Evolving role of magnetic resonance techniques in primary sclerosing cholangitis
2019-02-21EmmanuelSelvarajEmmaCulverHelenBungayAdamBaileyRogerChapmanMichaelPavlides
Emmanuel A Selvaraj, Emma L Culver, Helen Bungay, Adam Bailey, Roger W Chapman, Michael Pavlides
Abstract Development of non-invasive methods to risk-stratify patients and predict clinical endpoints have been identified as one of the key research priorities in primary sclerosing cholangitis (PSC). In addition to serum and histological biomarkers, there has been much recent interest in developing imaging biomarkers that can predict disease course and clinical outcomes in PSC.Magnetic resonance imaging/magnetic resonance cholangiopancreatography(MRI/MRCP) continue to play a central role in the diagnosis and follow-up of PSC patients. Magnetic resonance (MR) techniques have undergone significant advancement over the last three decades both in MR data acquisition and interpretation. The progression from a qualitative to quantitative approach in MR acquisition techniques and data interpretation, offers the opportunity for the development of objective and reproducible imaging biomarkers that can potentially be incorporated as an additional endpoint in clinical trials. This review article will discuss how the role of MR techniques have evolved over the last three decades from emerging as an alternative diagnostic tool to endoscopic retrograde cholangiopancreatography, to being instrumental in the ongoing search for imaging biomarker of disease stage, progression and prognosis in PSC.
Key words: Primary sclerosing cholangitis; Magnetic resonance imaging; Magnetic resonance cholangiopancreatography; Magnetic resonance elastography; Diffusion magnetic resonance imaging; Endoscopic retrograde cholangiopancreatography
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a rare, chronic, immune-mediated liver disease, characterised by intrahepatic and extrahepatic bile duct inflammation,leading to chronic cholestasis, biliary fibrosis and liver cirrhosis with portal hypertension[1]. It has a male preponderance, with a mean age of diagnosis of 40 years,and a strong association with concomitant inflammatory bowel disease (IBD)[2]. The population incidence ranges from 0 to 1.3 per 100000 persons annually, and the prevalence ranges from 0 to 16.2 per 100000 persons[3]. A clinical definition for PSC was based upon three landmark papers in the 1980s from the United States, United Kingdom and Norway[4-6]. Subsequently, four sub-types of PSC were described.‘Classical’ large-duct PSC (LD-PSC), accounting for 90% of patients, involves either the intrahepatic bile ducts, extra hepatic bile ducts or both. It is usually diagnosed on the basis of cholestatic liver biochemistry and characteristic changes in the bile ducts on cholangiography. Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) is the standard imaging modality to confirm a diagnosis of LD-PSC[7]. ‘Small duct’ PSC that has normal cholangiography but affects only the small intrahepatic bile ducts on liver histology, accounts for 5% of patients[8]. PSC with ‘autoimmune hepatitis (AIH) overlap’, confirmed histologically in those with elevated transaminases and/or immunoglobulin G levels and an abnormal cholangiogram, presents in 5% of patients[9]. Lastly, ‘PSC with high immunoglobulin G subclass 4 (IgG4) levels’ in the serum and/or tissue, is reported in 12%-18% of LD-PSC patients, with a distinct clinical phenotype and natural history of disease[10,11].
PSC is insidious, with nearly half of patients being asymptomatic at diagnosis,identified after investigation for abnormal liver biochemistry[12]. PSC is considered a premalignant condition, associated with the development of hepatobiliary and colorectal cancers, the most common being cholangiocarcinoma[13]. In the absence of effective medical therapies to date, liver transplantation is the only proven lifeextending intervention. PSC accounts for 10%-15% of all liver transplant activity in Europe and the median transplant-free survival of patients with PSC is 14.5 years[13].There is interest in developing non-invasive clinical risk-stratification methods and surrogate markers in the disease.
This article will review how the role of magnetic resonance (MR) techniques have evolved over the last three decades from emerging as an alternative diagnostic tool to endoscopic retrograde cholangiopancreatography (ERCP), to being instrumental in the ongoing search for an imaging biomarker of disease stage, progression and prognosis in PSC. A summary of the MR techniques that will be discussed in this review is presented in Table 1.
