High prevalence of occult hepatitis C infection in predialysis patients
2019-02-21LuHenriqueBezerraCavalcantiSetteEdmundoPessoadeAlmeidaLopesNathliaCampelloGuedesdosAnjosLucilaMariaValentevioAugustoVieiradeOliveiraNormaLucenaSilva
Luís Henrique Bezerra Cavalcanti Sette, Edmundo Pessoa de Almeida Lopes,Nathália Campello Guedes dos Anjos, Lucila Maria Valente, Sávio Augusto Vieira de Oliveira,Norma Lucena-Silva
Abstract BACKGROUND Occult hepatitis C virus (HCV) infection (OCI) may be associated with extrahepatic diseases and it is known that the patients with chronic kidney disease (CKD) who are on hemodialysis (HD) present a higher prevalence of this type of infection than the general population, with a worse clinical outcome.However, there are no data in the literature to assess the presence of OCI in patients prior to the initiation of renal replacement therapy (RRT). Therefore, this study aimed to evaluate the occurrence and epidemiological aspects of OCI in patients with Predialysis CKD. We hypothesize that this infection could occur before RRT initiation.AIM To research the status in predialysis patients when HD patients have high prevalence of OCI.METHODS A cross-sectional study was conducted between 2015 and 2017. Adults with creatinine clearance < 60 mL/min·1.73 m2 (predialysis patients) were recruited to the study. Pregnant and postpartum women, patients with glomerulopathies,and patients showing positivity for serological markers of hepatitis B virus(HBV), HCV or human immunodeficiency virus infection were excluded. Patients were diagnosed with OCI according to test results of anti-HCV antibody negativity and HCV RNA positivity in either ultracentrifuged serum or, if serum-negative, in peripheral blood mononuclear cells.RESULTS Among the 91 total patients included in the study, the prevalence of OCI was 16.5%. Among these 15 total OCI patients, 1 was diagnosed by 14 ultracentrifuged serum results and 14 were diagnosed by peripheral blood mononuclear cell results. Compared to the non-OCI group, the OCI patients presented higher frequency of older age (P = 0.002), patients with CKD of mixed etiology (P = 0.019), and patients with markers of previous HBV infection (i.e.,combined positivity for anti-hepatitis B core protein antibody and anti-hepatitis B surface protein antibody) (P = 0.001).CONCLUSION Among predialysis patients, OCI involved the elderly, patients with CKD of mixed etiology, and patients with previous HBV infection.
Key words: Occult hepatitis infection; Chronic hepatitis C; Chronic kidney disease;Hemodialysis; Hepatitis C virus-RNA; Peripheral blood mononuclear cells
INTRODUCTION
Occult hepatitis C virus (HCV) infection (OCI) is characterized by presence of HCV genetic material (HCV RNA) in a patient’s peripheral blood mononuclear cells(PBMCs), ultracentrifuged serum, or hepatic tissue, along with the absence of HCV antibodies (anti-HCV) and HCV RNA in the non-ultracentrifuged serum[1]. This form of viral hepatitis may be associated with tissue damage in the liver (i.e., chronic cryptogenic liver disease and nonalcoholic fatty liver disease)[2-4]and extrahepatic diseases (i.e., glomerulopathies, lymphoproliferative diseases, and end-stage renal disease)[5-7].
It has been suggested that a specific cellular immune response underlies OCI, one that is less effective than the response in individuals without infection but which is more effective than that in individuals with the classic form of the disease (HCV RNA-positive serum)[8]. It is known that the patients with chronic kidney disease(CKD) who are on hemodialysis (HD) present a higher prevalence of HCV infection than the general population, both in its classical form[9]and the occult form, reaching up to 45% prevalence among patients with OCI[7,10].
Nowadays, due to universal precautions in HD units and the reduced need for blood transfusion, some authors suggest that HCV infection may occur in the predialytic period[11]. Indeed, patients with predialysis (PD)CKD have a higher prevalence of infection with HCV and hepatitis B virus (HBV), as compared to the general population[12-14]. However, there are no data in the literature to assess the presence of OCI in patients prior to the initiation of renal replacement therapy.
