardia and the tunnel is difficult to extend. The main operation procedure is as follows: (1) Submucosal injection and tunnel entry creation: The fluid applied in the ESD (such as saline) is injected to form a liquid mat 8-10 cm above the oesophageal sphincter and the mucosa is incised to establish the tunnel entry site; (2) establishment of the tunnel: A submucosal dissection is performed from the entry incision and a tunnel is established between the mucosa and the MP. The tunnel is extended as far as possible until it cannot be continued because of the severe adhesion; (3) incision of the MP: First, a 1-3 cm fullthickness myotomy is performed after establishing a super-short tunnel. Then, the submucosa and MP are incised simultaneously to 2-3 cm below the dentate line; and(4) tunnel entry incision sealing: This step is performed in the same way as the standard-tunnel POEM.
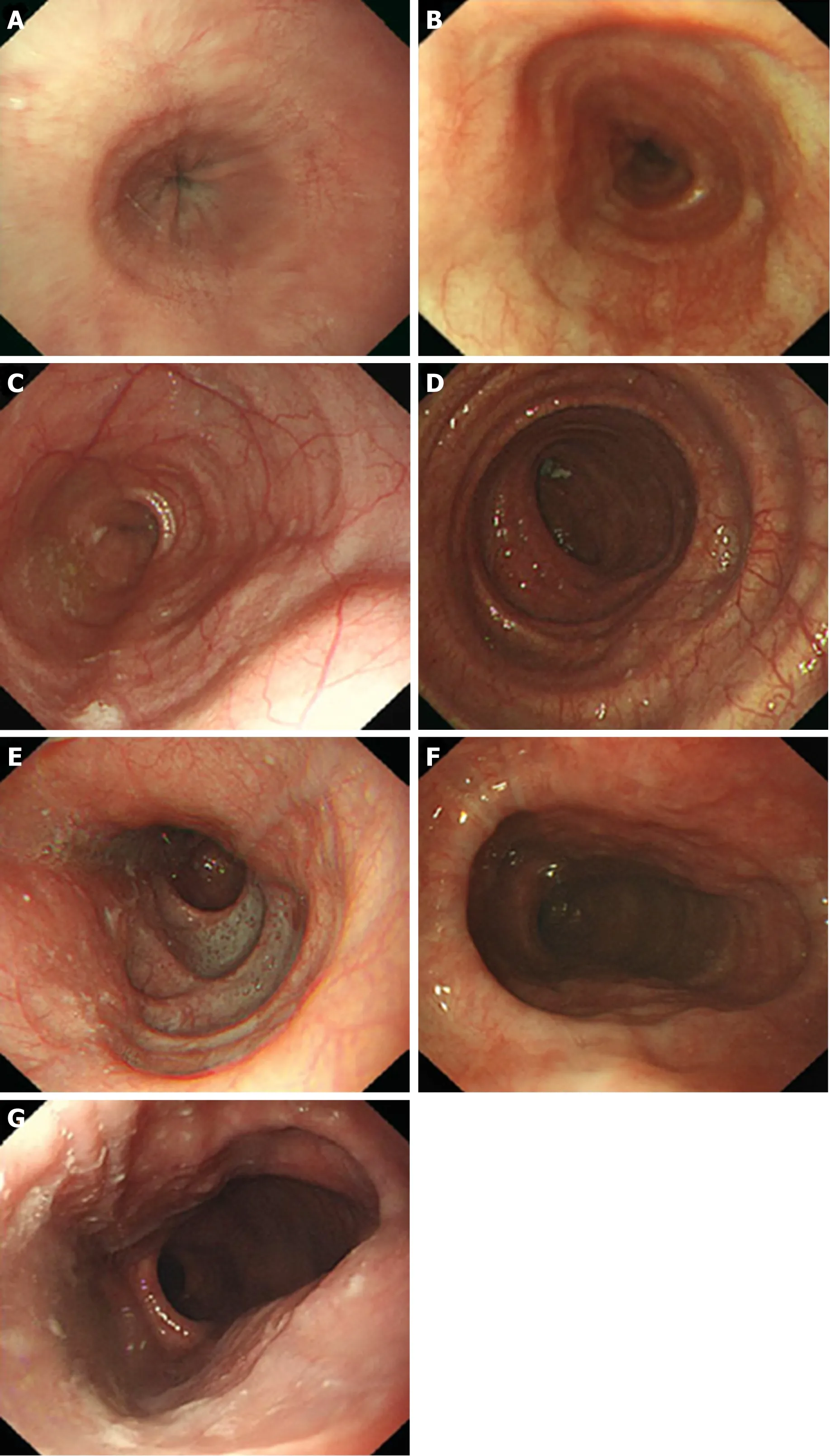
Figure 5 Endoscopic images of the Ling classification of achalasia cardia. A: Ling I; B: Ling IIa; C: Ling IIb; D:Ling IIc, E: Ling III1; F: Ling IIIr; G: Ling IIIlr.
Ling classification of the oesophageal morphology by endoscopy can help the operator to select an appropriate surgical method. The oesophageal lumen in Ling I, Ling IIa, and Ling IIb cases is relatively straight; therefore, it is appropriate to adopt standard-tunnel POEM. For types Ling IIc and Ling III, we find that there is a high risk of losing one's bearings and damaging the tunnel mucosa when establishing a standard tunnel to cross the “ridge” formed by the crescent-like structure due to severe tortuosity and dilation of the lower oesophagus (Figure 11A). Thus, shorttunnel POEM is considered to be a good choice. The entry incision of the tunnel is established at a relatively flat location on the oesophageal wall (Figure 11B), which facilitates the endoscope to bypass the "ridge" via the portal into the oesophageal submucosa (Figure 11C). As indicated in relevant studies, there is no significant difference in the efficacy between short-tunnel POEM and standard-tunnel POEM for AC patients with LingIIc and Ling III classifications[67-69].
