Roles of Na+/Ca2+ exchanger 1 in digestive system physiology and pathophysiology
2019-01-29QiuShiLiaoQianDuJunLouJingYuXuRuiXie
Qiu-Shi Liao, Qian Du, Jun Lou, Jing-Yu Xu, Rui Xie
Abstract The Na+/Ca2+ exchanger (NCX) protein family is a part of the cation/Ca2+exchanger superfamily and participates in the regulation of cellular Ca2+homeostasis. NCX1, the most important subtype in the NCX family, is expressed widely in various organs and tissues in mammals and plays an especially important role in the physiological and pathological processes of nerves and the cardiovascular system. In the past few years, the function of NCX1 in the digestive system has received increasing attention; NCX1 not only participates in the healing process of gastric ulcer and gastric mucosal injury but also mediates the development of digestive cancer, acute pancreatitis, and intestinal absorption.This review aims to explore the roles of NCX1 in digestive system physiology and pathophysiology in order to guide clinical treatments.
Key words: Na+/Ca2+ exchanger; Digestive system diseases; Ion channel; Sodium; Calcium
INTRODUCTION
Ca2+is an important cellular signal. Changes in intracellular Ca2+control various cellular processes that are relevant to the regulation of normal function and to the development of diseases. These processes include muscle contraction, blood coagulation, nerve excitation, angiogenesis, cell apoptosis[1-3], and the development of cancer[4,5]. The homeostasis of intracellular calcium is controlled by a variety of proteins and ion channels, including the plasma membrane Na+/Ca2+exchanger(NCX). Members of the NCX family can exchange Na+and Ca2+in either direction depending on the transmembrane electrochemical gradients and membrane potential[6], and these exchangers have a two-way transport mode such that under physiological conditions, one Ca2+ion exits and three Na+ions enter the cell but the reverse transport occurs under special conditions (such as cancer or inflammation),that is, three Na+ions exit and one Ca2+ion enters[7]. The NCX family contains three separate gene products exhibiting differential expression: NCX1, NCX2, and NCX3.NCX1 is widely expressed in mammalian organs and tissues[8], and NCX2 and NCX3 are expressed mainly in nerves and skeletal muscle[9]. Numerous studies have shown that NCX1 is involved in a variety of physiological and pathophysiological processes.For example, in the cardiovascular system, NCX1 can control the contraction and relaxation of vascular smooth muscle[10], while NCX1 can regulate heart rhythm,which is related to arrhythmia[11,12], and participate in the regulation of myocardial ischemia-reperfusion injury[13]. In the nervous system, NCX1 regulates neurotransmitter release[14]and microglia-related functions[15], which is associated with cerebral ischemia-reperfusion and Alzheimer's disease[16,17]. In the urinary system,NCX1 is involved in renal Ca2+reabsorption and associated with renal ischemiareperfusion[18,19]. In the endocrine system, NCX1 can regulate insulin secretion[20]. In the immune system, NCX1 is associated with the development of systemic lupus erythematosus[21]. In recent years, NCX1 has been found to be expressed in all of the organs of the digestive system and play important roles in the physiological processes and digestive diseases (such as pancreatitis, gastric ulcer, and gastrointestinal cancers)[22-24], However, the mechanism and function of NCX1 in the gastrointestinal tract have not yet been completely elucidated, particularly relating to certain digestive diseases and tumors. This review intends to explore the roles of NCX1 in digestive system physiology and pathophysiology as well as current treatments utilizing NCX1-based therapeutics.
STRUCTURAL FEATURES OF NCX1
NCX1 is a transmembrane bidirectional transporter with a molecular weight of 110 kDa and consists of 970 amino acids. NCX1 has 9 transmembrane segments, forming a large central cytoplasmic loop between the 5th and 6th transmembrane segments[25,26].In addition, the NCX1 transmembrane segment has two internal repeat regions, the α1 and α2 repeat regions[27]. The first half of the transmembrane segment, including the α-repeat region, may be involved in ion transport[28-30]. In contrast, the second half,which contains the central cytoplasmic ring, has an inhibitory effect on the entire sodium-calcium exchanger[31,32]. In addition, there are two binding sites that can regulate Na+and Ca2+[33,34], and there is a secondary Ca2+adapter site[35](Figure 1).
NCX1 AND THE ESOPHAGUS
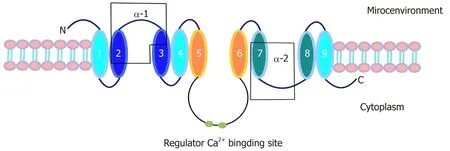
Figure 1 Structural features of NCX1.