The study of the epidemiology of OCI in patients with PDCKD is relevant according to: (1) the higher prevalence of classic HCV infection in HD patients[9,15]; (2)the outlook for the use of diagnostic OCI techniques in these patients before the onset of HD[1]; (3) the greater difficulty in identifying hepatic aggression in patients with CKD due to reduced serum aminotransferase levels resulting from hemodilution[16];and, finally; and (4) the possibility of HCV treatment with the new direct-acting antiviral agents that have few side effects and high efficacy[17].
Therefore, this study aimed to evaluate the occurrence and epidemiological aspects of OCI in patients with PDCKD.
MATERIALS AND METHODS
Study design
A cross-sectional study was carried out from October 2015 to April 2017, in which patients with PDCKD were evaluated in the CKD Outpatient Clinic of the Nephrology Service of the Federal University of Pernambuco (Brazil). Adult patients with a follow-up period of more than 3 mo and with an estimated creatinine clearance of less than 60 mL/min·1.73m2were included. Pregnant and postpartum women,patients with glomerulopathies, and patients with serological markers of infection with either HBV [hepatitis B surface antigen (HBsAg)-positive], HCV (anti-HCV-positive) or human immunodeficiency virus (commonly known as HIV) (anti-HIV-positive) were excluded.
Each study participant provided written informed consent following the explanation that study participation would: (1) not disrupt the patient’s normal attendance at the outpatient clinic; (2) include a copy of all exam results being placed in their medical record, so that the doctor accompanying the patient in clinic will know the results; and (3) provide information for research for publication, with any exam results being published without the patient name. The risks and benefits of study participation were also explained. Samples were only taken from patients after agreement to study participation and signing of the informed consent form.
Each study participant enrolled in the study filled out a questionnaire on demographic characteristics (sex, age, ethnicity), clinical diagnoses of CKD (stage,etiology) and risk factors for viral hepatitis acquisition (transfusion, tattooing,piercing, intravenous drug use). A blood sample (20 mL in vacutainer tube) was drawn from each participant’s peripheral vein, for use in biochemical, serological and virological laboratory tests.
Serum levels of aminotransferases (aspartate aminotransferase, alanine aminotransferase) and creatinine were determined by automated method (UV Diasys-Architect c8000; Abbott Diagnostics, Lake Forest, IL, United States). The creatinine clearance rate was evaluated by the Chronic Kidney Disease Epidemiology Collaboration formula (commonly known as the CKD-EPI equation)[18].
Evaluation of viral infections included third-generation ELISA serum screening for anti-HCV, rapid immunochromatographic assay for the qualitative determination of HBsAg, and immunoassays for anti-HBs detection by direct chemiluminescence(ADVIA Centaur®automated system; Siemens Healthcare Diagnostics S.A., Sao Paulo,Brazil) and for total qualitative anti-hepatitis B core protein (HBc) (IMMUNITE 2000®Imunoassay; Siemens Healthcare Diagnostics S.A.). Patients with negative results on anti-HCV and HBsAg screening were included in the study and evaluated for OCI.
Patients were diagnosed with OCI if they were positive for HCV RNA by PCR screening of ultracentrifuged serum or PBMCs. If the screening was positive for HCV RNA in the ultracentrifuged serum, the patient’s sample of non-ultracentrifuged serum was screened using the same procedure to confirm the diagnosis of OCI.
Detection of HCV RNA
Each peripheral blood sample was initially centrifuged at 3000 x g for 7 min at room temperature, to obtain serum. Then, a 2 mL aliquot of the serum was overlaid by a 10% sucrose buffer, in a ratio of 1:1, and ultracentrifuged at 100000 x g for 17 h at 4 °C.The precipitate obtained by ultracentrifugation was eluted in 200 μL of diethylpyrocarbonate (DEPC; Invitrogen, Carlsbad, CA, United States), to generate an RNase-free sample. A separate aliquot of the peripheral blood with anticoagulant)was subjected to the density gradient centrifugation with Ficoll-Paque (GE Healthcare, Little Chalfont, United Kingdom), to isolate PBMCs. The cDNA synthesis was performed with random primers having as template RNA strands extracted from peripheral blood mononuclear cells and/or ultracentrifuged serum, using the enzyme M-MVL reverse transcriptase (InvitrogenTM), following the manufacturer's specifications.