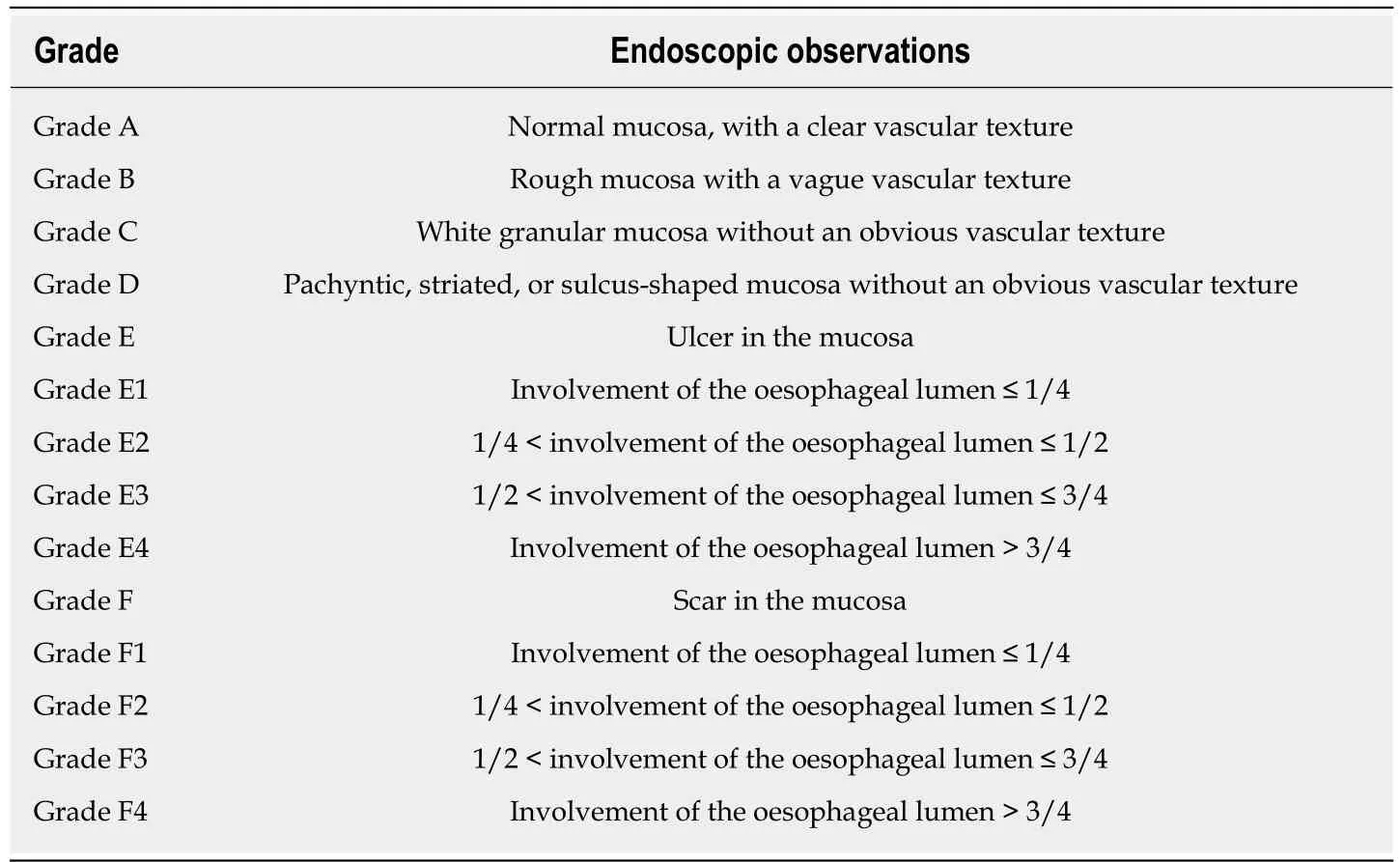
Table 4 Degree of inflammation of the oesophageal mucosa in achalasia cardia
Types of myotomy in the tunnel
The myotomy types commonly reported include inner circular muscle myotomy, fullthickness myotomy, glasses-style myotomy, inner circular muscle myotomy + balloon plasty, and progressive full-thickness myotomy[70-76]. At present, full-thickness myotomy and progressive full-thickness myotomy are widely applied in clinical practice.
Inner circular muscle myotomy(Figure 12): The circular muscle is incised at 1 cm above the narrow part with electro-surgical knives, which is pushed forward step by step until all of the inner circular muscle is cut apart. At the bottom of the circular muscle, part of it is lifted up and an incision is progressively created to the end point of the tunnel, aiming to mutilate the muscle totally. This technique is inefficient in some patients.
Full-thickness myotomy(Figure 13): The circular and longitudinal muscles are completely incised from the narrow part to the location below the cardia with electrosurgical knives. The method is always associated with a higher incidence of postoperative gastro-oesophageal reflux disease (GERD).
Glasses-style myotomy(Figure 14): The MP is fully incised from the oral side to the anal side, with only an approximately 1 cm-long muscle left at the relative position of the dentate line, which appears as a glasses-type structure under an endoscope, to prevent postoperative GERD.
Circular muscle myotomy + balloon plasty(Figure 15): The oesophageal lumen is dilated with a cylindrical expansion balloon to detach some longitudinal muscle on the basis of the inner circular muscle myotomy. The method may achieve the goal of mutilating the MP fully, while the procedure appears to be much more complex.
Progressive full-thickness myotomy(Figure 16): The muscle is incised from 1 cm above the first narrow ring to the end of the tunnel, following the sequence of incising part of the inner circular muscle, full inner circular muscle myotomy, and then fullthickness myotomy of the MP from superficial to deep. This method appears to be the most valid way to relieve the symptoms of dysphagia effectively without causing GERD-relative problems.
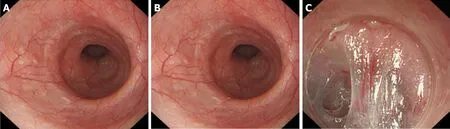
Figure 6 Correlation between grade A or grade B mucosal inflammation and mild oesophageal submucosal adhesion. A: Grade A mucosal inflammation; B:Grade B mucosal inflammation; C: Mild oesophageal submucosal adhesion: The fibre filaments are distributed in bundles.
Indications, relative indications, and relative and absolute contraindications of POEM
Indications: Patients diagnosed with AC[77]. Ling types I, IIa, and IIb are the best indications. With the development of endoscopic technology and the appearance of new endoscopic procedures, Ling types IIc and III, which previously were contraindications, are now considered indications for POEM[78-88].
Relative indications: Patients with a significantly dilated and tortuous oesophageal lumen, diffuse oesophageal spasm, nutcracker oesophagus, or prior treatment by Heller myotomy[89]. Cutting part of the circular muscle is recommended in patients with diffuse oesophageal spasm or nutcracker oesophagus.
Relative contraindications: Patients with Grade E, Grade F (endoscopic classification of oesophageal mucosa), or severe submucosal adhesion.
Absolute contraindications: Patients who have contraindications for endoscopic examination or exhibit severe cardiopulmonary dysfunction.
