Current status, problems, and perspectives of non-alcoholic fatty liver disease research
2019-01-29NaokiTanakaTakefumiKimuraNaoyukiFujimoriTadanobuNagayaMichiharuKomatsuEijiTanaka
Naoki Tanaka, Takefumi Kimura, Naoyuki Fujimori, Tadanobu Nagaya, Michiharu Komatsu, Eiji Tanaka
Abstract Non-alcoholic fatty liver disease (NAFLD) is a major chronic liver disease that can lead to liver cirrhosis, liver cancer, and ultimately death. NAFLD is pathologically classified as non-alcoholic fatty liver (NAFL) or non-alcoholic steatohepatitis (NASH) based on the existence of ballooned hepatocytes,although the states have been known to transform into each other. Moreover,since the detection of ballooned hepatocytes may be difficult with limited biopsied specimens, its clinical significance needs reconsideration. Repeated liver biopsy to assess histological NAFLD activity for therapeutic response is also impractical, creating the need for body fluid biomarkers and less invasive imaging modalities. Recent longitudinal observational studies have emphasized the importance of advanced fibrosis as a determinant of NAFLD outcome. Thus,identifying predictors of fibrosis progression and developing better screening methods will enable clinicians to isolate high-risk NAFLD patients requiring early intensive intervention. Despite the considerable heterogeneity of NAFLD with regard to underlying disease, patient age, and fibrosis stage, several clinical trials are underway to develop a first-in-class drug. In this review, we summarize the present status and future direction of NAFLD/NASH research towards solving unmet medical needs.
Key words: Non-alcoholic steatohepatitis; Fibrosis; Steatosis; Ballooning; Biomarker;Outcome; Treatment
INTRODUCTION
With the worldwide spread of sedentary lifestyle and diet westernization, the prevalence of non-alcoholic fatty liver disease (NAFLD) has increased in many countries among children and the elderly alike[1,2]. Approximately 25% of adults in the United States have fatty liver in the absence of excessive ethanol consumption. In Japan, roughly a third of individuals were found to have NAFLD in annual health checkups[3], translating to an estimated 20 million NAFLD patients. In China, fatty liver disease is increasing at a rate of 0.594% per year and is expected to afflict 20% of Chinese by 2020[4]. NAFLD is becoming the most common liver disease worldwide.
NAFLD was originally considered as non-progressive and fundamentally benign until Dr. Jurgen Ludwig, a pathologist at the Mayo Clinic, proposed the concept of non-alcoholic steatohepatitis (NASH) in 1980[5]. They observed that 20 patients without a drinking habit displayed histological findings similar to those in alcoholic steatohepatitis, such as fatty changes, focal hepatocyte necrosis, ballooned hepatocytes with Mallory-Denk inclusion bodies, lobular inflammation, and perisinusoidal/perivenular fibrosis. These patients frequently had diabetes,dyslipidemia, hypertension, and/or obesity. Thereafter, worldwide increases in obesity have led to a rapid spread of the concept of NASH, with many cases of fatty liver progressing to liver cirrhosis and hepatocellular carcinoma (HCC) being reported[6-8]. At present, NAFLD is classified into two categories according to liver pathology: non-alcoholic fatty liver (NAFL), also designated as simple steatosis or isolated steatosis, and NASH. Whereas NASH is defined as the presence of macrovesicular steatosis in addition to hepatocyte ballooning degeneration, lobular inflammation, and/or fibrosis, NAFL is characterized as macrovesicular steatosis without ballooned hepatocytes[9-12].
NAFLD is frequently associated with increased visceral adiposity (obesity) and ensuing metabolic abnormalities, including insulin resistance, diabetes, hypertension,dyslipidemia, atherosclerosis, and systemic micro-inflammation. A recent metaanalysis involving over 8.5 million individuals from 22 countries showed that more than 80% of NASH patients were overweight or obese, 72% had dyslipidemia, and 44% had type 2 diabetes mellitus[13]. Therefore, NAFLD can also be regarded as a hepatic manifestation of metabolic syndrome. Although it remains controversial whether NAFLD is a cause or a result of glucose intolerance and insulin resistance, a prospective study has demonstrated higher risks of diabetes and cardiovascular events in non-diabetic NAFLD patients than non-NAFLD ones[14]. Therefore, NAFLD is a detrimental condition necessitating appropriate interventions.