Detection of HCV RNA in the ultracentrifuged serum and PBMCs was performed by PCR prepared with 100 ng of cDNA, 5 μM of the primers specific for amplification of the HCV genome (UTRLC1: 5'-CAAGCACCCTATCAGGCAGT-3'; UTRLC2: 5'-CTTCACGCAGAAAGCGTCTA-3'), 1 x PCR Rxn buffer (Invitrogen), 5 mmol/L MgCl2, and 10 pmol dNTPs. The reaction conditions consisted of an initial cycle of 10 min at 95 °C, followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s,and with a final 5-min extension at 72 °C, performed in the SimpliAmp Thermal Cycler (Applied Biosystems Inc., Foster City, CA, United States). Positive and negative controls consisted of a sample of patients known to be positive for classic hepatitis C and the PCR mix without DNA addition, respectively. The amplified product was subjected to 2% agarose gel electrophoresis and visualized on the SYBR-safe gel through the L-PIX Transilluminator (Loccus, São Paulo, Brazil). The presence of a fragment of approxiately 230 base pairs in the absence of nonspecific bands was considered a positive result. The positive result was confirmed by a new PCR from another aliquot of the patient’s sample, using the same procedure.
Statistical analysis
Numerical variables were represented by measures of central tendency and dispersion measures. The categorical variables were submitted to χ2test and Fisher's exact test to evaluate the occurrence of association between them. The Kolmogorov-Smirnov normality test was applied to the quantitative variables. When the normal distribution was evidenced, the Student t test was used. When the variable was nonnormal the comparison was performed using Mann-Whitney (Non-Normal).STATA/SE 12.0 and Excel 2010 were used for data storage and processing. In all tests,95%CIs were applied.
RESULTS
We initially recruited 154 patients with creatinine clearance < 60 mL/min·1.73 m2as potential participants for the study; of those, 130 were enrolled, including 24 patients who had follow-up time of < 3 mo. Ultimately, 39 of the enrolled patients were excluded from study participation, for presence of glomerulopathies (n = 23), refusal to participate (n = 7), positivity in HIV serology (n = 3), positivity for anti-HCV (n = 3),positivity for HBsAg (n = 2), and impossibility of venipuncture (n = 1).
The demographic, clinical and laboratory characteristics of the 91 study participants are described in Table 1. The prevalence of OCI among them was 16.5%(15/91), including 14 cases for who the HCV RNA positivity was identified in the PBMCs and 1 with positivity in the ultracentrifuged serum. Figure 1 shows an electrophoresis of a patients positive for OCI.
OCI showed an age relationship, since older patients presented a higher risk of infection compared to younger patients (69.4 ± 7.9 vs 60.9 ± 13.5, P = 0.002). CKD of mixed etiology was observed in 11 of the 15 cases (73.3%) of OCI and was much more frequent than in cases without OCI (P = 0.019). No relationship was found between the occurrence of OCI and sex, skin color or stage of CKD. There was no difference between serum levels of aminotransferases between the groups with and without OCI neither with albumin, bilirubin or gamma glutamyl transferase serum levels.
Risk factors associated with viral hepatitis transmission were also tested (previous HD, use of injectable drugs, occupational exposure, contaminated family member,history of hemotransfusion, piercing, tattoo, acupuncture, sexually transmitted infection, and condom use). None showed an association to the occurrence of OCI.
Of the 91 total patients evaluated, 2 provided an insufficient sample for assessment of HBV antibodies. Thus, of the 89 patients analyzed, 75 (84.2%) were anti-HBc negative, 9 (9.8%) were anti-HBc positive, and 5 (5.5%) were undetermined. A total of 4 out of the 5 patients with undetermined anti-HBc were positive for anti-HBs and were considered as having had previous contact with HBV. A total of 31 patients were anti-HBs positive only and this antibody was not found more frequently in OCI patients (P = 0.59); however, the presence of total anti-HBc and anti-HBs in combination (indicating previous HBV infection) was more frequent in patients with OCI, when compared to uninfected patients (P = 0.001), as shown in Table 2.
DISCUSSION
This was the first study to describe the occurrence of OCI in patients with PDCKD. It was previously known that patients with PDCKD present a higher occurrence of HCV infection in the classic form than the general population, with reported prevalence rates range from 3.9% to 7.3% and being up to seven times higher than in the control populations studied[12-14].