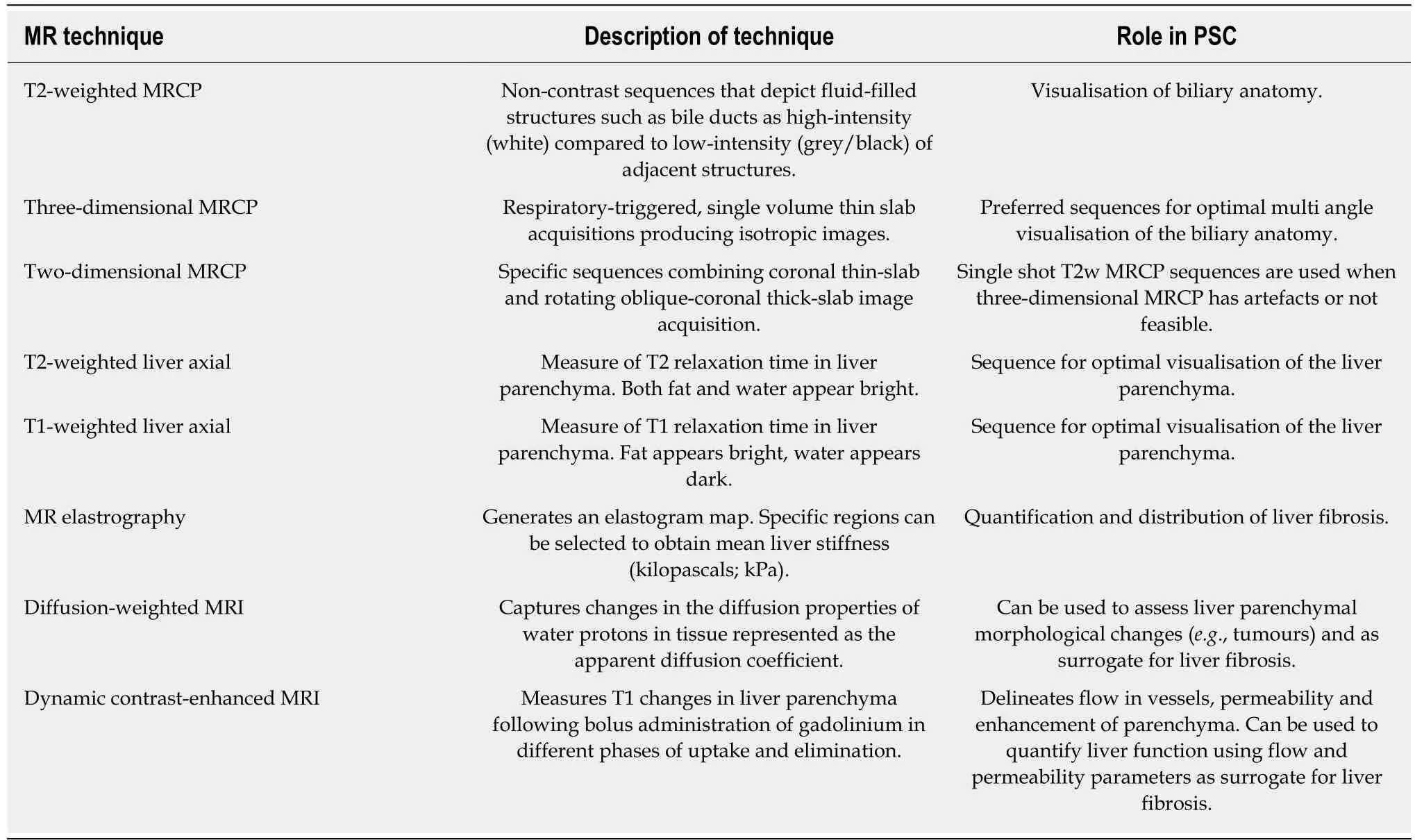
Table 1 Summary of magnetic resonance techniques used in primary sclerosing cholangitis
EVOLUTION OF PSC DIAGNOSIS
Pathology and cholangiography
The earliest description of PSC was found in a publication by Hofmann 1867[14]. The German pathologist reported two post-mortem descriptions of obstruction of the common hepatic duct by thickening of the duct walls with the absence of malignancy and stones. However, it was not until 1924-1925 that the first well-documented case was reported by the French surgeons Delbet[15]and Lafourcade[16]. The first in the English literature was reported by Miller in 1927[17]. In 1958, Schwarts and Dale reported 13 cases who they felt were consistent with a diagnosis of PSC on review of worldwide literature[18].
PSC was historically a condition recognised intra-operatively when the accessible portion of the extrahepatic biliary system was involved. Subsequently, with the development of operative cholangiography, surgeons were better able to visualise the intra- and extrahepatic bile ducts, extent of the disease and plan optimum site for biliary drainage using T-tube cholangiograms. Reported series of PSC cases were small prior to 1980[5]. The advent of percutaneous transhepatic cholangiography (PTC)and ERCP paved the way for non-operative methods of obtaining a cholangiogram.This led to more detailed description of PSC with a rise in reporting in the medical literature worldwide. The first classification of intrahepatic and extrahepatic features of PSC using cholangiograms obtained from T-tube, PTC, or ERCP was reported in 1984 by Chen and Goldberg using a case series of 19 patients[19].