The esophagus is a muscular portion of the digestive tract that transports food from the pharynx to the stomach depending on the contraction of muscle. In normal conditions, the tension of the lower esophageal smooth muscle (LES) depends mainly on the intracellular Ca2+concentration. A high concentration of intracellular Ca2+can cause smooth muscle contraction, and a low concentration of Ca2+causes smooth muscle relaxation[36]. However, the contraction of the esophageal body is mainly dependent on the gradient of extracellular calcium. The occurrence of esophagitis is also closely related to the regulation of Ca2+in pathological conditions. Calcium channel blockers (CCBs) have been used in the treatment of esophageal-related diseases, such as achalasia[36,37]. Achalasia is a kind of neuropathy where the smooth muscle fiber is not relaxed or cannot relax completely, a partial loss of esophageal body peristalsis occurs, and the motility is not coordinated. Gelfond et al[38]first reported that use of the L-type CCB nifedipine can relax the esophageal smooth muscle to reduce the pressure of the lower esophageal sphincter by blocking the flow of calcium ions into the cells and intracellular calcium release, thereby achieving the purpose of treating achalasia. However, taking CCBs for a long time will cause the LES to become too relaxed and will lead to reflux esophagitis (RE)[39]. There is no definitive evidence as to whether NCX1 plays a regulatory role in RE. However, Kim et al[40]found that NCX1 is widely expressed in the esophageal muscle layer.Furthermore, the estrogen E2-induced inhibition of smooth muscle contraction in the esophagus and the mucus secretion in the esophageal mucosa are mainly achieved by downregulating the expression of calcium-related genes such as NCX1, CaBP-9k, and PMCA1 and decreasing the intracellular calcium level. It is suggested that NCX1 may play an important role in the regulation of contraction and relaxation in the LES. In addition, at present, our research group also confirmed that NCX1 expression was significantly increased along with the expression of TRPC6, TRPV4, and other acidsensitive calcium channels that have a regulatory role in Barrett's esophagus or reflux esophagitis caused by acid reflux or bile reflux. Interfering with NCX1 can significantly inhibit the release of inflammatory mediators and the expression of intestinal metaplasia genes caused by the aforementioned pathogenic factors.Therefore, NCX1 is likely to be an important treatment target for esophageal functional diseases.
NCX1 also plays an important role in the correlation between smoking and the pathogenesis of esophageal squamous cell carcinoma (ESCC)[41]. Clinical evidence showed that the expression of NCX1 in ESCC tissue was significantly higher than that in esophageal noncancerous tissue and demonstrated a positive correlation between the NCX1 expression level and the smoking status of ESCC patients. The tobaccoderived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) can significantly enhance NCX1 expression in normal esophageal cells and human ESCC cells. NNK mediates an increase in the intracellular Ca2+concentration through NCX1 activation and promotes the proliferation and migration of human ESCC cells[41].These findings indicate that tobacco smoking could cause Ca2+entry through enhanced expression and function of NCX1, finally resulting in the pathogenesis of ESCC. Furthermore, NCX1 is also involved in the proliferation and migration of ESCC cells. To elucidate the mechanism of NCX1 in ESCC, it is necessary to study the function of NCX1 in ESCC in the future.
EXPRESSION AND FUNCTION OF NCX1 IN THE STOMACH
The stomach, the main digestive organ of the human body, is connected to the esophagus and the duodenum. It is known that the plasma membrane Ca2+-ATPase(PMCA), NCX, and the endoplasmic reticulum (ER) Ca2+-ATPase are the main mechanisms for the transport of intracellular Ca2+to the extracellular space of gastric smooth muscle cells[42,43]. Studies have shown that NCX1 is widely expressed in the antrum of guinea pigs, while NCX2 has higher expression in the fundus[44].Researchers believe that different NCX subtypes, which have different physiological functions, are expressed in different parts of the stomach and regulate each other.NCX1 is mainly involved in gastric antral motility, while NCX2 mainly changes the intracellular Ca2+homeostasis in fundus smooth muscle cells to control the contraction and relaxation of the fundus smooth muscle[44]. This information suggests that the NCX family may control the movement of the whole stomach by controlling the movement of the gastric antrum and fundus smooth muscle.