Table 1 Distribution of clinical parameters according to the occurrence of occult hepatitis C virus infection in 91 patients with chronic kidney disease: 2015 to 2017
One of the possible reasons underlying the high prevalence of OCI (16.5%) in our study could be the low sensitivity of the ELISA tests used, that is, false negative anti-HCV results. However, in this study, patients underwent at least two anti-HCV screenings, with the first one in the diagnosis of CKD and the second as an inclusion criterion for this study. Along with both having negative results, a third-generation immunoenzymatic assay was employed, which shows close to 100% accuracy[19]. The other possibility would be the immunological deficiency of patients with PDCKD who would not be able to produce anti-HCV antibodies in detectable titers.
We could still consider the possibility of a false positive result in the PCR reaction to detect the HCV RNA in the PBMCs or ultracentrifuged serum; however, this is unlikely since the different steps in the procedure were performed in different environments under laminar flow with quality reagents. In addition, positive results were confirmed by a new PCR from another aliquot of the patient's blood sample,using the same technique for detection of HCV RNA.
In this study, we did not perform HCV RNA screening by PCR in the serum (nonultracentrifuged) of all patients since our objective was not to identify patients with serum anti-HCV negativity and HCV RNA positivity. However, HCV RNA screening was performed in all patients’ non-ultracentrifuged serum when HCV RNA was positive in PBMCs or in ultracentrifuged serum. Moreover, it would be unlikely to find patients with anti-HCV negativity and HCV RNA negativity in both PBMCs and in ultracentrifuged serum, but with positivity in non-ultracentrifuged serum.According to these parameters, then, we question the need for performing the HCV RNA screening by PCR of serum before RNA screening of PBMCs and ultracentrifuged plasma for diagnostic confirmation of OCI.

Figure 1 Electrophoresis showing a positive sample visualized on the SYBR-safe gel through the L-PIX Transilluminator (Loccus, São Paulo, Brazil).
The practical relevance of the findings from our study is uncertain. However, the high frequency of OCI found in our study population may play a significant role in the transmissibility of the disease within HD units once the patients do not have detectable antibodies and circulating virions, when they could potentially be infectious[20]. Moreover, the vast majority of patients (14/15) with OCI had viral genome components at detectable levels in PBMCs, making them potential transmitters of the infection[21]. These are intriguing findings since, in general, about 50% of HCV-infected patients do not have identifiable risk factors for HCV infection[22].
Epidemiologically, a phylogenetic study conducted by Castillo et al[23]found that patients with OCI have high intrafamilial transmissibility. Moreover, Roque-Cuellar et al[24]evaluated the immunological response in the presence of OCI in heterosexual partners of patients with the classic form of infection; they found a specific CD4+ and CD8+ immune response and a higher prevalence of OCI in the partners compared to those with uninfected heterosexual partners. These findings were corroborated by Shazly et al[25], who evaluated 50 partners of patients with positive anti-HCV in serum and found a about 4% prevalence rate for OCI, higher than expected in the general population.
In addition to the exclusion of patients with glomerulopathies (since this group may present a prevalence of OCI of up to 39%), our results are strengthened by the fact that two techniques were used to identify OCIboth the serum ultracentrifugation and the identification of the viral genome in PBMCs, which increases the chance of detection of OCI. In fact, Castillo et al[5]demonstrated that positivity for HCV in PBMCs by PCR corresponded to 82% of positive cases for OCI; in addition, when using ultracentrifugation, the number of positive patients increased. In none of the cases of that study was the examination of OCI positive by both techniques.
In our study, OCI was associated with age, mixed etiology CKD, and positivity for HBV antibodies (anti-HBc and anti-HBs), suggesting previous infection.
Certainly, older patients are more exposed to contamination during their longer lifetimes. These patients also have more comorbidities, as described in a meta-analysis published in 2016 by Alvarez et al[26]. Age has been characterized as an independent risk factor for acquisition of HCV infection[27]. In addition, a North American study found that about 75% of HCV-infected patients were born between the years of 1945 and 1965, representing the so-called Baby Boomer generation[28].