Emergence of MRCP
ERCP had been the standard of reference for obtaining a cholangiogram in diagnosing PSC until the emergence of MRCP. Whilst MRI of the liver was being performed for liver disease, it was not until 1991 that the first “MR cholangiography” sequence was performed by Wallner et al[20]. They developed a T2-weighted rapid sequential gradient-echo two-dimensional (2D) acquisition and a three-dimensional (3D) postprocessing technique to produce coronal and sagittal images, without the need for ionising radiation or intravenous biliary contrast. Static fluid-filled structures in the abdomen have long T2 relaxation time in comparison to adjacent tissue. MRCP exploits these differences by using heavily T2-weighted sequences that depicts higher signal intensity (white) of slow-moving or static fluid within the bile and pancreatic ducts in comparison to lower signal intensity (grey/black) of adjacent solid structures.Specific image acquisition sequences ensure that flowing blood has minimal or no measurable signal in order not to mistake blood vessels for bile or pancreatic ducts.Acquisition is performed in a fasted state (often for at least 4 h) to reduce signal overlap from fluid in the surrounding stomach and duodenum, reduce peristalsis and promote gallbladder distension. Some centres use a negative oral contrast agent, such as 200-400 mL of pineapple juice, 20-30 min prior to MRCP. The high concentration of manganese in pineapple juice is thought to have a paramagnetic effect, suppressing signal from overlapping fluid in the stomach and duodenum[21]. 2D and 3D MRCP has undergone significant advances since its first description with shorter acquisition time, better image quality and improved reconstruction algorithms. Optimal imaging protocol depends on the specific scanner used and parameters including field strength(e.g., 1.5 or 3T), the manufacturer, institutional preference and experience. Good quality 3D MRCP acquisition also depends on good navigator-based respiratorytriggering of the diaphragm.
MRI/MRCP technical review
The International PSC Study Group has recently published a position statement from multidisciplinary experts offering recommendations on the minimum standard for performing MRI/MRCP in PSC as well as a more complete workup[22].
Bile duct imaging:T2-weighted MRCP is preferred to T1-weighted for improved visualisation of biliary ducts. 3D MRCP is preferred over 2D MRCP as the thinner 1mm sections result in higher spatial resolution with excellent signal-to-noise ratio.Post-processing 3D reconstruction using isotropic data allows creation of multiple projections. However, the trade-off for this is longer acquisition time and motion artefact. If a hepatobiliary contrast agent is used, 3D MRCP sequences should be acquired first or else the bile signal will be suppressed.
Liver parenchyma imaging:Cross sectional T2 and T1-weighted acquisition is recommended. T2-weighted coronal plane acquisitions covering most of the liver from anterior to posterior is important for evaluation of peripheral intrahepatic ducts.Fat-suppressed T1-weighted image is acquired as it adds information on the liver parenchyma. Gadolinium-based intravenous contrast agents form part of a more complete workup of PSC patients to detect and differentiate mass lesions and inflammation. MR contrast agents can be classified as purely extracellular or extracellular with a hepatocyte-specific (hepatobiliary) component. Depending on the contrast agent used, post-contrast images are acquired in different phases including arterial, portal venous, equilibrium (parenchyma), delayed and hepatobiliary phases.Some centres perform diffusion-weighted imaging (DWI) routinely for parenchymal and lesion characterisation.
Diagnosis of PSC
Cholangiography is required to make a diagnosis of LD-PSC. ERCP is invasive with potential serious complications including pancreatitis, cholangitis, perforation and bleeding[23]. Over the last twenty years, MRCP has replaced ERCP as the first line imaging method for the diagnosis of PSC. A large meta-analysis, including 189 PSC patients, compared the diagnostic accuracy of MRCP against combined clinical,biochemical, and ERCP or PTC endpoint as the reference standard for diagnosis. The study concluded that the sensitivity and specificity of MRCP for the diagnosis of PSC were 0.86 (95%CI: 0.80-0.90) and 0.94 (95%CI: 0.86-0.98) respectively, with an area under the receiver operating curve of 0.91, supporting a high diagnostic accuracy[24].Advancement in imaging techniques with higher quality images and spatial resolution is likely to have increased the diagnostic accuracy further. Both the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend MRCP as the first-choice imaging modality in PSC[7,25]. Performing MRCP first has been shown to be a more costeffective strategy[26,27]. Moreover, incomplete biliary tract distension mimicking the ductal irregularities of PSC can give rise to false-positive diagnosis on ERCP cholangiogram and false-negative diagnosis if a high-grade stricture causes inadequate opacification of the intrahepatic ducts[28,29]. However, ERCP is still performed when diagnostic doubt exists after MRCP scanning.
MRI/MRCP features of PSC
Identification of multifocal fibrotic strictures and areas of dilatation and ductal wall thickening of the intrahepatic or extrahepatic biliary systems, or both, underpins the diagnosis of LD-PSC. The majority of patients have involvement of both the intra- and extrahepatic bile ducts, with less than 25% with intrahepatic duct disease only[25].Exclusive involvement of the extrahepatic duct is uncommon (less than 5%) and should prompt a search for an alternative cause[1]. Figure 1 illustrates typical MRCP features of LD-PSC. The MRI/MRCP features of PSC that have been reported in the literature are summarised in Table 2[22,30-32].