Perioperative management
All patients should complete HRM, 24-h pH monitoring, EGD, and/or a chest CT scan. POEM is performed after the patients have undergone fasting for 48 h, water restriction for 6 h, and EGD to ensure that no food residue remains in the oesophageal lumen on the day of the operation. Gargling with sterile water or saline is recommended before anaesthesia. Anticoagulant and antiplatelet agents are stopped for at least 5-7 d, and blood coagulation should be performed before the operation[90].
Postoperative treatment is similar to that for ESTD. The details are as follows: (1)Diet: All patients fast for 2-3 d after the procedure. The patients' diets progress gradually from a clear liquid diet to a semi-liquid diet and to a normal diet in 1 mo if no obvious postoperative complications are observed; (2) acid inhibitors: A PPI is administered intravenously for 3 d. An oral PPI is required for at least 4 wk; (3) antiinfection: Antibiotics are used for 2-3 d if there are no clinical signs of infection.Otherwise, a prolonged duration of antibiotic therapy or higher grade antibiotics should be considered; (4) routine blood tests, chest and abdomen X-ray, or CT are performed 3 d after POEM to determine whether there are signs of infection,emphysema, or pneumothorax; (5) dietary guidance: A solid food is given first,liquids are administered sequentially, and chewing well is also important; and (6)patients are scheduled to follow-up at the centre 1 mo, 3 mo, 6 mo, and 1 yr postoperatively and then yearly afterward, during which symptom assessments,physical examinations, and objective tests including EGD, HRM, and 24-h pH monitoring are performed.
Management of complications
The major complications mainly include: (1) Gas-related complications[91-94]: The examples are pneumomediastinum (approximately 4.9%), pneumothorax (0.2%-14.3%), pneumoperitoneum (13%-16.2%), and subcutaneous emphysema (11%-21.8%).Because of the debate over whether minor pneumatosis should be defined as a postoperative complication, few studies have been reported on them; (2) gastrooesophageal reflux: The incidence of GERD is 5%-72.5%, which is mainly related to the differences in the specific definition in various studies[95-99]; and (3) other relatively rare complications, such as delayed bleeding, incision tears, mediastinal abscess,oesophageal stenosis, hydrothorax, and oesophagitis[100].
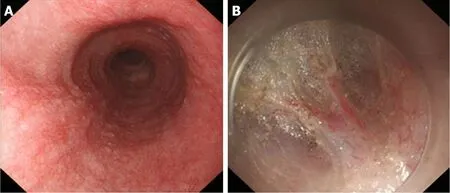
Figure 7 Correlation between Grade C mucosal inflammation and moderate oesophageal submucosal adhesion. A: Grade C mucosal inflammation; B: Moderate oesophageal submucosal adhesion. The fibres are arranged in disorder, with fusion and decreased transparency.
Gas-related complications:Patients with mild subcutaneous emphysema,mediastinal emphysema, pneumothorax, and pneumoperitoneum are left without interventions because slight pneumatosis can be absorbed spontaneously. However,massive pneumatosis should be solved in a timely manner because it may cause blood oxygen desaturation and affect the vital signs directly. For patients with severe pneumothorax and pneumomediastinum, thoracic closed drainage can be performed under imaging guidance if possible. For severe pneumoperitoneum, an injector is often used for abdominocentesis to exhaust gas. In addition, multiple injectors are used if necessary to speed up the rate of pressure reduction.
In addition, the following advice can reduce the incidence of gas-related complications resulting from POEM: (1) CO2is used during the whole procedure, as this gas can be absorbed quickly by the human body; (2) when full-thickness myotomy is performed, the integrity of the oesophageal outer membrane should be retained as much as possible; and (3) the duration of the myotomy is minimized.
The following measures are useful for preventing infection: (1) Fasting for 48 h before POEM; (2) a tracheal cannula is recommended to avoid aspiration; (3) the tunnel is cleaned with saline before closing the tunnel entry; and (4) the entry is closed tightly to avoid large gaps.
Postoperative GERD can be relieved by using a PPI and GI prokinetic drugs. When the symptoms of GERD are not relieved by the above measures, a high dose of PPI is recommended; endoscopic therapy, such as cardiac constriction and fundoplication, is also considered.
When severe delayed bleeding occurs in the tunnel, endoscopic electrocoagulation should be performed immediately after removing the clips and cleaning the tunnel with saline. Then, the tunnel entry is closed again after successful haemostasis. Clips and porcine fibrin glue are both used to close mucosal injury[101-103].
The following measures can be used to treat mediastinal abscess: (1) Cleaning the oesophageal lumen, tunnel, and mediastinum, placing a drainage tube in the mediastinum, and fixing it onto the nose; (2) keeping the tunnel entry open and prolonging the time of fasting; and (3) using antibiotics intravenously and placing a jejunal nutritional tube for enteral nutrition.
Clinical efficacy of POEM for AC
POEM can achieve significant short-term clinical efficacy for AC, and the treatment success rate (postprocedure Eckardt score ≤ 3) is 82.4% to 100%[15,53,92,104-109]. Recent data demonstrate that the long-term clinical success rate (follow-up period ≥ 5 yr) of POEM is 83% to 87.1%[110,111]. A significant reduction in LES pressure and alleviation of oesophageal dilatation can be observed in patients who are treated with POEM.
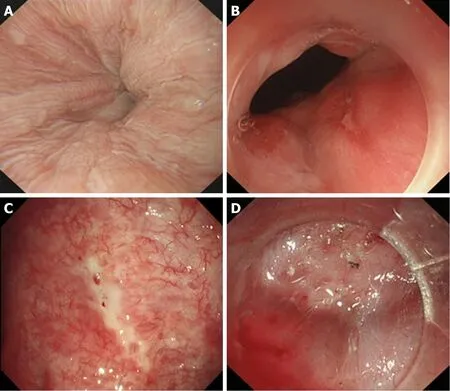
Figure 8 Correlation between Grade D, Grade E, or Grade F mucosal inflammation and severe oesophageal submucosal adhesion. A: Grade D mucosal inflammation; B: Grade E mucosal inflammation; C: Grade F mucosal inflammation; D: Severe oesophageal submucosal adhesion. The submucosa and muscularis propria are completely adherent.