In a study of gastrointestinal motility diseases, Hagi et al[45]found that NO and PACAP act as important mediators of the transient and sustained relaxation in the mouse gastric fundus. A change in functional coupling and/or collaborative functions between NO signaling and PACAP signaling may cause intracellular Ca2+concentration changes, thereby controlling gastric fundus relaxation in mice. The overexpression of NCX1 in smooth muscle may result in increased functional coupling and/or collaborative functions between NO signaling and PACAP signaling,resulting in the occurrence of functional gastrointestinal disorders[46]. Interestingly,studies on experimental gastric ulcer (GU) indicate that nitric oxide synthase (NOS)activity may be an important marker of neutrophil infiltration[47,48]. NO also contributes to ethanol-induced gastric ulceration and inflammatory bowel diseases(IBD) due to its role in the stimulation of cell proliferation in the gastric mucosa[49-51].However, the overexpression of NCX1 in gastrointestinal smooth muscle may affect the function of the NO signaling pathway, and whether this change will promote GU and IBD process through the NO signaling pathway needs further study. It has also been reported that electric field stimulation (EFS) can induce sustained relaxation of the stomach fundus, but not other intestinal regions[52-54]. The study suggested that this sustained status may be closely related to the regulatory functions of NCX1 and NCX2. In the past few years, studies have shown that NCX1 and NCX2 are expressed in smooth muscles and neurons to regulate the relaxation and motility of the gastric fundus. Upon NCX1 or NCX2 heterozygosity deletion, the fundus relaxation and the gastric peristalsis can be enhanced by EFS[55]. Therefore, the NCX family may be an important treatment target for functional gastrointestinal diseases. In addition, Lajos V also found that NCX family members (NCX1, NCX2, and NCX3) are expressed extensively in human gastric myofibroblasts and participate in the regulation of intracellular calcium oscillations. Knockdown of NCX1 significantly inhibited the migration and proliferation of gastric myofibroblasts induced by insulin-like growth factor II (IGF-II)[22]. Gastric myofibroblasts are a kind of contractile, nonexcitatory cell induced by inflammatory factors such as transforming growth factor (TGF-β), and these cells are localized to the subepithelium throughout the whole gastrointestinal tract[56,57]. It is known that gastric myofibroblasts can not only regulate the secretion of extracellular matrix proteins and the formation of new blood vessels, promoting the healing process of ulcers[58,59], but also participate in the development of chronic gastritis and gastric cancer cell invasion and metastasis[60,61]. Further study on the role of NCX in gastrointestinal smooth muscle may elucidate the regulatory mechanisms of ulcer healing and tumor invasion and metastasis.
NCX1 AND THE INTESTINE
NCX1 and the small intestine
The expression of NCX1 protein has been detected in the small intestine, colon, and rectum[62,63], and it participates in the physiological regulation mechanism of intestinal calcium absorption, bicarbonate secretion, ileal smooth muscle movement and so on.The small intestine absorbs 90% of calcium[3,64,65]. Additionally, NCX1 mainly participates in the process of extracellular discharge of calcium ions from the basal membrane of intestinal epithelial cells. NCX1 can transport three Na+ions into the cell and transport one Ca2+ion out of the cell; this transport is regulated by vitamin D and 1,25-(OH)2D3[66], which can enhance the expression and activity of NCX on the basal membrane and promote the transport of Ca2+from the cell to the outside[66]. Moreover,Wongdee et al found that vitamin D can upregulate the expression of the Ca2+transporter gene NCX1, thus enhancing the Ca2+transmembrane transport[67].
It is well known that the gastric acid defense barrier involves bicarbonate, and the hyposecretion of bicarbonate is one of the key mechanisms for the pathogenesis of duodenal ulcers. It has been reported that intracellular calcium signals can promote HCO3-secretion depending on the activation of the HCO3-secreting channel cystic fibrosis transmembrane conductance regulator (CFTR) or on the activation of the intermediate-conductance Ca2+activated K+channel (IKCa2+), which provide a driving force for HCO3-secretion[68]. However, NCX1 may be the key to the regulation of intracellular calcium changes. Dong et al found that the NCX1 protein is functionally expressed in the mouse duodenal mucosal epithelium, and a dynamic calcium ion experiment determined that the reverse regulation mode of NCX1 occurs, causing Na+efflux and Ca2+entry to regulate HCO3-secretion[69]. Subsequent research confirmed that the muscarinic receptor agonists carbachol and 5-hydroxytryptamine (5-HT) can increase the intracellular calcium concentration and promote duodenal bicarbonate secretion after stimulating mouse duodenal mucosal or epithelial cells and confirmed that disturbing or inhibiting the function of NCX1 can obviously block the intracellular calcium change and promote bicarbonate secretion[70]. These results suggest that NCX1 and its mediated Ca2+influx play a critical and extensive role in regulating the secretion of HCO3-in the duodenal mucosa. Other studies have shown that NCX1 and NCX2 are also involved in ileal smooth muscle contraction and ileal motility regulation[71]. Nishiyama et al found that NCX2 regulates ileal motility primarily by controlling the sensitivity of acetylcholine (AchE) and substance P (SP) in smooth muscle[71]. Compared with that in the wild-type model, the contraction amplitude induced by AchE and SP after NCX2 knockout was significantly reduced;although NCX1 also plays a role in the regulation of ileal contraction, this decline was not evident in the NCX1 knockout model[71]. This finding suggests that NCX2 plays a more important role than NCX1 in ileal movement.