As for any other imaging modality, MRI/MRCP is subject to inter-observer variability. The cholangiographic features of PSC on its own do not necessarily distinguish PSC from secondary sclerosing cholangitis, particularly in the absence of IBD diagnosis. Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) can often mimic PSC on MRCP. Features that support a diagnosis of IgG4-SC over PSC include dilatation proximal to confluent stricture, symmetrical bile duct wall thickening with smoother outer and inner margins, presence of continuous as opposed to skip disease in the bile ducts, common bile duct thickness greater than 2.5 mm, gallbladder and pancreatic involvement[33,34]. Other mimickers include ischaemic cholangiopathy,acquired immune deficiency syndrome-related cholangiopathy, secondary sclerosing cholangitis after repeated ascending cholangitis and portal biliopathy.
STAGING OF LIVER FIBROSIS
Liver biopsy
Liver biopsy, assessed using Ludwig staging system, has been shown to be an independent predictor of survival in PSC[35,36]. More recently, in a multicentre PSC cohort, three separate histological scoring systems (Nakanuma, Ishak and Ludwig),have been shown to have independent prognostic value in monitoring disease progression[37]. However, liver biopsy is not recommended in the current EASL and AASLD guidelines for the diagnosis and follow-up of PSC due to its invasive nature and risk of complications[7,25]. Moreover, distribution of disease in PSC is patchy and liver biopsy is prone to sampling variability[38]. A liver biopsy is usually performed when MRCP/ERCP is normal to diagnose small-duct PSC and/or there is suspicion of an autoimmune overlap syndrome or IgG4-SC.
Serum markers of fibrosis
Several promising serum biomarkers have been studied as surrogate markers for liver fibrosis. The ELF test is based on three direct markers of fibrogenesis: hyaluronic acid(HA), tissue inhibitor of metalloproteinases-1 (TIMP-1) and procollagen III amino terminal peptide (PIIINP). It has been reported in two large, retrospective, cohort studies to be a strong predictor of clinical outcomes defined as liver-transplant or death, and independent of other risk factors or prognostic scores that predict outcomes[39,40]. Fibrosis-4 index (FIB-4) and aspartate aminotransferase to platelet ratio index (APRI) have been studied in other chronic liver diseases as a marker of liver fibrosis, but their roles in PSC have not been reported to date.
Liver stiffness measurement
Liver stiffness has also been shown to be a surrogate marker for liver fibrosis. Studies have shown correlation with stages of liver fibrosis, liver decompensation and survival[41,42]. Liver stiffness can be measured using shear-wave-based technology such as vibration controlled transient elastography (VCTE) or magnetic resonance elastography (MRE). Two retrospective studies have shown good correlation between baseline VCTE measurements and changes in VCTE measurements with stages of fibrosis and clinical outcomes[43,44]. Although widely available with relatively low-cost,false positive elevation of VCTE measurements can be caused by biliary obstruction and active inflammation such as occurs in PSC patients[45,46]. Technical failures and unreliable results have been reported to be as high as 10% in PSC patients[43]. There is limited data reported on the use of other ultrasound-based techniques such as point shear wave elastography and 2D shear wave elastography in PSC.
MRE has been shown to predict liver decompensation in a large single-centre retrospective study involving 266 PSC patients with median follow-up of 2 years[47]. In a small sub analysis of this study, MRE was also shown to correlate with different stages of liver fibrosis. In comparison to VCTE, it has the added advantage of being able to visualise the whole liver and identify patchy areas of fibrosis in PSC. MRE is able to assess more than 1000 times the volume of liver than VCTE[48]. Unlike VCTE,MRE can be performed regardless of patient’s body habitus or presence of ascites.However, MRE is not widely available, is more costly and time-consuming[49]. Whilst MRE was found to have better diagnostic accuracy than VCTE for staging of liver fibrosis in non-alcoholic fatty liver disease[50], there has been no head-to-head comparison performed in a chronic cholestatic disease such as PSC. MRE has also been shown to correlate better with Mayo PSC risk score than VCTE and liver stiffness quantified by MRE is an independent predictor of worse score[51].
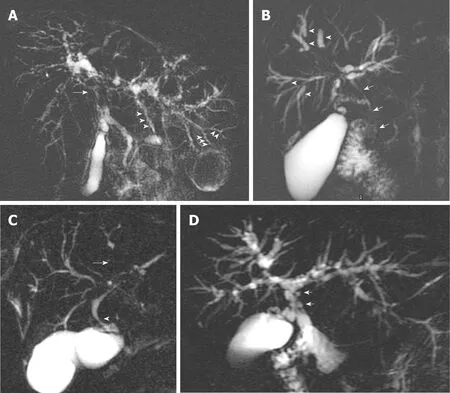
Figure 1 Magnetic resonance cholangiopancreatography annotating typical features of large-duct-primary sclerosing cholangitis. A: Common hepatic duct stricture (arrow) and intrahepatic duct beading (arrowheads); B:Dominant stricture (arrow) and dilated proximal intrahepatic ducts secondary to distal strictures (arrowheads); C: Area of non-filling of intrahepatic ducts indicating tight stricture (arrow) and common hepatic duct stricture (arrowhead); D:Pseudodiverticula.