In conclusion, compared with other endoscopic surgeries, POEM is characterized by fixed operation steps. Therefore, it is essential to formulate a set of standard operating procedures to regulate its procedures. The following steps are recommended: (1) Patients should fast for 48 h from solid and liquid diets and 6 h from water. The oesophageal lumen is cleaned with normal saline under preoperative gastroscopic guidance. The patient should be placed in the supine position with the right shoulder raised and should undergo intratracheal intubation anaesthesia with CO2applied during the entire surgical process (level of evidence, II; strength of recommendation, B); (2) standard tunnels should be established for AC patients with types Ling I, IIa, and IIb, while short tunnels are recommended for Ling IIc and III cases (level of evidence, II; strength of recommendation, B); (3) it is recommended to apply inversed T entry incisions (level of evidence, III; strength of recommendation,B); (4) it is recommended to apply progressive full-thickness myotomy (level of evidence, IVa; strength of recommendation, B); (5) it is recommended to apply clips or porcine fibrin glue for occlusion in case of mucosal injury (level of evidence, IVa;strength of recommendation, B); (6) in case of the occurrence of complications, the abovementioned treatment methods help to perform conservative treatments safely and successfully[5](level of evidence, III; strength of recommendation, B); and (7)according to preliminary data, the mid- to long-term treatment effects of POEM are similar to those of surgical treatment[53,107-109,111-113], and POEM is expected to be the future clinical first-line therapeutic choice[99](level of evidence, I; strength of recommendation, A).
Extended application of POEM
POEM for oesophageal diverticula (D-POEM):With the development of POEM, the scope of its treatment has been expanded. POEM has been reported to be used in the treatment of oesophageal diverticula by establishing a submucosal tunnel to reach the diverticulum, where the proper muscular layer is incised to make the diverticulum smaller, and symptoms such as dysphagia and weight loss can be relieved[114-117]. This is a novel technique for the treatment of diverticula, but further studies on clinical efficacy are needed. The main indication for D-POEM is the formation of a sacked bag diverticulum that significantly affects swallowing and food flow. The pre-procedure preparation of D-POEM is similar to that of routine endoscopy, including fasting for 12 h and water prohibition for 4 h before the procedure.
POEM for gastric disease (G-POEM):In recent years, there have been reports on applying gastric POEM (G-POEM) in the treatment of diabetic gastroparesis (DGP)and delayed gastric emptying (DGE) after subtotal gastrectomy[15,118,119]. G-POEM is essentially similar to POEM for oesophageal achalasia, including submucosal tunnelling to the pyloric ring, myotomy involving the full thickness of the pyloric sphincter, and finally, closure of the incision using clips, with the aim of alleviating the symptoms of patients with emptying dysfunction caused by various aetiologies and unidentified idiopathic gastroparesis.
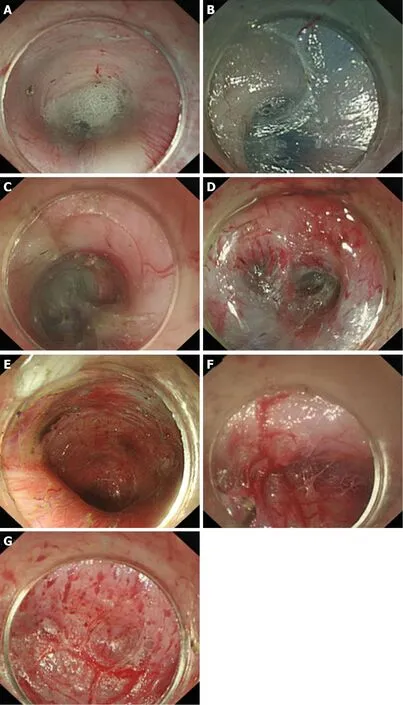
Figure 9 Anatomical landmark in the tunnel from the lower oesophagus to the cardia. A: Grid-like blood vessels in the cardia; B: Crescent-like structure visible at the proximal cardia; C: Ampulla-like structure appearing after entering the crescent-like structure; D: Branching vessels with bulky vascular roots in the ampulla-like structure;E: Tunnel below the cardia, showing a steep downward form; F: Stubby and multi-branched vessels below the cardia;G: Beadlike vessels below the cardia.
The indications for G-POEM include gastric emptying disorders caused by diabetes, surgery, infection, and idiopathic gastroparesis with an unknown aetiology,accompanied by symptoms associated with severe gastric emptying dysfunction such as nausea, vomiting, abdominal pain, stomach fullness, early satiety, loss of appetite,nausea, postprandial fullness, and weight loss. The contraindications are similar to those for POEM. The pre-procedure preparation for G-POEM is similar to that for POEM for AC, except that G-POEM requires a gastric emptying test.
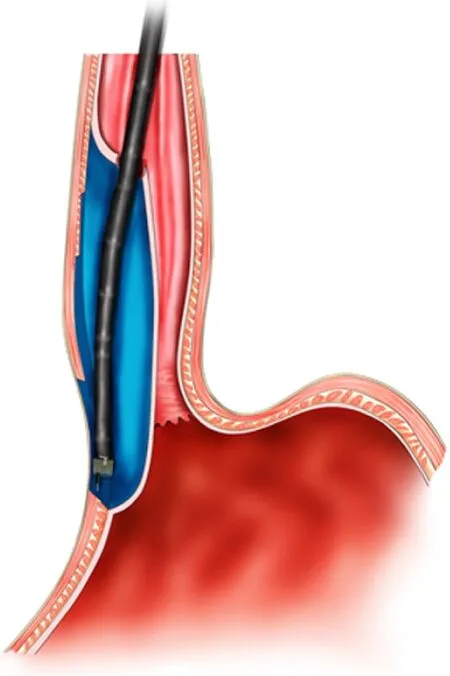
Figure 10 Schema chart of per-oral endoscopic myotomy, demonstrating a tunnel that is created to incise the muscularis propria.
Similar to oral endoscopic myotomy (POEM), the G-POEM operation is also divided into four steps: Establishing a tunnel opening; creating a submucosal tunnel;performing a pyloromyotomy; and closing the tunnel entrance. It should be noted that there is no uniform standard for the operation due to the short development time of the technology and because the current total number of cases is small.
G-POEM is an emerging minimally invasive therapy for patients with gastric emptying disorders. After being successfully applied in the treatment of patients with DGP who failed medical treatment for the first time in 2013[120], the feasibility, safety,and effectiveness of G-POEM have been confirmed by a series of studies. The feasibility of the G-POEM operation process is reproducible and can be completed by experienced endoscopists with a success rate of up to 100%. G-POEM has been demonstrated to be a safe procedure. Intra-procedural and postprocedural complications such as haemorrhage, mucosal tears, and perforation are rarely reported, and the incidence of complications is low. In terms of effectiveness, after GPOEM treatment, the clinical symptom relief rate associated with gastric emptying disorder can reach more than 80%, and the Gastroparesis Cardinal Symptom Index at 1, 6, and 12 mo is significantly decreased. Gastric electrical stimulation shows that the gastric emptying ability becomes normal or improves significantly, and the quality of life of patients also improves[119-122].