NAFLD also occurs in children and adolescents. The prevalence of NAFLD among junior high school students was estimated at approximately 4% in certain areas of Japan, and one student had obesity, diabetes, dyslipidemia, and NASH with mild fibrosis[15]. A study of more than 250000 Danish children showed that high body mass index (BMI) in childhood increased the risk of HCC in adulthood[16]. The above findings suggest that chronic obesity and NAFLD from childhood may produce a higher risk of liver fibrosis, cancer, and decompensation requiring liver transplantation at older ages. The economic burden of NAFLD in the United States is already enormous, with more than $100 billion in annual direct medical costs primarily for NASH and its sequelae[13].
NAFLD/NASH currently stands at the crossroads of gastroenterology,cardiovascular disease, endocrinology/metabolism, and oncology. The path of NAFLD/NASH research appears long and diverse, but progress on improved screening techniques and therapeutic agents is ongoing. In this review, we consolidate the broad clinical picture of NAFLD/NASH and outline the unresolved problems surrounding NAFLD/NASH research and treatment.
CURRENT STATUS OF NAFLD/NASH
Pathogenesis
Understanding the pathogenesis of NAFLD/NASH is essential to establish proper therapeutic interventions. However, disease development is so complicated that it has been designated as “multiple hit and organ theory”[17].
Mechanism of steatogenesis:An initial step in NAFLD onset is triacylglycerol (TAG)accumulation in hepatocytes. TAG is synthesized from fatty acid (FA) and glycerol.FA is usually absorbed from the circulation into the hepatocytes or produced from glucose in the liver via de novo lipogenesis. FA is catabolized primarily by β-oxidation in the mitochondria or peroxisomes, and excess amounts are converted into TAG and stored as lipid droplets in hepatocytes. The TAG in lipid droplets is hydrolyzed or secreted into the circulation as very-low-density lipoprotein particles. Disruption of those pathways can result in hepatosteatosis[18].
FA β-oxidation in hepatocytes is mainly regulated by the nuclear receptor peroxisome proliferator-activated receptor (PPAR) α. PPARα down-regulation has been associated with NAFLD/NASH[18]. Hyperglycemia enhances de novo lipogenesis,which is strongly regulated by insulin through activation of transcriptional factor sterol regulatory element-binding protein 1c (SREBP-1c). This mechanism may partially explain the close relationship between NAFLD/NASH and insulin resistance.
Dynamic changes in hepatocyte lipid droplets are also important considerations in the mechanism of hepatic steatosis. Indeed, hepatocyte-specific disruption of fatspecific protein 27 [FSP27, human cell death-inducing DFF45-like effector C (CIDEC)],a lipid-coating protein stabilizing TAG in lipid droplets, in ob/ob mice attenuates fatty liver through increased TAG hydrolysis and FA utilization[19].
As approximately 60% of FA in the liver originates from white adipose tissue[20],adipocyte dysfunction may lead to the FA overflow and NAFLD/NASH. Adipocytespecific FSP27-disrupted mice aggravate high-fat diet-induced hepatic steatosis because of impaired fat storage and enhanced lipolysis in white adipose tissue[21].Enhanced white adipose lipolysis to steatotic mice induced by choline-deficient diet promotes FA mobilization from adipose to liver and increases hepatic oxidative stress,leading to development of steatohepatitis[22]. Additionally, NAFLD/NASH is frequently accompanied in lipodystrophic patients[23]and humans having CIDEC mutation exhibit lipodystrophy, marked insulin resistance, and NASH with advanced fibrosis[24]. These findings indicate the importance of liver-adipose axis for the occurrence of NAFLD/NASH.