MONITORING DISEASE PROGRESSION AND CLINICAL OUTCOMES
Disease progression in PSC can affect the biliary tree and/or the liver. A third domain of disease complications or progression includes extra-hepatic manifestations and symptoms, which can be independent of the stage of the biliary or liver disease. The complications of disease progression in PSC are summarised in Figure 2. Predicting which patients are at risk of developing these complications is challenging in clinical practice. Development of non-invasive methods to risk-stratify patients with PSC and predict clinical events was identified as a research priority in a 2016 survey by PSC Support, a registered United Kingdom charity[52]. It highlighted that patients experience significant anxiety due to the uncertainty about the future of their disease;in particular, the risk of disease progression, malignancy, and liver transplantation. In addition to the stage of liver fibrosis, a combination of serum biomarkers, clinical risk prediction model and cholangiographic features can be used to risk-stratify patients.
Serum prognostic markers
Serum alkaline phosphatase (ALP) has been the most widely studied serum biomarker in PSC. ALP levels can fluctuate throughout the disease course but persistently low ALP has been shown to correlate with better clinical outcomes. A cutoff value of 1.5 × the upper limit of normal (ULN) has been demonstrated in several patient cohorts to have prognostic implications[53-57]. However, patients can have normal serum ALP with advanced liver disease[58]. In one study with 10-year followup, 62% of patients did not experience any liver-related endpoints despite having a serum ALP that did not improve to levels less than 1.5 × ULN[57].
Clinical risk score and prognostic model
Several clinical risk scores have been developed to predict disease progression and clinical outcomes in PSC. The revised Mayo PSC risk score, based on age, bilirubin,albumin, AST and variceal bleeding, is the most commonly used clinical risk model[59].The model was developed using multi-centre large cohort data (n = 405) and was subsequently validated in a separate cohort (n = 105). The risk score provides survival estimates up to 4-year follow-up but does not include time to liver transplant. Given that it is made up of markers predictive of advanced disease, it is not surprising that it has insufficient power and is not clinically useful in discriminating and predicting the clinical course of early disease.
The Amsterdam-Oxford risk score based on seven variables (PSC subtype, age at diagnosis, albumin, platelet, AST, ALP and bilirubin) predicted long-term transplantfree survival in a large derivation cohort (n = 692) and external validation cohort (n =264)[60]. The PSC risk estimate tool (PREsTo) was recently developed using machine learning techniques. It consists of 9 variables (bilirubin, albumin, ALP times the ULN,platelet, AST, haemoglobin, sodium, age and number of years since diagnosis)[61]. The model was derived using 509 patients and validated in an international multicentre cohort (278 patients) who did not have markers of advanced disease. It accurately predicted the 5-year risk of liver decompensation. None of the prognostic scores that have been developed to date has entered radiological features as a variable into their modelling methods, probably because of the significant inter-observer variability in radiological interpretation even among experienced experts[62].

Table 2 Descriptive features of primary sclerosing cholangitis on magnetic resonance imaging/ magnetic resonance cholangiopancreatography[22,30-32]
Cholangiography
Given that cholangiography is required for the diagnosis of the majority cases of PSC,it would seem intuitive to use cholangiographic features as predictors of disease stage and prognosis. Whilst there are limited studies evaluating the use of ERCP cholangiogram findings, there is an increasing trend of utilising MR techniques to study both the liver parenchyma and cholangiography of PSC patients simultaneously to propose imaging biomarkers in PSC. The non-invasive nature of MR techniques makes this an attractive option as a surrogate marker.
ERCP:Craig et al[63]retrospectively reviewed ERCP cholangiograms of a cohort of 174 PSC patients with relatively advanced disease and found that both high-grade intrahepatic duct strictures and diffuse intrahepatic duct strictures were associated with a lower 3-year survival[63]. Similarly, Olsson et al[64]concluded that high-grade intrahepatic strictures predicted shorter survival in a study involving 94 PSC patients.The Amsterdam cholangiographic classification system was developed by Ponsioen et al[65], incorporating the previously reported classifications by Majoie et al[66]and Chen-Goldberg[19]. It is based on scoring intrahepatic and extrahepatic stricture and dilatation severity on ERCP cholangiograms as outlined in Table 3. In a large singlecentre study with a long follow-up period, 133 patients’ cholangiograms were scored.Cholangiographic scores were inversely correlated to survival, and together with age at ERCP, a prognostic model was derived[65]. It remains to be externally validated,perhaps reflecting the shift away from invasive biomarkers of disease.
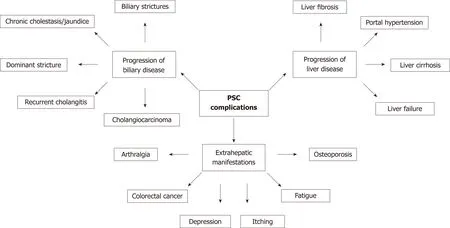
Figure 2 Summary of complications resulting from disease progression in primary sclerosing cholangitis. PSC: Primary sclerosing cholangitis.