NCX1 and the colon
NCXs (NCX1 and NCX2) are also widely expressed in colonic smooth muscle and the myenteric plexus layers[72]. Nishiyama et al showed that NCX1 overexpression in the mouse distal colon enhanced the relaxation amplitude induced by EFS, suggesting that NCX1 can affect the distal colonic smooth muscle movement in mice[71].Furthermore, it was found that the secretion of the mucin MUC5AC induced by ATP depends on the influx of Ca2+into colonic goblet cells and that ATP requires the activation of TRPM5 channels to increase intracellular Na+, which activates the NCX reverse transport mode and increases intracellular Ca2+uptake; thus, inhibiting NCX can significantly reduce the MUC5AC secretion in goblet cells[73]. There is also evidence that the NCX family may also be involved in the pathogenesis of diarrhea.Although NCX1 and NCX2 were found to be expressed in the myenteric nerve plexus of the proximal colon and the colon transversum as well as longitudinal and annular muscular layers, the function of NCX1 and NCX2 in intermuscular neurons may be different from that in smooth muscle[74]. Kazuhiro et al have found that in a diarrhea model induced with magnesium sulfate or 5-HT, the diarrhea in NCX2 heterozygous knockout mice (NCX2 HET) was more serious than that in wild-type mice (WT), but the diarrhea in NCX1 heterozygous knockout mice (NCX1 HET) showed no significant changes from that of WT[75]. Magnesium sulfate-induced diarrhea was exacerbated in NCX2 HET by decreasing normal and soft fecal materials and increasing watery fecal materials, however, PGE2-induced diarrhea in NCX1 HET and NCX2 HET was similar to that in the WT[75]. The researchers believe that this finding may be due to the mechanism of 5-HT-induced diarrhea involving stimulation of the 5-HT3 receptor in myenteric plexus neurons and its downstream cholinergic and tachykinin excitatory pathways[76,77]. However, PGE2 acts directly on smooth muscle and stimulates fluid accumulation to induce diarrhea[78,79]. Therefore, NCX2 rather than NCX1 in the myenteric plexus may play a critical role in the occurrence and development of diarrhea. Further study of NCX may provide new targets for the diarrhea caused by gastrointestinal dysfunction.
NCX1 AND THE PANCREAS
Expression and distribution of NCX in the pancreas
NCX1 is functionally expressed in the β-cells, acinar cells, and ductal cells of the rat pancreas and has a distinct pattern of distribution in pancreatic ducts depending on their size and proximity to acini[80]. Two splicing variants of NCX1, NCX1.3 and NCX1.7, are mainly expressed in rat pancreatic cells[81-84]; however, three other variants, NCX1.2, NCX1.9, and NCX1.13, were also found in guinea pigs, hamsters and mice. In the past few years, studies have proved that different types of NCX1 have different expression levels between different species.
Role of NCX1 in pancreatic physiological processes
The physiological functions of the pancreas include secreting variousdigestive enzymes and insulin. Normally, the intracellular ATP/ADP ratio increases after pancreatic β-cells uptake glucose, via the closure of K+-ATP channels, causing β-cell depolarization and inducing extracellular calcium influx through calcium channels in the membrane; the intracellular calcium increase causes fusion of the vesicular membrane containing insulin with the cytoplasmic membrane and the subsequent secretion of insulin from cells via vesicular exocytosis[85]. It has been found that both the voltage-dependent calcium channel (CaV) and the intracellular IP3-sensitive calcium pool are important in insulin secretion regulation in the past few years[86], but the role of the NCX family in this process is just beginning to be evaluated.
In normal pancreatic islet β-cells, NCX1 is mainly responsible for Ca2+efflux from cells. The aim is to control the Ca2+concentration within the normal physiological range in order to accurately control the insulin release level[83,87]. In native pancreatic ducts, the NCX1 expression level is downregulated by acetylcholine and secretin but upregulated by insulin[80]; as the main physiological stimulant of insulin release,glucose has the reverse regulatory effect on the transcription, expression, and activity of NCX[88].
NCX1 and pancreatic diseases
Pathologically, NCX also has a regulatory mechanism affecting the insulin secretion from the β-cells of diabetic patients. In the past few years, research has shown that NCX overexpression can lead to ER stress and Ca2+release from the ER, thus promoting β-cell apoptosis, reducing β-cell proliferation, and decreasing insulin secretion[88]. Herchuelz et al[89]found that heterozygous inactivation of NCX1 (Ncx1+/-) leads to an increase in β-cell function and a 5-fold increase in both β-cell mass and proliferation. The mutation also increases the β-cell resistance to hypoxia, and Ncx1+/- islets show a 2-4 times higher rate of curing diabetes than Ncx1+/+ islets when transplanted into diabetic animals. However, in some cases, NCX may change into the reverse regulation mode to promote Ca2+entry, prolonging the duration of the peak electrical activity associated with glucose and increasing insulin release[90]. In summary, the different NCX1 expression and transport modes can regulate insulin secretion, so selective inhibition of NCX1 may improve insulin secretion, which provides more theoretical evidence for novel glucose-sensitive insulinotropic drugs for type 2 diabetes that target NCX1.