Mechanisms promoting hepatocyte injury and inflammation:TAG stored as lipid droplets are not strongly toxic to hepatocytes. Several studies have demonstrated the absence of a correlation between the degree of TAG accumulation and NAFLD severity, and hepatosteatosis is known to attenuate with fibrosis progression.Therefore, TAG precursors and intermediates, such as palmitate, diacylglycerol(DAG), and ceramide, are likely detrimental for hepatocytes. Palmitate increases oxidative and endoplasmic reticulum (ER) stress, leading to c-jun N-terminal kinase(JNK) activation and lipoapoptosis[25-27]. DAG activates protein kinase C and disrupts insulin signaling. Ceramide up-regulates the expression of SREBP-1c and promotes the production of palmitate[28]. Moreover, fat-rich cells are prone to lipid peroxidation,leading to mitochondrial and ER dysfunction. Increased free cholesterol also causes mitochondrial dysfunction and inflammasome activation[29]. Cytotoxicities mediated by these specific lipids (i.e., lipotoxicity) are one of the major causes of hepatocyte injury in NASH.
Damaged hepatocytes release several pro-inflammatory mediators that include damage-associated molecular patterns and pathogen-associated molecular patterns to recruit immune cells and activate Kupffer cells. Activated immune cells release bioactive molecules that further damage hepatocytes or render them more sensitive to various substances, such as microbiome-derived lipopolysaccharides and secondary bile acids as well as food contaminants from gut, thus amplifying cell death and inflammation[30,31].
Mechanisms of fibrogenesis:Damaged hepatocytes and activated immune cells also promote hepatic stellate cell (HSC) activation. During the normal repair/regeneration process, healthy hepatocytes occupy voids created by sporadic hepatocyte death.Chronic hepatocyte death or impaired hepatocyte regeneration leads to alternative replacement by fibers and extracellular matrix, causing significant scarring and remodeling of the normal architecture of hepatic lobules[32]. Although HSC activation is a key event in liver fibrogenesis regardless of etiology, fibrogenesis mainly in the perisinusoidal space is relatively specific to steatohepatitis.
Mechanism of hepatocarcinogenesis:Several epidemiological studies have revealed obesity and diabetes as risk factors for HCC. In the context of Asian populations, the impact of occult hepatitis B virus (HBV) infection should also be taken into consideration when discussing NAFLD-related HCC since HBV has carcinogenic properties due to its DNA integration[33]. Kimura et al[34]analyzed 77 Japanese patients with HCC who underwent surgical resection and were negative for serum anti-HBV core/surface antibodies, HBV surface antigen, and anti-hepatitis C virus (HCV)antibody. The NAFLD-related HCC subjects had a higher BMI and prevalence of diabetes, although 30%-40% had none-to-mild fibrosis in non-cancerous tissue.Multivariate analysis revealed that the presence of diabetes was associated with NAFLD-HCC with none-to-mild fibrosis. In agreement with other reports[8,35],NAFLD-HCC may occur not only in fibrotic/cirrhotic livers (i.e., through the classical inflammation-fibrosis-HCC sequence), but also from none-to-mild hepatic fibrosis even in the absence of past HBV infection. Although the mechanism of how obesity and diabetes influence hepatocarcinogenesis is not fully clarified, insulin resistance,increased circulating advanced glycation end products and ensuing disruption of cell proliferation signals, and genetic background including PNPLA3, TERT, and MBOAT may have prominent roles in HCC development[36].
Diagnosis
Diagnosis of NAFLD:NAFLD is often asymptomatic and detected only by abnormal liver function or imaging results in health checkups or during follow-up for other diseases. Patients with persistent elevation of serum aspartate aminotransferase (AST)and alanine aminotransferase (ALT) and fatty change on ultrasonography (US) or computed tomography (CT) without a history of habitual drug/ethanol intake or positive hepatitis virus markers or autoantibodies can be suspected as having NAFLD. NAFLD/NASH may also develop after gastrointestinal surgery, including pancreaticoduodenectomy and intestinal bypass[37,38]. Since some genetic diseases,including Wilson's disease, citrin deficiency[39-41], and cholesteryl ester storage disease[42], exhibit hepatic steatosis mimicking NAFLD, careful exclusion of these disorders is important. In cirrhotic NASH, serum AST/ALT levels and hepatic TAG accumulation are markedly reduced, such as in burned-out NASH, which may be diagnosed as cryptogenic liver cirrhosis[7,43]. It should be noted that normal ALT levels cannot exclude the possibility of NASH with advanced fibrosis; the combination of liver function testing with fibrosis markers and/or imaging modalities is indispensable for an accurate diagnosis.