Patients with CKD of mixed etiology are reported to have more comorbidities and,therefore, have greater chance of exposures to other diseased patients, such as those infected with hepatitis, in hospital settings and to diagnostic and therapeutic procedures, all known risk factors for HCV infection[29]. In fact, a case-control study conducted by Perez et al[30]in the United States, involving acute and symptomatic HCV infections in individuals over 55 years of age, found that exposure in a healthcare environment with intravenous drug infusion was related to a higher risk of HCV infection; namely, 2.7-fold higher than in the controls, with risk attributable to exposure of 37% (OR = 2.7, 95%CI: 1.3-5.8).
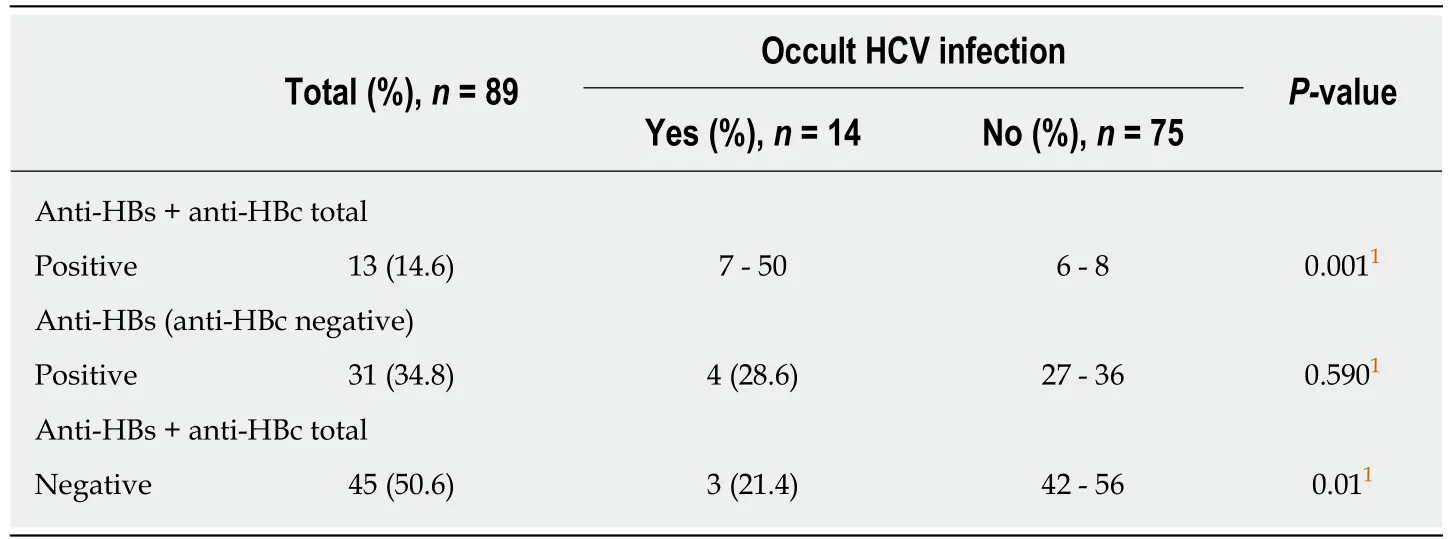
Table 2 Distribution of antibodies against hepatitis B virus according to the occurrence of hepatitis C virus occult infection in 89 patients with chronic kidney disease: 2015 to 2017
In our study, patients with OCI showed a higher frequency of positivity for anti-HBc and anti-HBs, when compared to those not infected. It is also known that patients with HCV infection present a higher chance of HBV contamination, since the risk factors for transmission of these infections are similar[31].
Our study had some limitations that must be considered when interpreting our findings. Specifically, by not quantifying the viral load and not genotyping the patients, the epidemiological and transmissibility factors could not be determined for our study population of patients with OCI.
The reasons underlying the emergence of the occult form of HCV infection are not known. We can speculate that virus-related factors may be involved in this form of infection, as described in genomic studies where genetic variations between different viral strains of HCV constitute a mechanism of viral survival, contributing to their pathogenicity and resistance to the host's immune system[32,33]. We can also speculate that host-related factors may be involved in the occult form of HCV infection. Of course, the specific cellular immune response (CD4+ and CD8+) in patients with OCI would be less effective than in individuals without infection but more intense than in those with the classic form of disease, presenting with HCV RNA positivity in the serum[8].