In addition to regulating physiological insulin secretion, the Ca2+homeostasis is also a key factor leading to pancreatitis, hypercalcemia, pancreatic cancer, and other diseases. Pancreatitis is one of the most common acute abdomen problems, and the pathogenesis is the abnormal accumulation of intracellular Ca2+(calcium overload) to promote excessive activation of trypsinogen, resulting in pancreatic autodigestive injury[91]. Previous studies have reported that the calcium overload in acute pancreatitis may be related to calcium channels such as CRAC/TRPV1/TRPV3[92,93];however, it has recently been found that the NCX1 reverse regulation mode may also be involved in this overload. Yu et al confirmed that the mRNA and protein expression of NCX1 in tissues of acute pancreatitis induced by cerulein was significantly increased in cell experiments and animal experiments[94]. The authors found that the expression of inflammatory mediators such as TNF-α and interleukin-6(IL-6) caused by cerulein was decreased significantly after treatment with KB-R7943 (a specific inhibitor of NCX1)[94]. This finding suggests that NCX1 may play a critical role in the occurrence and development of acute pancreatitis. In addition, pancreatic cancer is a kind of cancer with high malignancy and poor prognosis, and duct cell carcinoma is the main pathological type of pancreatic cancer[95,96]. However, it is generally accepted that alterations in TGF-β signaling and its downstream SMAD pathway play an important role in pancreatic cancer development[97]. The study by Chow et al found that TRPC1 and NCX1 are expressed and functional in pancreatic cancer cells. TGF-β activates TRPC1 and NCX1 channels to mediate a cytoplasmic Ca2+concentration increase in pancreatic cancer cells, which activates the downstream PKC/SMAD4 pathway to regulate pancreatic cancer cell motility[98]. These results suggest that NCX1 may be involved in the malignant biological behavior regulation of pancreatic cancer (Figure 2).
NCX1 AND THE LIVER
NCX1 and hepatic ischemia-reperfusion
Although NCX1 is expressed in normal livers, liver fibrosis, and liver cancer, the transcription levels and regulation modes are different under these three conditions,suggesting that NCX may have different functions and effects in the development of hepatitis, liver fibrosis, and liver cancer[99]. In the study of liver ischemia-reperfusion injury, intracellular calcium accumulation is a critical mechanism of cell apoptosis and injury[100]. NCX mainly adopts the forward control mode in ischemic-reperfusion injury. Trisulfated disaccharide (TD) can transport excess intracellular calcium out of the cell by activating NCX1, thus reducing the serum levels of inflammation markers(TNF-α, IL-6, and IL-10) and the lipid peroxidation after liver injury[101].
NCX1 and liver fibrosis
Hepatic fibrosis is a necessary process in the progression from chronic hepatitis to cirrhosis. The activation and proliferation of hepatic stellate cells (HSCs) are the central link of hepatic fibrosis. Nakamura et al found that the NCX mRNA and protein expression levels were significantly upregulated in response to the activation of rat HSCs induced by CCl4[102]. It was also reported that NCX expression is upregulated in cirrhotic tissue, although the specific mechanism is not clear; NCX may be a new target in liver fibrosis or cirrhosis research[93].