Borderline between non-alcoholic and alcoholic status:The threshold of ethanol consumption amount for differentiating between NAFLD and alcoholic liver disease is problematic because the impact of ethanol on the liver differs among individuals with regards to race, sex, aldehyde dehydrogenase 2 gene polymorphisms, mode of drinking (binging or persistent small amounts), and lifestyle. The precise amount of ethanol intake is also sometimes hard to estimate. Some reports have shown that mild drinking attenuates hepatic steatosis, while in our cohort, NAFLD patients with a mild drinking habit (< 20 g/d) had a higher male prevalence, increased gammaglutamyl transpeptidase, and more frequent liver cirrhosis[44]. Furthermore, the occurrence rate of HCC in advanced fibrosis was higher in those patients. More attention is necessary on the impact of mild drinking on NAFLD outcome.
Evaluation of hepatic steatosis using imaging modalities:US is a simple method to detect fatty liver, but the lack of quantitative performance guidelines causes interobserver differences and complicates the monitoring of fat accumulation changes during interventions[45,46]. As calculated by non-enhanced abdominal CT liver/spleen Hounsfield unit (HU) and liver HU scores of < 40 or a liver/spleen HU ratio of < 0.9 indicates the presence of hepatic steatosis. Although CT has greater quantitative performance, objectivity, and reproducibility compared with US, it also carries the disadvantages of radiation exposure, cost, HU variability depending devices set-up,and inaccuracy from accompanying iron/copper depositions[45,46]. Magnetic resonance imaging (MRI) can quantify hepatic fat accumulation almost perfectly. The correlation coefficient between the area of lipid droplets in liver biopsy sections and the amount of fat estimated by MR is reportedly more than 0.9[47]. Although such equipment is prohibitively expensive for many primary care clinics and other facilities. The recently introduced Fibroscan®can quantify the degree of hepatic fat accumulation and fibrosis as controlled attenuation parameter (CAP) and E values, respectively.However, CAP values correlated with the area of lipid droplets in liver histology only in NAFLD patients with BMI < 28 kg/m2and none-to-mild fibrosis, suggesting limited applicability outside those parameters[48]. Further improvements in diagnostic performance are needed, especially for severely obese patients.
Clinical significance of liver biopsy and detection of ballooned hepatocytes:The discrimination of NASH from NAFL and assessment of the histological severity of NAFLD are routinely performed using pathological findings of the liver, but repeated liver biopsy is somewhat invasive, costly, and ultimately unrealistic. Sampling error and inter-rater discrepancies in pathological diagnosis are also problematic[12,49,50]. In the search for less invasive and more accurate methods to assess NAFLD pathology,several serum biomarkers to detect the presence of ballooned hepatocytes have been evaluated[51-55]. For instance, cytokeratin 18 (CK18) accumulates in ballooned hepatocytes with Mallory-Denk body-like inclusion bodies. Circulating CK18 fragment concentrations were significantly increased in NASH compared with NAFL and healthy controls and correlated with the incidence of ballooned hepatocytes and histological NAFLD activity score (NAS)[51].
Multiple studies have emphasized the importance of ballooned hepatocytes in NAFLD/NASH. The need to discriminate NASH from NAFL stems from the notion that the prognosis of the former (steatosis plus ballooned hepatocytes) is poorer than that of the latter (steatosis without ballooned hepatocytes). However, ballooned hepatocytes sometimes disappear and NASH may transform into NAFL and vice versa; some NAFL cases have progressed to liver cirrhosis presumably through NASH. We earlier described a NAFLD patient who underwent careful 27-year followup[7]. The patient was diagnosed as having NAFL at the first liver biopsy, which gradually progressed to cirrhosis and HCC over 20 years. This case teaches us that NAFL is not always benign. Moreover, HCC may develop from NAFL regardless of the absence of advanced fibrosis, past HBV infection, or regular ethanol consumption[34]. More importantly, recent studies have demonstrated that the presence of advanced fibrosis, but not ballooned hepatocytes, was a determinant of poor prognosis in NAFLD patients[56,57]. Taken together, it appears that the clinical significance of ballooned hepatocytes has given way to that of fibrosis in NAFLD/NASH.