DETT FOR MP LESIONS: STER
Previously, submucosal tumours (SMTs) were treated by open surgery, thoracoscopy,or laparoscopy, which were associated with high invasiveness. Along with the development of endoscopic techniques, the availability of endoscopic submucosal excavation (ESE) and endoscopic full-thickness resection (EFR) makes it possible to perform endoscopic resection of SMTs originating from the MP layer. However, these procedures do not maintain the mucosal integrity, which could result in perforation,infection, and postoperative strictures.
In 2011, Linghu et al[123]reported a case involving the resection of MP tissue in two pigs using a tunnel, which initially proved that it was feasible to resect SMTs originating from the MP layer using a tunnel technique. STER is a novel technique named by Xu et al[124]who reported resecting SMTs by establishing a tunnel between the submucosal and MP layer in patients in 2012. STER was preliminarily proved to be superior to ESE and EFR for the treatment of SMTs originating from the MP layer due to its ability to maintain the integrity of the mucosa[125-127].
Indications, relative indications, and contraindications for STER[128-133]
Indications: Considering that the lesion to be excised would pass several narrow places and the limit of the tunnel width, it is advised to choose SMTs with a transverse diameter of 2.5 cm or less.
Relative indications: 2.5 cm ≤ transverse diameter ≤ 3.5 cm.

Figure 11 Endoscope crosses the “ridge” via short-tunnel per-oral endoscopic myotomy. A: Type Ling IIc oesophagus. The arrow indicates a “ridge” structure formed by the crescent-like structure; B: The short-tunnel entry incision established on a relatively flat oesophageal wall at the oral side of the “ridge”; C: It is easy to cross the “ridge” within the tunnel.
Relative contraindications: (1) The surface of the SMT mucosa is not intact with ulcer,which eliminates the significance of establishing a tunnel to maintain mucosal integrity; there are submucosal adhesions in the ulcer area due to inflammation, and it is difficult to create a submucosal tunnel; (2) the tumour is located at the entrance of the oesophagus, and there is no room to create a submucosal tunnel; and (3) the transverse diameter of the tumour is > 3.5 cm, and it cannot be completely removed from the tunnel.
Contraindications: (1) The patients with severe cardiopulmonary dysfunction cannot undergo an endoscopic operation; (2) blood coagulation dysfunction is evident; (3)there is a large area of scarring or anastomosis at the tunnel area; and (4) there is a suspected malignant tumour.
STER procedures
Standard STER is conducted as follows:
Submucosal injection and mucosal incision:A submucosal fluid cushion is made with an injection needle 3-5 cm proximal to the tumour, and then a mucosal incision is made as the entry point. There are three types of incisions, including a longitudinal mucosal incision, transverse incision, and inverted T incision. A transverse incision is suggested for SMTs that are located in the upper oesophagus, which are closed to the oesophagus inlet, where there is a limited length to establish a tunnel.
Creation of a submucosal tunnel:A tunnel is created from the oral side to the anal side between the mucosal and the MP layer and ends at the distal side of the tumour.Repeated submucosal injections contribute to avoiding accidental injury to the tunnel mucosa. The exposed vessels and small bleeds should be treated in time to ensure a satisfactory endoscopic view. To obtain the integrity of the mucosa and reduce the bleeding, submucosal dissection should be conducted close to the MP where the vascular networks are absent.
Resection of the tumour:The tumour is resected with an intact capsule from the MP layer after being completely exposed using a knife or a snare. The submucosal tunnel is lavaged with normal saline solution after the removal of the tumour. The resection edge is carefully treated with haemostatic forceps and APC to reduce haemostasis and prevent delayed bleeding and postoperative infection.
Closure of the mucosal incision site:Clips are used for incision closure. The closure method of STER is similar to that of POEM. The transverse incision and inverse T incision are closed in a longitudinal shape. The resected specimen is sent for pathological evaluation. SMT < 1.5 cm in diameter could be extracted directly by endoscopic aspiration, while SMT ≥ 1.5 cm in diameter should be extracted with a snare or basket. The schema chart of STER is shown in Figure 17.
When resecting cardial SMTs, the direction of the tunnel is difficult to identify because of the anatomical structure of the cardia. Methylene blue or indigo carmine can be used to locate the tumour and guide the direction of the tunnel following submucosal incision[127,134-136]. The procedures for STER treatment of cardial SMTs are shown in Figure 18.
Special considerations
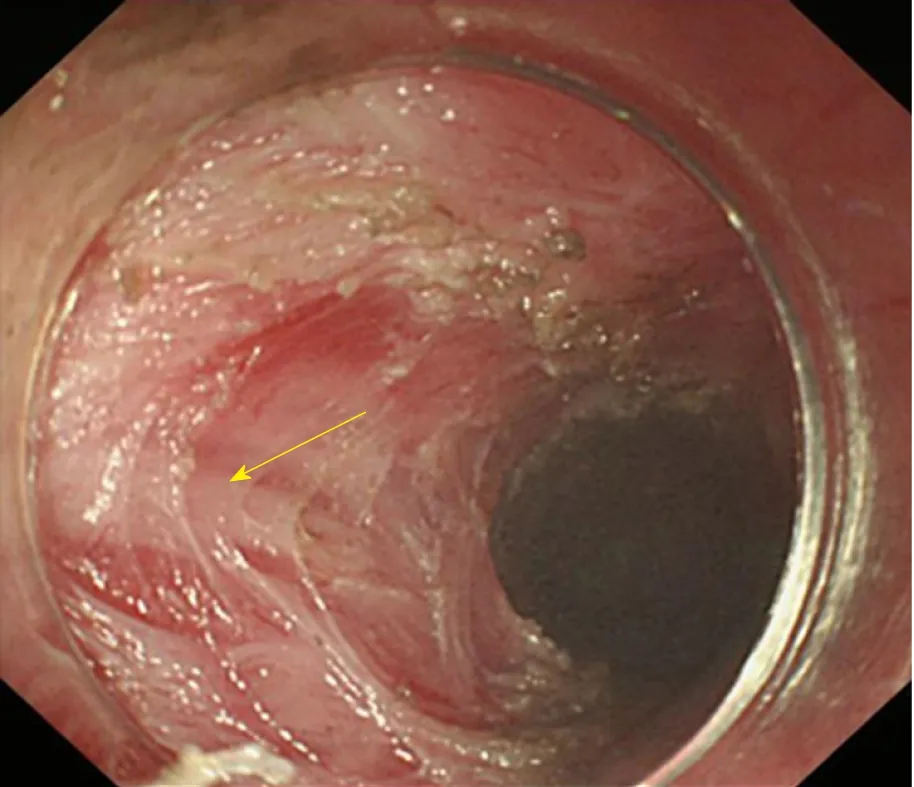
Figure 12 Inner circular muscle myotomy. The arrow shows the well-retained longitudinal muscle.