MRI/MRCP:MRI/MRCP presents a more favourable option than ERCP as a marker of disease activity and prognosis in PSC as it allows co-assessment of liver parenchyma and biliary abnormalities. Petrovic et al[67]retrospectively examined the relationship between MRI/MRCP features and survival as predicted by the Mayo risk score. The severity of biliary stricture was graded using the Amsterdam cholangiographic classification. In this study involving 47 patients with PSC, delayed(3-min post contrast) peribiliary hyperenhancement in the liver parenchyma using extracellular gadolinium contrast, showed weak correlation with Mayo risk score.There was no correlation with peribiliary hyperenhancement at 2-min post contrast,intrahepatic or extrahepatic duct grading of strictures. Extrapolating ERCP-based cholangiographic findings to MR-based cholangiography cast doubts on the reproducibility of stricture grading, particularly with variability in contrast injection technique and volume used during ERCP. Tenca et al[68]reported only moderate agreement between ERCP and MRCP cholangiograms using a modified Amsterdam scoring system. Weak correlations were demonstrated between severity of biliary changes and serum ALP as well as clinical endpoints defined by liver transplantation or death.
Change in the morphological appearance of the biliary tree and liver on interval MRI/MRCP is often used to comment on whether the disease is stable or has progressed. Ruiz et al[69]designed the first MRI-based score to determine radiological disease progression on follow-up MRI/MRCP of 142 well-characterised PSC patients.They designed an interpretation standard model that converted radiological descriptors into categorical variables in this study, which allowed them to systematically analyse the bile ducts and liver parenchyma. An MRI progression risk score model was built using factors that predicted radiological progression between two successive MRIs, as shown in Figure 3.
This study demonstrated radiological progression in 58% of patients (n = 37) over a 4-year follow-up period. Both scores had area under receiver operating characteristic curve of 80% and 83% respectively for predicting radiological progression. However,the study did not take into account inter-observer variability, had no correlation with clinical outcomes and did not have ERCP as the reference standard. The MRI score is awaiting external validation. Kitzing et al[70]subsequently examined serial MRI/MRCP images and reported that liver morphological changes on surveillance imaging,specifically liver atrophy, was associated with adverse clinical outcome and shorter transplant-free survival over a mean intervening period of 5 years.
Several studies have evaluated the changes seen on contrast-enhanced MRI sequences in PSC with mixed evidence. Bader et al[71]studied 52 patients with PSC and reported that there were no correlations between liver parenchymal signal abnormalities or biliary ductal features and Childs-Pugh or Model for End-stage Liver Disease (MELD) score in a retrospective single time point analysis of MR images.Whilst delayed phase peribiliary hyperenhancement showed weak correlation with Mayo risk score as described earlier, Ni Mhuircheatiagh et al[72]reported that the presence and extent of arterial phase peribiliary hyperenhancement on MRI was
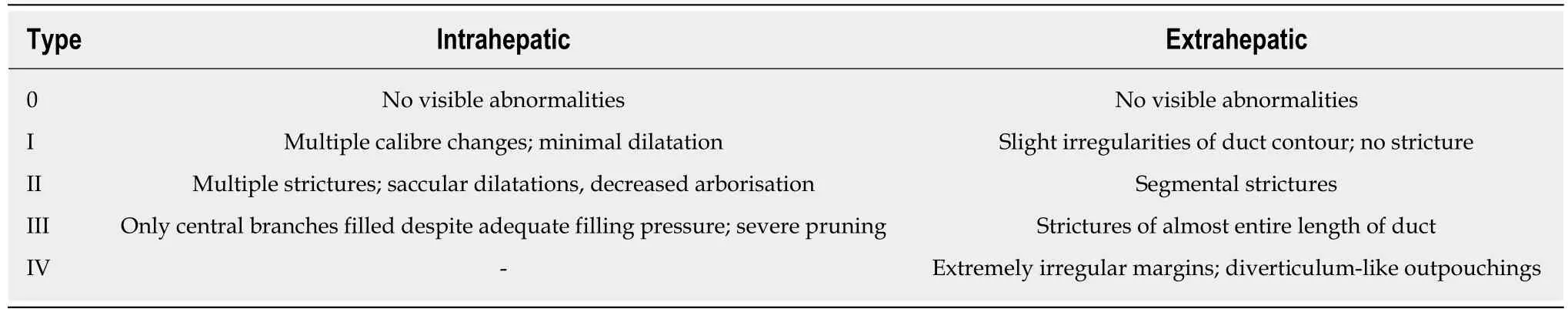
Table 3 The Amsterdam classification of endoscopic retrograde cholangiopancreatography cholangiographic changes in primary sclerosing cholangitis[65]
associated with a higher Mayo risk score in a cohort of 60 PSC patients. They postulated that this is potentially a marker of active biliary inflammation and poorer prognosis. Bookwalter et al[73]retrospectively reviewed MRI that included dynamic contrast enhanced sequences, MRCP and MRE of 55 PSC patients to examine the relationship between liver parenchymal changes, biliary features and liver stiffness at a segmental, lobar and global level. They found weak correlation at segmental level between liver stiffness and liver parenchymal signal changes and ductal strictures.However, they found no significant correlation between the presence and absence of periductal enhancement in any of the three contrast enhanced phases with Mayo risk score or MELD score.