Evaluation of liver fibrosis:Considering recent trends, the need for less invasive,more accurate methods of assessing liver fibrosis has produced several potential fibrosis indicators. Platelet count and serum levels of hyaluronic acid, type 4 collagen 7S, Mac2-binding protein, and autotaxin are promising biomarkers that predict advanced fibrosis (Table 1)[58-63]. Although those single indicators are simple,convenient, and useful for busy clinicians, it should be emphasized that results may be influenced by underlying conditions, such as co-existing collagen disease, systemic inflammation, and renal dysfunction. NAFLD fibrosis score, AST-to-platelet ratio index (APRI), FIB-4 index, BARD, CA index, ELF, and FibroTest have also been proposed as indices to predict advanced fibrosis in NAFLD patients (Table 2)[64-71]. ELF and FibroTest use direct markers of collagen synthesis and degradation, but such measurements are uncommon in clinical situations. In contrast, NAFLD fibrosis score,APRI, and FIB-4 exploit the biochemical test components of age, AST, ALT, glucose,BMI, platelets and albumin, all of which are routinely obtained in clinical practice.However, the scores of these indices tend to be increased in the elderly, and it is also unclear whether changes in AST, ALT, and BMI are correlated with the degree of actual fibrosis. For more global applicability, the cut-off values of single biomarkers and panels will require optimization according to country, race, sex, age, and other factors.
In addition to serum biochemical analysis, repeated quantification of the severity of liver fibrosis using imaging modalities is considered ideal for monitoring NAFLD progression and assessing therapeutic response. MR elastography has a significantly higher diagnostic accuracy than does transient elastography fort the detection of fibrosis stage[72]. Since US is more widespread than MRI, two-dimensional shear wave elastography might compensate for an inability for MR elastography at some institutions.
Treatment
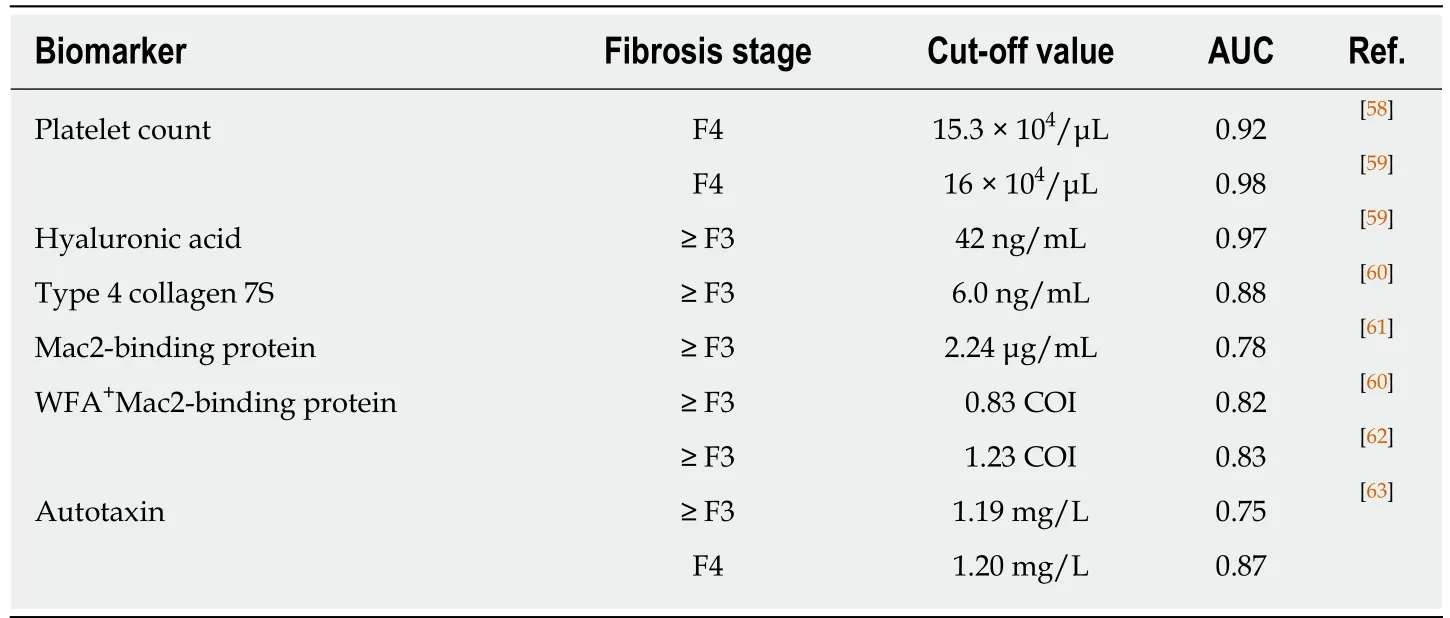
Table 1 Biomarkers predicting ≥ F3 fibrosis in Japanese non-alcoholic fatty liver disease patients
Body weight reduction:Since early-stage NAFLD/NASH is basically resolved by weight loss, lifestyle modifications geared towards weight reduction are routinely prescribed. Vilar-Gomez et al[73]analyzed NASH patients who received repeated biopsy and revealed that weight loss, the absence of diabetes, ALT normalization,young age, and baseline NAS ≤ 5 as independent predictors of NASH resolution without fibrosis worsening after 1-year of lifestyle intervention. Weight loss