There are several factors to which attention should be paid during the operation. First,because of the polygon or ginger shape of cardial SMTs, it is necessary to detach the lesion edge while dissecting muscle fibres in the tunnel. If the lesion is so large that it affects the vision in the tunnel, it can be partly resected with a snare after the exposure of some parts of the lesion and the above procedures repeated until the lesions are completely resected. Second, the resection of a large SMT may lead to the absence of a large area of MP at the cardia, which may cause GERD because of the low pressure of the LES after the cicatricial healing of the MP. However, further studies with large sample sizes are warranted to confirm this conclusion.
Preoperative preparations and postoperative care
EUS, enhanced CT, and other imaging examinations should be performed before STER to evaluate the size, location, shape, depth, and blood supply of the tumour and to eliminate the possibility of metastasis and/or invasion outside the digestive tract(level of evidence, I; strength of recommendation, A).
The general condition of the patient should be assessed to evaluate whether he or she is suitable for anaesthesia. A coagulation function test should be performed before the procedures. Patients should stop using anticoagulant drugs, antiplatelet drugs,and drugs that affect blood function at least 5-7 d prior to STER[38]. Patients should be fasted from food for 12 h and from water for 4 h. Medicines that dispel mucus and foam should be administered 30 min before the procedure for patients with upper GI lesions, while a good preparation to keep the bowel clean is indispensable for patients with lower GI lesions. Any discomfort such as fever, abdominal pain, perforation,haematemesis, and haematochezia is closely monitored after STER. A complete blood count is performed on the morning after STER.
The postoperative treatments for STER are similar to those for ESTD and POEM.Patients are fasted for 2-3 d, consume a liquid diet for 3 d, and return gradually to a normal diet within 2 wk. Intravenous PPIs are used for 2-3 d, followed by oral PPI therapy for 4 wk. The intravenous antibiotics are stopped after 2-3 d if there are no signs of infection, otherwise prolonged antibiotics or strong antibiotics should be used to control infection. Chest/abdominal X-ray or CT is performed in cases of severe chest and/or abdominal pain. The majority of the gas-related complications resolve spontaneously without the need for intervention. Thoracic drainage is required for pneumothorax with collapse of greater than 30% of the lung. A 20-gauge needle is inserted into the right lower quadrant to confirm the presence of the complication and the release of the gas for suspected pneumoperitoneum during and/or after the procedure[137]. Patients are discharged when they can take a semi-liquid diet.
Surveillance endoscopy is performed at 3, 6, and 12 mo after the operation and then annually[125,138,139]. Contrast-enhanced CT is performed for patients with GI stromal tumours (GISTs) every 3-6 mo for 3-5 yr. A less-frequent follow-up period is recommended for very-low-risk GISTs[140].
Safety outcomes and treatment of complications of STER
The incidence of STER-related complications is 0-66.7%, and gas-related complications are the most common complications, with an incidence of 0 to 66.7%[137,141,142]. The incidence of STER-related complications mostly ranges from 5% to 25%[123,124,126,127,129,139,143-148]. The oesophagus is the safest area for performing STER, with a low incidence of complications, namely, 9.5% to 16.7%[129,145]. The incidence of complications is 15.3% to 42.9% in the cardia[125,127,144,149], 11.1% to 21.9% in the stomach[139,147], and 62.5% in the rectum[150]. A meta-analysis showed that the incidence rates of STER-related mediastinal/subcutaneous emphysema, pneumothorax, and pneumoperitoneum are 14.8% [95% confidence interval (CI): 10.5% to 20.5%], 6.1%(95% CI: 4.0% to 9%), and 6.8% (95% CI: 4.7% to 9.6%)[151], respectively.
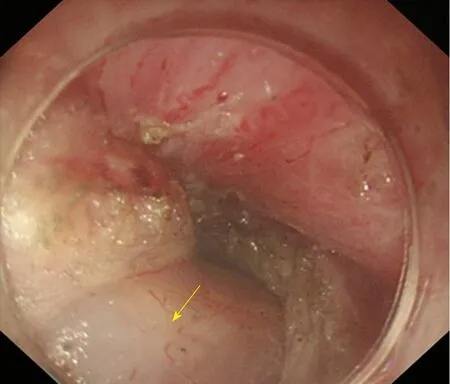
Figure 13 Full-thickness myotomy. The arrow shows the tunica adventitia of the oesophagus.
Treatment methods for common complications are as follows: (1) Subcutaneous emphysema, a small amount of mediastinal emphysema, and a small number of pneumothorax cases do not require special treatment and can be absorbed spontaneously. Compared with air, CO2is more easily absorbed by the body;consequently, the use of CO2perfusion can reduce the occurrence of gas-related complications during the operation. Chest pain is a common symptom after surgery.A chest X-ray or chest CT scan should be performed in a timely manner for suspected pneumothorax. A small amount of pneumothorax cannot be prevented temporarily and should be closely monitored. Thoracentesis is feasible for obvious pneumothorax(lung tissue collapse > 30%); (2) postoperative fever can be treated with an antipyretic combined with physical cooling. If infection happens, timely anti-infection treatment is required; (3) mucous membrane breakage can be clipped with a titanium clip or plugged with a biological fibrin glue. Repeated submucosal injection can help reduce mucosal damage; (4) for patients with pneumoperitoneum, if the patient's abdominal distension is not obvious and the gas is less, the condition can be closely monitored and the gas can be absorbed spontaneously. If the patient has obvious abdominal distension or obvious abdominal distension, puncture and release of the gas can be performed in the right lower abdomen after the operation. No other special treatments are needed.
Effectiveness of STER
The total en bloc resection rate of STER for GI SMTs is 83.3%-100%[130,152,153]. The en bloc resection rates for oesophageal and cardial SMTs are 84.6%-98.6%[129,145]and 74.5%-100%[125,127,139,144,154], respectively.