NCX1 and liver cancer
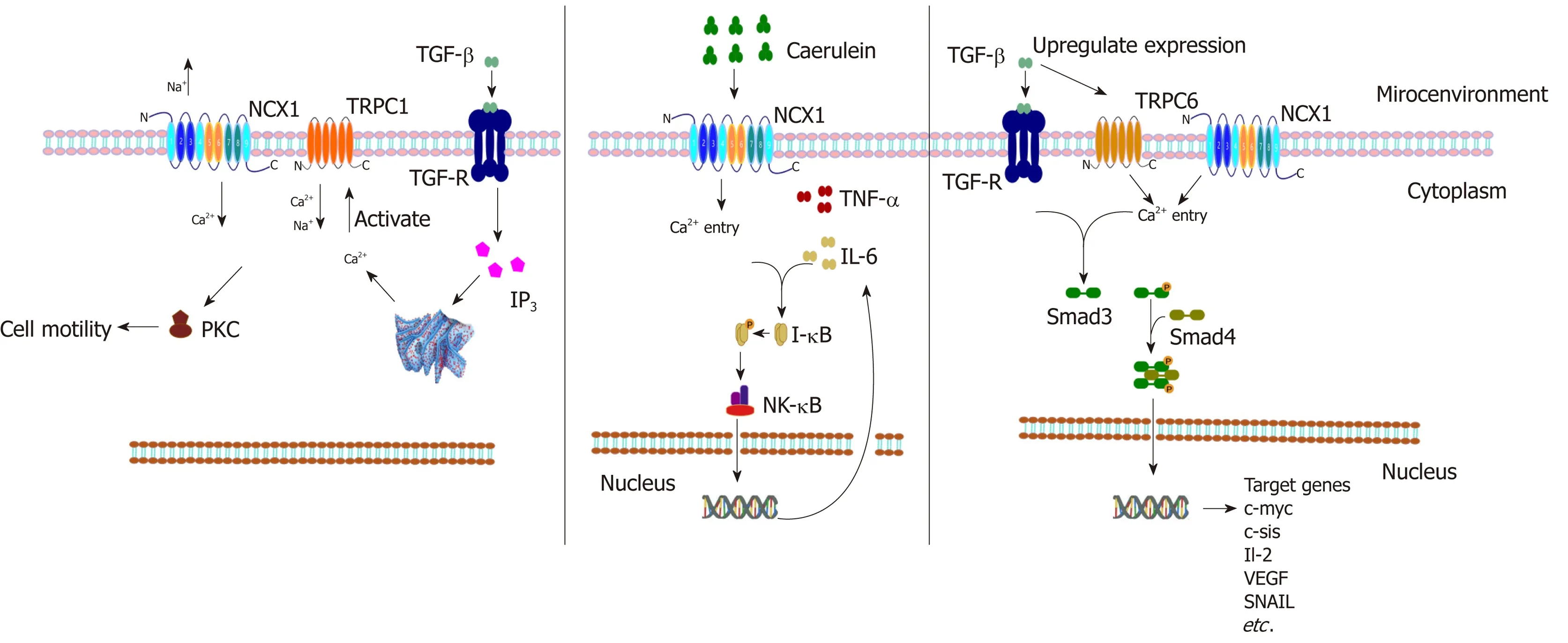
Figure 2 The regulated signal pathways and transcription factors of NCX1 in digestive diseases. Transforming growth factor-β (TGF-β) stimulates the activation of PLC-IP3 and Ca2+ release from the endoplasmic reticulum, which activates TRPC1 and the reverse mode of NCX1 resulting in Ca2+ influx, and the increase of Ca2+ mediates cell motility directly or indirectly via activation of Ca2+-dependent PKC in pancreatic cancer. Cerulein activates NCX1 and induces activation of inflammatory factors TNF-α and IL-6 and the downstream NK-κB pathway in pancreatic cells. TGF-β can upregulate the expression of NCX1 and TRPC6 and activate the downstream SMAD pathway to regulate the migration and invasion of hepatocellular carcinoma cells.
Finally, in hepatocellular carcinoma (HCC) research, our research group has published articles confirming that the expression of NCX1 is obviously upregulated in hepatoma cells and tissues and that NCX1 can affect the intracellular calcium level to affect the cytokines TGF-β or IL-6 in the malignant behavior of hepatoma cells. A study found that TGF-β can upregulate the expression of NCX1 and the transient receptor potential channel TRPC6 in hepatoma cells and further induce intracellular calcium activation in HCC to promote the formation of a complex between NCX1 and TRPC6, thus activating the downstream SMAD pathway, which can regulate HCC malignant biological behaviors such as migration and invasion[103]. Not only was the phosphorylation of Smad proteins dependent on TRPC6 and NCX1, but also the Smad signaling, especially the phosphorylation of Smad2, augmented the expression of TRPC6 and NCX1. At the same time the upregulated expression of TRPC6 and NCX1 can be strongly correlated with the stage and pathologic grade of HCC, which may become useful biomarkers for monitoring disease progression of liver cancer patients[103,104]. In a related study of IL-6 and liver cancer, it was also confirmed that an intracellular pH regulator (NHE1), NCX1, and calmodulin (CaM) coexisted in the same lipid-crossing structure of the cell membrane and that their expression levels were upregulated in liver cancer tissues[105](Figure 2). Moreover, IL-6 activated NHE1 to promote H+excretion and an NCX1-induced external Ca2+influx, and NHE1 pumped H+in exchange for Na+influx to promote NCX1 activation, which enhanced the interaction between NCX1 and CaM, thus promoting the occurrence and development of liver cancer[106]. The above findings provide the basis for the important role of NCX in hepatic carcinogenesis and also provide a new possibility for drug development for early intervention in inflammation-associated tumors.
CONCLUSION
In summary, the NCX1 channel protein regulates the Ca2+signaling pathway via its forward/reverse modes in the digestive system and regulates the cell function, thus participating in the occurrence and development of digestive system diseases (Figures 3 and 4). The functions of NCX1 in inflammation-associated digestive diseases (such as inflammatory bowel disease and hepatitis ) will become a new research hotspot.NCX1 could be a new molecular marker for the digestive system disease diagnosis and treatment, and drug development targeting NCX1 will represent a new direction of the treatment of digestive system diseases.