of 5% and 7%-10% attenuated steatosis and steatohepatitis, respectively[74,75]. However, it is sometimes difficult for diet and exercise regimens to achieve and maintain a 10%weight loss. To facilitate this, close multi-disciplinary cooperation between doctors(gastroenterologists, cardiologists, endocrinologists, etc.), nurses, dietitians, and exercise therapists is needed. In cases of morbid obesity with unsuccessful weight reduction, bariatric surgery is a promising option. Although it was documented that bariatric surgery can significantly improve NASH[76], its long-term safety and effectiveness remain under debate.
Pharmacological interventions for underlying disorders:Since NAFLD/NASH is accompanied by dyslipidemia, hyperglycemia, and insulin resistance, the correction of these disorders is beneficial for disease management. The therapeutic agents recommended by the Japan Society of Gastroenterology (JSG) and the Japan Society of Hepatology (JSH) are vitamin E, pioglitazone (for NAFLD/NASH with diabetes), and statin (for NAFLD/NASH with dyslipidemia)[77]. The American Association for the Study of Liver Disease (AASLD) guidelines 2017 have included these substances as well[78]. On the contrary, metformin and ursodeoxycholic acid are not recommended.Vitamin E is a lipid-soluble vitamin that scavenges free radicals to reduce oxidative stress in NAFLD/NASH livers. In PIVENS trials, vitamin E significantly improved NASH histology in non-diabetic and non-cirrhotic adult NASH patients compared with a placebo[79]. However, the safety of long-term, high-dose vitamin E treatment has not been confirmed. A PPARγ activator, pioglitazone increases circulating adiponectin and attenuates insulin resistance, steatosis, lobular inflammation, and fibrosis in diabetic/pre-diabetic NASH patients (ClinicalTrials.gov Identifier:NCT00994682)[80]. PPARγ agonist might also reduce HCC prevalence in diabetic patients[81], but fluid retention (edema, heart failure) and osteoporosis were observed as major adverse effects[82]. For all pharmacological agents, the balance of long-term benefits and risks along with improvements to minimize adverse effects remain a constant challenge.
Novel agents under clinical trials:Several promising agents undergoing clinical trials are listed in Table 3. Among them, obeticholic acid, elafibranor, selonsertib, and cenicriviroc are now in phase III trials[83]. It is noteworthy that these trials evaluate not only histological improvement of NASH, but also the benefit of long-term outcome for NASH patients, such as prevention of progression into cirrhosis, hepatic decompensation, and death.