Comparison of STER and surgery
Compared with surgical treatment, STER is superior with less invasion, faster postoperative recovery, lower hospitalization expenses, higher acceptability, and other advantages[130,145,155]. However, STER cannot completely replace surgical treatment. The optimal treatment method should be selected according to the disease itself, thus benefiting the patient.
In conclusion, STER is not suitable for all SMTs, and surgeons should strictly assess the indications. STER is indicated for benign lesions with a diameter less than 3.5 cm and free from an abundant blood supply as determined by EUS, CT, or other imaging tools[138,155](level of evidence, II; strength of recommendation, A). It is challenging to gain a clear view in the tunnel when treating cardial SMTs, especially when they originate from the deeper MP layer; therefore, methylene blue is recommended to locate the tumour and guide the direction of the tunnel, thereby shortening the time required for lesion detection and increasing efficiency[127](level of evidence, III;strength of recommendation, B).
SURGERIES ON THE EXTERNAL DIGESTIVE TRACT WALL
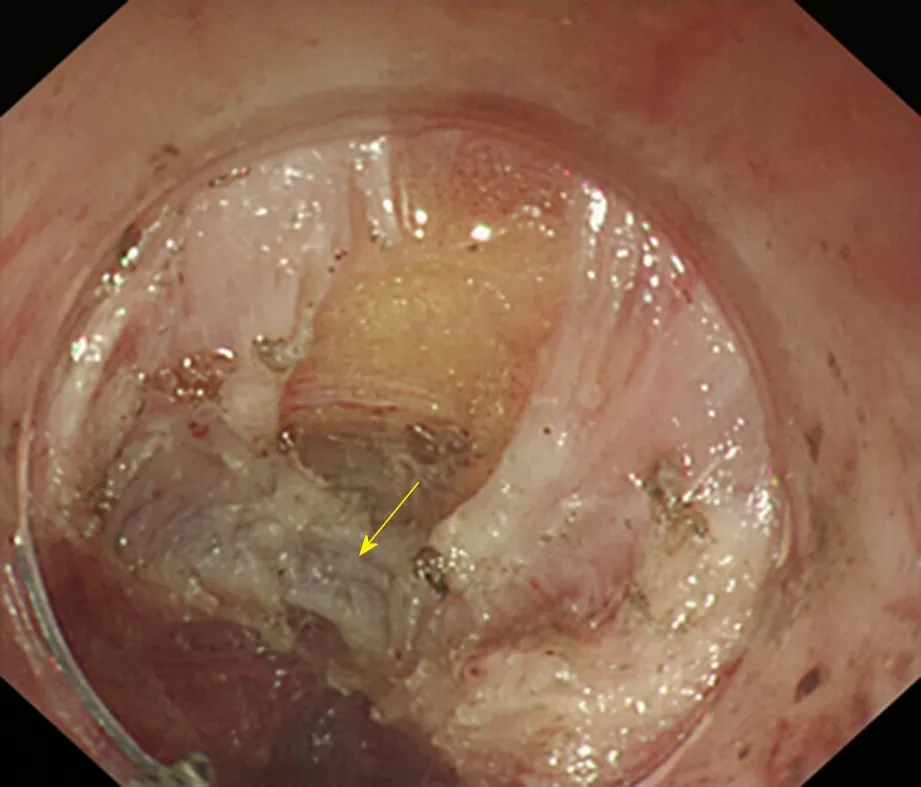
Figure 14 Glasses-style myotomy. The arrow shows the muscles remaining at the cardia.
Currently, surgeries on the external digestive tract wall are performed on the basis of the establishment of tunnel targeting in experimental subjects and human cadavers[11],but many controversies remain, and no consensus, such as on how to control abdominal infection, has been reached. With the constant development of DETT and improvement of related endoscopic techniques and instruments, surgery on the external digestive tract performed by endoscopy via a tunnel will soon become a reality. The schema chart of DETT on the external digestive tract wall is shown in Figure 19.
CONCLUSION
DETT has removed the obstacles between internal medical and surgical treatments, in line with the principles of endoscopic treatment centred on diseases. In the near future, endoscopy is set to follow the basic principles of complete lacunas, sterility,use of natural orifices, and the absence of chemical stimulation, thus providing patients with better treatment methods.
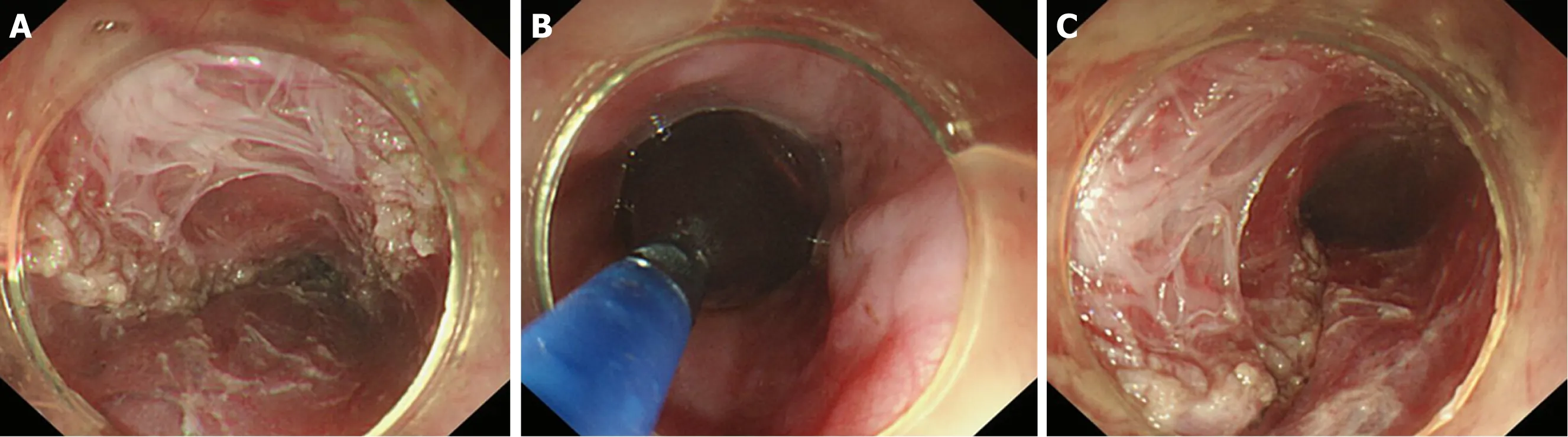
Figure 15 Circular muscle myotomy + balloon plasty. A: The width of the incision should be 1/3 of the circumferential oesophagus; B: Balloon-dilation in the oesophagus; C: The width of the incision after expansion should be 2/3 of the circumferential oesophagus.