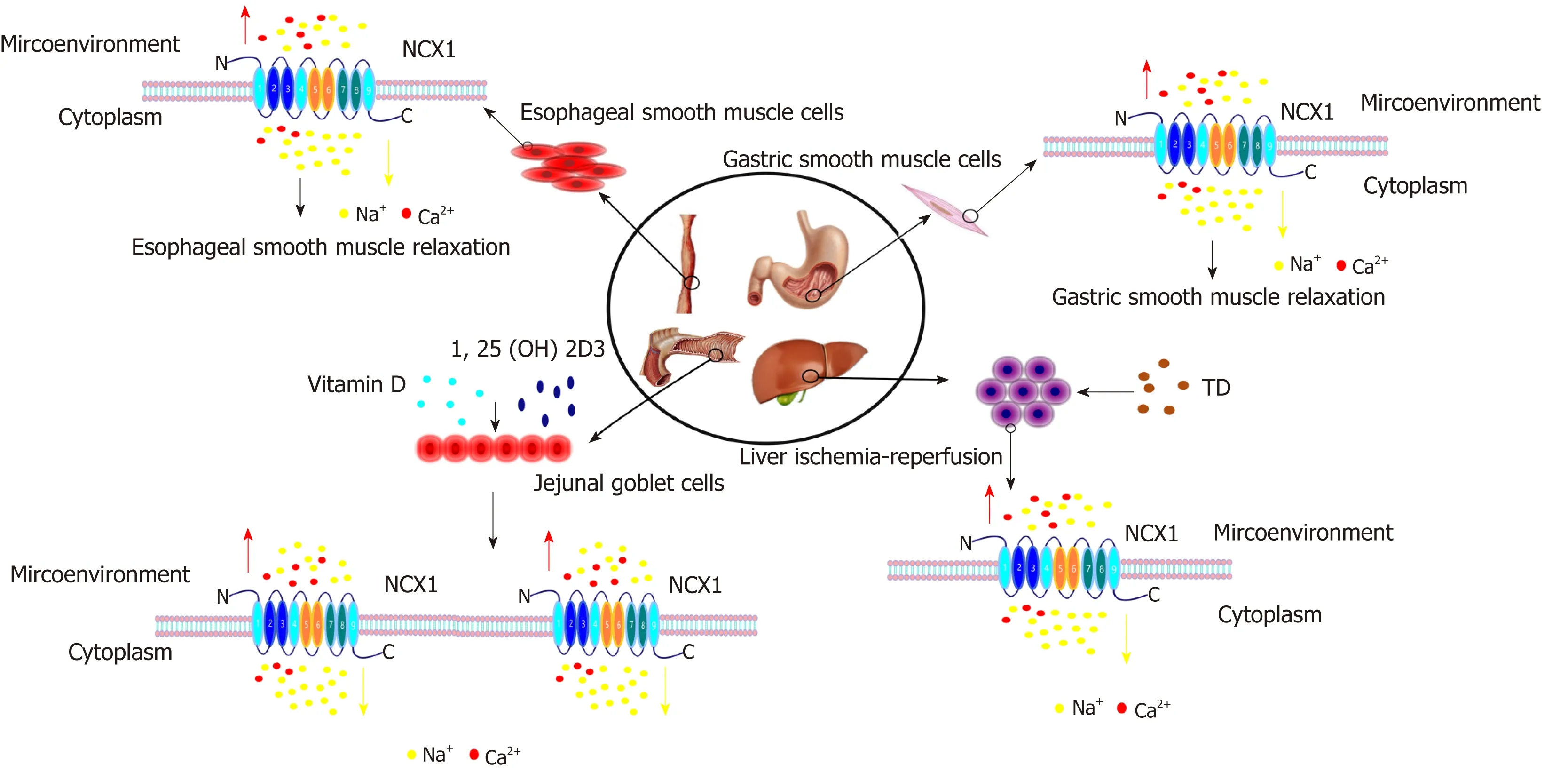
Figure 3 The effects of NCX1 positive mode in the digestive system. Under normal circumstances, NCX1 adopts the positive mode in esophageal smooth muscle and gastric smooth muscle, excreting Ca2+ from the cells, reducing intracalcium concentration and inducing smooth muscle relaxation. In the jejunum, vitamin D and 1,25-(OH)2D3 can enhance the expression and activity of NCX1 to increase the excretion of Ca2+. NCX1 mainly adopts the forward control mode in ischemicreperfusion injury. Trisulfated disaccharide (TD) can transport excess intracellular Ca2+ out of the cell by activating NCX1.