Obeticholic acid is a potent whole-body farnesoid X receptor (FXR) agonist[18]. The drug improved necroinflammation without worsening fibrosis compared with a placebo in the large-scale FLINT trial of NASH patients[84]. At present, an international phase III trial is ongoing (REGENERATE study, NCT02548351). However, obeticholic acid significantly increased blood triglyceride and low-density-lipoprotein-cholesterol levels and decreased high-density-lipoprotein-cholesterol concentrations, whichmight raise the risk of cardiovascular diseases.
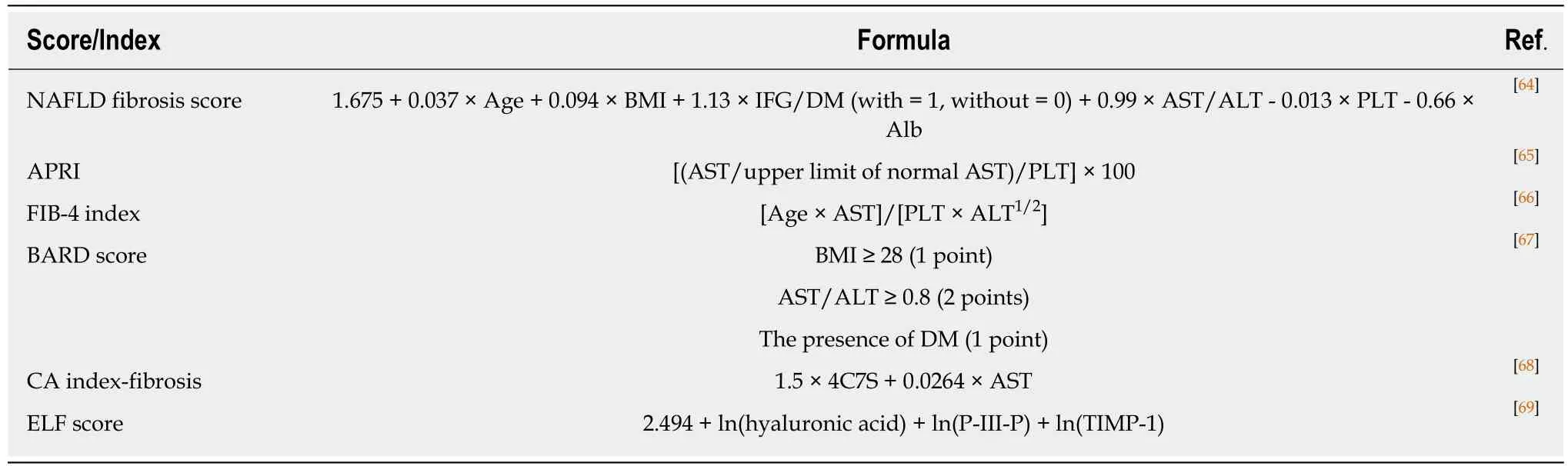
Table 2 Representative indices predicting ≥ F3 fibrosis in non-alcoholic fatty liver disease patients
Elafibranor (GFT-505) is a PPARα/δ dual agonist. While PPARα activation attenuates hepatic steatosis and inflammation, PPARδ stimulation can ameliorate hepatic inflammation and fibrosis[18]. In the GOLDEN-505 trial, 120 mg elafibranor resolved NASH and improved liver enzymes, glucose, and lipid profiles in larger proportions of NASH patients with NAS ≥ 4 compared with a placebo. Patients with NASH resolution after receiving the drug exhibited lower liver fibrosis stages compared with those without resolution[85]. A phase III trial verifying the effect of 120 mg elafibranor is underway for NASH patients with NAS ≥ 4 and stage 2/3 fibrosis(RESOLVE-IT study, NCT02704403).
Selonsertib (GS-4997) is an apoptosis signal-regulating kinase 1 (ASK1) inhibitor.ASK1 is activated by various stimuli, including hyperglycemia, transforming growth factor-β, and oxidative stimulus to induce apoptosis and fibrosis through p38 and JNK. Up-regulated ASK1-JNK1 axis aggravates insulin resistance, steatosis, and inflammation and further activates ASK1, resulting in a vicious cycle. In an animal NASH model, ASK1 inhibition reduced body weight along with hepatic fat and fibrosis and improved insulin resistance[86]. In a phase II study, selonsertib ameliorated NASH activity and fibrosis[87]. An international phase III trial is ongoing for stage 3 fibrosis and cirrhotic NASH patients (STELLAR3 study, NCT03053050 and STELLAR4 study, NCT03053063, respectively).