Dominant stricture and cholangiocarcinoma
A dominant bile duct stricture in PSC is defined as a stricture less than 1.5 mm diameter in the common bile duct, or less than 1 mm in the left or right main hepatic ducts on cholangiography[7]. However, there are currently no validated criteria for MR definition of a dominant stricture. Deterioration in clinical and biochemical parameters prompts evaluation for a dominant stricture and/or cholangiocarcinoma on MRI/MRCP. The presence of a dominant stricture either at diagnosis or follow-up is associated with an increased risk of developing cholangiocarcinoma and mortality[74,75].
Over one third of cholangiocarcinoma cases were detected within the first year following PSC diagnosis in a retrospective, international, observational cohort study involving 594 PSC patients[13]. This is likely due to length-time bias and detection of cholangiocarcinoma only when it becomes clinically overt. Serum tumour marker carbohydrate antigen (CA)19-9 is widely used in surveillance strategy but it lacks both sensitivity and specificity for the detection of cholangiocarcinoma[76,77]. The cholangiocarcinoma is usually too advanced for curable treatment by the time CA19-9 becomes persistently elevated[78]. Annual MRI/MRCP as a surveillance strategy is often performed, but with limited proven benefit. Nevertheless, the current recommendation for cholangiocarcinoma surveillance is annual CA19-9 and MRI/MRCP[79]. There is an unmet need for earlier detection of cholangiocarcinoma and closer monitoring of newly diagnosed patients.
Subclinical PSC
A population-based study of long-term IBD patients in Norway reported that 8.1% of 322 patients had MRCP lesions indicating PSC, a 3-fold higher prevalence than detected clinically before MRCP screening. Nearly two-thirds of these detected cases had ‘subclinical’ PSC with mild changes on cholangiography and no biochemical abnormalities[80]. A prospective controlled UK study demonstrated 14% of 51 patients with extensive ulcerative colitis and normal liver biochemistry had biliary abnormalities suggestive of PSC on MRCP, and over long-term (10-year) follow-up,one-third developed abnormal liver biochemistry, one-fifth developed progressive bile duct disease and over half developed malignancy, including two biliary and one colorectal carcinoma[81].
QUANTITATIVE LIVER IMAGING
DW-MRI
DW-MRI manipulates the altered diffusion properties of water protons in fibrotic tissue and allows assessment of liver fibrosis[82]. Addition of the short sequence to routine MRI/MRCP enables whole liver assessment of fibrosis distribution,particularly useful in a patchy disease such as PSC[32,83]. Since TE and MRE have shown better diagnostic accuracy for the staging of liver fibrosis[83,84], DWI has fallen out of favour but is still performed for better characterisation of lesions involving the liver parenchyma. In a recent prospective study involving 47 PSC patients, DWI-MRI performed better than dynamic contrast-enhanced MRI (DCE-MRI) in detecting and staging liver fibrosis using VCTE as the reference standard[85].

Figure 3 Magnetic resonance imaging progression risk score[69]. MRI: Magnetic resonance imaging.
DCE-MRI
Administration of hepatocyte-specific contrast agents such as Gd-BOPTA (gadobenate dimeglumine) and Gd-EOB-DTPA (gadoxetate disodium) allows assessment of liver function by analysing the liver uptake and elimination of contrast. Several studies have attempted to quantify liver parenchymal changes with the administration of a contrast agent.
Ringe et al[86]demonstrated that hepatobiliary excretion of hepatocyte-specific contrast is significantly reduced in patients with PSC when compared to normal controls and correlated with bilirubin levels in PSC. Noren et al[87]quantitatively compared hepatocyte-specific contrast uptake with histopathological stage of fibrosis in a prospective study involving 38 patients with compensated chronic liver diseases of varying aetiology. They demonstrated that quantitative measurement of signal intensities using DCE-MRI was able to distinguish advanced fibrosis (F3-4) from no and moderate fibrosis (F0-2). Nilsson et al[88]developed a non-invasive imaging-based method using DCE-MRI to assess liver function at the segmental and global level, and showed significantly heterogeneity in the liver parenchyma of PSC patients compared to controls. This small study (involving PSC patients with mild disease) reported correlation between MRI-derived liver function indices and disease severity using Mayo risk score. Segmental liver function correlated with level of downstream biliary obstruction.
Hinrichs et al[89]reported that reduction in T1 relaxation time after hepatocytespecific contrast administration correlated with liver biochemistry tests, MELD and Mayo risk score. They proposed that global liver function could be non-invasively assessed using this specific T1 mapping sequence technique in PSC. Nolz et al[90]performed retrospective quantitative analysis of liver parenchymal enhancement in T1-weighted MRCP images in a small cohort of PSC patients, and calculated the difference in signal intensity (SI) ratio between the hepatobiliary phase and unenhanced parenchyma [termed relative enhancement (RE)]. They demonstrated significant reduction in RE in localised areas of impaired liver parenchyma in comparison to normal areas, thus allowing regional functional assessment.