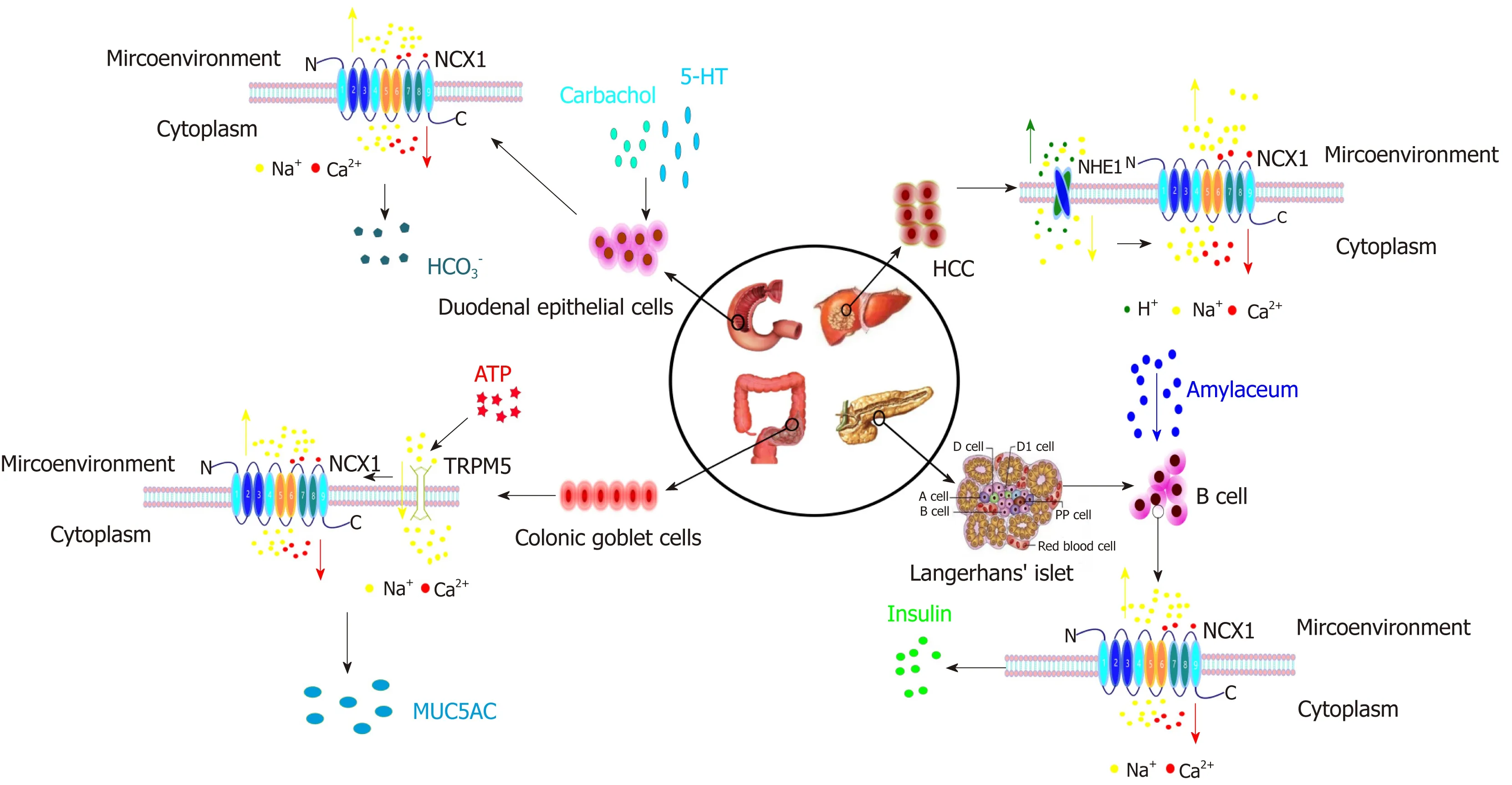
Figure 4 The roles of NCX1 reverse mode in the digestive system. In duodenal epithelial cells, carbachol and 5-HT can activate the reverse mode of NCX1,enhancing Ca2+ influx to release HCO3-. In hepatoma cells, NHE1 can promote H+ excretion and Na+ influx and activate the reverse mode of NCX1 to induce Ca2+influx. In colon goblet cells, ATP activates the TRPM5 channel to induce Na+ influx, and an increase of Na+ concentration starts the NCX1 reverse mode and increases Ca2+ influx and MUC5AC expression. In pancreatic islet β cells, under glucose stimulation, NCX1 can be converted to a reverse mode to promote Ca2+influx to increase insulin secretion.
ACKNOWLEDGEMENTS
We thank Professor Biguang Tuo (Department of Gastroenterology, Affiliated Hospital to Zunyi Medical College) for highly professional services.
杂志排行
World Journal of Gastroenterology的其它文章
- Chronic hepatitis B and metabolic risk factors: A call for rigorous longitudinal studies
- Endoscopic resection techniques for colorectal neoplasia: Current developments
- Elastography-based screening for esophageal varices in patients with advanced chronic liver disease
- NKX6.3 protects against gastric mucosal atrophy by downregulating β-amyloid production
- Effects of positive acceleration (+Gz stress) on liver enzymes,energy metabolism, and liver histology in rats
- Incidence and treatment of mediastinal leakage after esophagectomy: Insights from the multicenter study on mediastinal leaks