In addition, although most infected patients show a specific cellular immune response to HCV, only 20% of infected individuals are able to achieve viral clearance in the acute phase of the disease[34,35]. Indeed, Burke et al[36]observed that patients who were unable to achieve viral clearance had lower cell responses and decreased numbers of CD4+ and CD8+ T lymphocytes. In addition, existing differences between the human leukocyte antigen (commonly known as HLA) molecules can target poorly effective immune responses, as some HLA super types affect the recognition and binding affinity of T lymphocytes to antigenic epitopes[37].
Treatment of OCI can be performed with antiviral therapy, as reported by Pardo et al[38]. Those authors administrated pegylated-interferon and ribavirin for 6 mo to 10 patients who presented with OCI and abnormalities in serum levels of aminotransferases that were associated with chronic hepatitis (diagnosed by biopsy).At the end of treatment, 6 patients presented normalization of the serum alanine aminotransferase levels and in 7 cases the HCV RNA was no longer detectable in the PBMCs, suggesting viral behavior similar to that of the classic HCV infection[38].
In our study, presented herein, occurrence of OCI was high among patients with PDCKD. OCI in this patient population was more common in the elderly, in patients with CKD of mixed etiology and in patients with serological markers of HBV(indicating previous infection). Further studies will be needed to evaluate the influence of this form of infection on the transmissibility of HCV in the HD setting and the role of treatment for HD patients with OCI.
ARTICLE HIGHLIGHTS
Research background
Patients with chronic kidney disease (CKD) and who are on hemodialysis (HD) have a higher prevalence of hepatitis C virus (HCV) infection, both in its classical form and the occult (OCI)form. However, no studies in the literature have evaluated the occurrence of OCI in predialysis patients.
Research motivation
The motivation of this study was borne of the new lines of evidence showing OCI having implications on transmissibility, prognosis and progression of CKD in patients on dialysis, and knowledge that this infection could be diagnosticated and treated before end-stage kidney disease is reached.
Research objectives
The main objective of this study was to evaluate the prevalence of OCI in predialysis CKD patients. The findings of this study could help in future efforts to trace the transmissibility of OCI and even to develop a treatment to improve these patients’ prognosis.
Research methods
This was a cross-sectional study, wherein patients were allocated if they had glomerular filtration rate < 60 mL/min·1.73 m2and were anti-HCV negative. PCR was performed on patient samples of ultracentrifuged plasma and peripheral blood mononuclear cells.
Research results
OCI was found in 16.5% of the study population of CKD patients. It was more common in the elderly, in patients with CKD of mixed etiology, and in patients with previous hepatitis B virus infection.
Research conclusions
This study found a high prevalence of OCI among CKD patients, suggesting that patients with low kidney function may be reservoirs for HCV. This high prevalence appears to be associated with infection occurring before the beginning of dialysis. Moreover, OCI could be an important transmission route of HCV infection in dialysis centers. The collective findings from this study could help future studies evaluating the transmissibility of hepatitis infection and the progression of CKD to need for dialysis.
Research perspectives
This is the first study to evaluate the prevalence of OCI in this specific group of patients, who are more vulnerable to viral infections. Future research should evaluate the influence of this form of infection on the transmissibility of HCV in the HD setting and the role of treatment for these OCI patients. A prospective study evaluating the clinical behavior of patients with OCI will be insightful.
ACKNOWLEDGEMENTS
We also thank the Aggeu Magalhães Laboratory, for making materials and staff available for this study.
杂志排行
World Journal of Hepatology的其它文章
- Caval replacement with parietal peritoneum tube graft for septic thrombophlebitis after hepatectomy: A case report
- Non-uremic calciphylaxis associated with alcoholic hepatitis: A case report
- Multidisciplinary approach for multifocal, bilobar hepatocellular carcinoma: A case report and literature review
- Low platelet count: Predictor of death and graft loss after liver transplantation
- Clinical factors associated with hepatitis B screening and vaccination in high-risk adults
- Temporal trends of cirrhosis associated conditions