RE = [(hepatobiliary SI - unenhanced SI)/unenhanced SI × 100]
Keller et al[91]adapted the above technique to MRI scans performed with extracellular gadolinium-based instead of hepatocyte-specific contrast. They retrospectively reviewed scans and liver biopsies of 40 PSC patients to evaluate the utility of several quantitative MRI-derived parameters as markers of liver inflammation and fibrosis (LIF). Relative liver enhancement (RLE) in the delayed phase of T1-weighted MRI was shown to strongly correlate with stage of liver fibrosis.The same group also demonstrated an increased RLE within T2 hyperintense areas in the liver parenchyma on T2-weighted MRI and postulated that this could be early changes of patchy inflammation[92]. Schulze et al[93]calculated RLE in the hepatobiliary MRI phase in a prospective study using hepatocyte-specific contrast agent and evaluated its role as a prognostic marker. Moderate correlation was demonstrated with serum markers (ALP, albumin, bilirubin, INR) and prognostic scorings systems(MELD, Mayo risk score, Amsterdam-Oxford). They proposed a cut-off RLE value that predicted clinical endpoints with low sensitivity (74%) and reasonable specificity(94%), which remains to be externally validated.
Non-contrast T1 mapping
LiverMultiscan™ (Perspectum Diagnostics, Oxford, United Kingdom) is a software product that enables post-processing of liver MRI using T1 and T2* maps[94,95]. In a small proof of principle study, the LIF score derived from the iron corrected T1 (cT1)measurements, has been shown to strongly correlate with clinical outcomes in patients with chronic liver disease of mixed aetiologies[96]. This technique looks promising for the evaluation of patients with non-alcoholic fatty liver disease[97].
More recently, Arndtz et al[98,99]reported the distribution of imaging metrics derived from quantitative maps of T1, T2* and cT1 of LiverMultiscan™ in autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and PSC. Using a machine-learning technique to analyse the skewness and kurtosis of the distribution as well as local regional variance, they demonstrated that addition of imaging metrics to serum ALT or ALP performed slightly better than serum ALT or ALP alone in disease differentiation between parenchymal liver disease (62 AIH patients) and biliary liver disease (124 PSC and PBC patients).
LIMITATIONS OF MRI/MRCP
Acquisition and 3D image reconstruction protocols still vary significantly across centres and there is no standard model for interpreting MRCP data. Current clinical utility of MRI/MRCP allows only qualitative assessment of the bile ducts and liver parenchyma, and is therefore susceptible to subjectivity in interpretation. Assessment of the distal common bile duct and subtle changes in the smaller peripheral intrahepatic bile ducts still remains a challenge despite the use of modern 3 Tesla (T)MRI scanners[22]. The position statement from the International PSC Study Group outlines areas of unmet need for imaging techniques in PSC, including (1) early detection of disease; (2) the determination of disease stage, activity and prognosis; (3)the assessment of treatment response; (4) a clinically meaningful definition of dominant bile duct stenosis; and (5) the early detection of cholangiocarcinoma.
CONCLUSION
The role of MRI/MRCP in establishing the diagnosis of PSC is well documented and has long superseded ERCP as the gold standard for obtaining cholangiography.Disease staging is based on the severity of the liver fibrosis component of PSC, which has prognostic implications. MRE appears to be a promising technique that generates a liver stiffness map of the whole liver for assessment of patchy liver fibrosis.However, there have been no comparative studies between MRE and other surrogate markers of fibrosis in PSC. Whilst there is some exciting work published on MR quantitative methods involving the liver parenchyma in PSC, there has been surprisingly little advancement in the last three decades on quantitative methods involving the bile ducts. Published studies to date have only proposed cholangiography-based scoring systems derived from interpretation of qualitative descriptors by two specialist radiologists assessing the morphological appearance of bile ducts and liver parenchyma. This method is highly variable even among experts and therefore limits its inclusion into reliable prognostic models. MRI/MRCP shows promising potential for prediction of disease course and clinical endpoints in PSC as MR techniques evolve towards ‘quantifying’ the disease. However, further development and validation of objective and reproducible MR-based parameters are needed before it can establish its role as an imaging biomarker in PSC.
杂志排行
World Journal of Gastroenterology的其它文章
- Cancer risk in primary sclerosing cholangitis: Epidemiology,prevention, and surveillance strategies
- Artificial intelligence in medical imaging of the liver
- Effect of Sheng-jiang powder on multiple-organ inflammatory injury in acute pancreatitis in rats fed a high-fat diet
- Preoperative rectosigmoid endoscopic ultrasonography predicts the need for bowel resection in endometriosis
- Short- and long-term outcomes of endoscopically treated superficial non-ampullary duodenal epithelial tumors
- Serum hepatitis B virus RNA is a predictor of HBeAg seroconversion and virological response with entecavir treatment in chronic hepatitis B patients