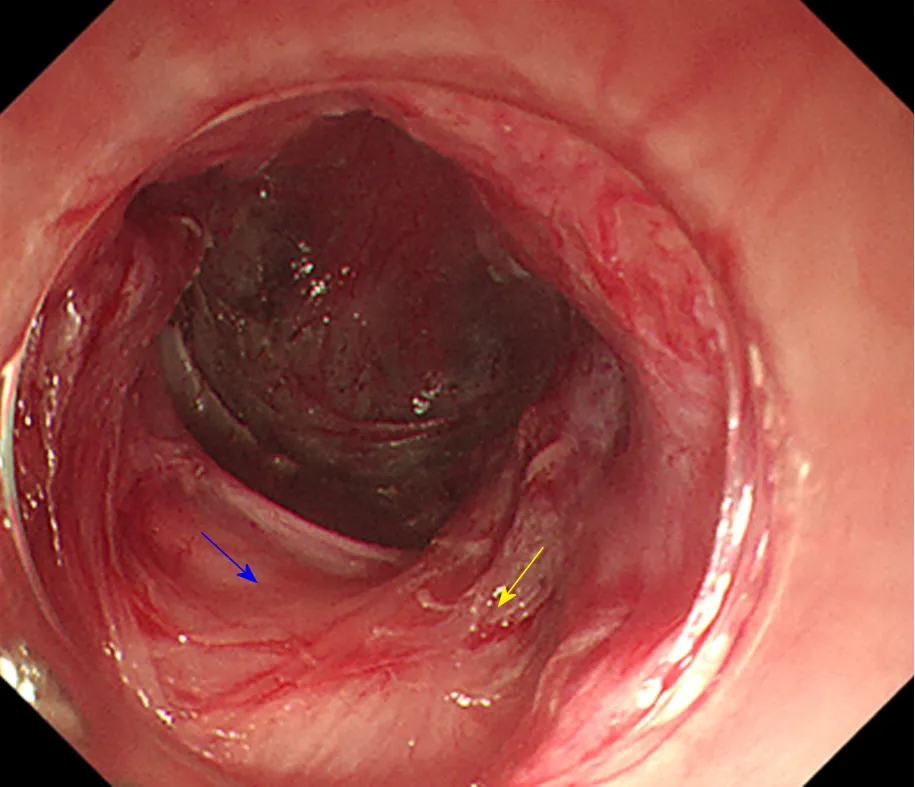
Figure 16 Progressive full-thickness myotomy. The yellow arrow shows the incision into the inner circular muscles from superficial to deep; the blue arrow shows the full incision into the muscularis propria.
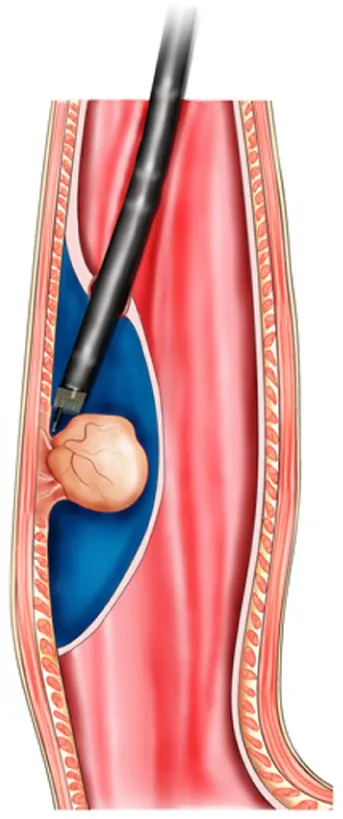
Figure 17 Schema chart of per-oral endoscopic myotomy, demonstrating a tunnel that is created to resect the lesion from the muscularis propria.
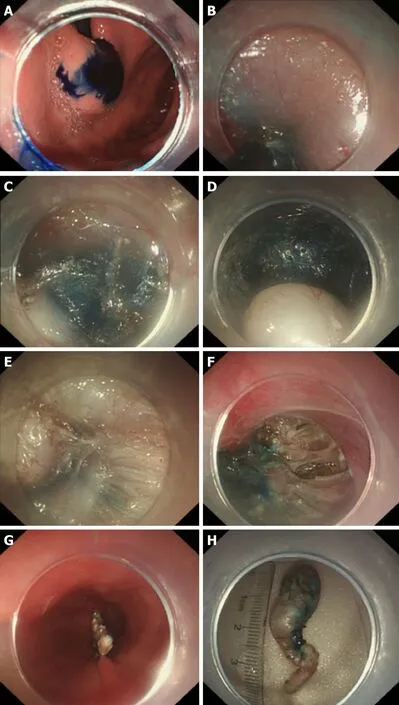
Figure 18 Key steps of submucosal tunnelling endoscopic resection for a cardial submucosal tumour. A: Injection of methylene blue into the lesion incision site for marking and positioning; B: Establishment of a tunnel; C: Finding of the marking and positioning with methylene blue in the tunnel; D: Exposure of the lesion;E: Resection of the lesion; F: Wound following the resection of the tumour; G: Closure of the mucosal incision; H: The resected specimen.

Figure 19 Schema chart of digestive endoscopic tunnel technique on the external digestive tract wall, demonstrating a tunnel that is created to resect a node outside the digestive tract.
ACKNOWLEDGEMENTS
We would like to thank the following doctors who made an contribution to this study in helping draft and edit the paper and contacting the authors in different hospitals:Du C, Feng XX, Jiang L, Li LS, Liu SZ, Ma Y, Qiu ST, Wang NJ, Wang N, Wang XY,Wang Y, Xiang JY, Zhang LY, Zhang WG, Zhong LS, and Zou JL.
杂志排行
World Journal of Gastroenterology的其它文章
- Solutions for submucosal injection: What to choose and how to do it
- Targeted and immune therapies for hepatocellular carcinoma:Predictions for 2019 and beyond
- Relationships among KRAS mutation status, expression of RAS pathway signaling molecules, and clinicopathological features and prognosis of patients with colorectal cancer
- Ubiquitin-specific protease 22 enhances intestinal cell proliferation and tissue regeneration after intestinal ischemia reperfusion injury
- Nutrient drink test: A promising new tool for irritable bowel syndrome diagnosis
- Aspiration therapy for acute embolic occlusion of the superior mesenteric artery