Lastly, cenicriviroc®is a C-C motif chemokine receptor (CCR) 2/5 antagonist. In a phase IIb trial (CENTAUR study), the agent attenuated liver fibrosis without worsening NASH compared with a placebo[88]. Currently, a phase III clinical trial evaluating the effect of cenicriviroc®for NASH patients with stage 2/3 fibrosis is underway (AURORA study, NCT03028740). Other clinical trials are listed in Table 3[89-93].
PROMBLEMS AND PERSPECTIVES IN NAFLD/NASH RESEARCH
Despite the dramatic gains in detection and treatment, there remain many unsolved issues in the field of NAFLD/NASH.
Pathogenesis
How animal data apply to humans? The pathogenesis of NASH/NAFLD is multifactorial and complicated. Still, reducing intracellular FA and free cholesterol while correcting obesity and insulin resistance may be fundamentally beneficial across species to attenuate NAFLD/NASH. Since NASH is both a metabolic disease and an inflammatory condition, strategies to inhibit inflammatory signaling and reduce oxidative and ER stress will be useful. However, lipid metabolism and immune mechanisms differ between rodents and humans, so any application of murine findings needs caution. Animal models that can precisely reproduce the human NASH condition are desired.
How do organs and cells crosstalk in NAFLD/NASH?Crosstalk among normal/steatotic hepatocytes, immune cells, HSCs and the organ network is anintriguing theme in the context of NAFLD/NASH pathogenesis. Especially,contribution of gut-liver axis and microbiota to NASH development is drawing much attention (Table 4)[94-96]. Although the merits of direct manipulation of the intestinal microbiota with antibiotics, prebiotics, or probiotics are debatable for human NAFLD/NASH due to the sheer diversity of microbiota, modulation of the gut-liver axis to target microbiota-derived metabolites is considered an attractive option. For instance, microbiota-derived deoxycholate evoked senescence-associated secretory phenotypes in HSCs and facilitated HCC development in hepatocarcinogen-primed genetically obese or high fat diet-fed mice[97]. Microbiota-derived taurocholate stimulated ceramide synthesis in enterocytes through FXR in mice, after which circulating ceramide promotes hepatic steatosis[28]. However, the applicability of these mechanisms to humans remains unclear. Recently, it was reported that microbiotaderived short-chain fatty acids lowered resting regulatory T-cells and associated with systemic T-cell activation in humans[96]. As such, discovering the pathogenic molecules that mediate organ/cell crosstalk and contribute to NAFLD/NASH development in humans will provide much needed insights into disease management[31,98].

Table 3 Novel therapeutic agents for non-alcoholic fatty liver disease/ non-alcoholic steatohepatitis under clinical trials
What should we learn from human genome analysis?Although a genome-wide association study clarified the genetic predisposition of NAFLD/NASH, the mechanism and functional changes by gene mutation/polymorphism require more precise assessment in genetically modified animals and human cells. Research is underway on the key genes involved in the development of NAFLD-related fibrosis and HCC.
Diagnosis
What is the simplest and most accurate surrogate marker of liver pathology in NAFLD/NASH?At present, NASH improvement is defined as the reduction of NAS and fibrosis stage as well as of scores for steatosis, ballooning, and lobular inflammation compared with baseline liver histology. There are several shortcomings to assessing NAFLD/NASH activity by liver biopsy only, the biggest of which is the impracticality of multiple procedures during follow-up. MRI can accurately quantify liver fat and stiffness, but is limited to large hospitals. Therefore, surrogate biomarkers that closely reflect liver pathology are needed for clinical trials and eventual adoption in monitoring clinical course and therapeutic response in realworld clinical situations. Moreover, the clinical significance of the conventional and widely-used biomarkers AST and ALT are in need of reconsideration in NAFLD/NASH.
How should we detect early-stage NAFLD/NASH?With the development of modern imaging modalities and biomarkers, it has become easier to detect advanced fibrosis stage of NAFLD/NASH. New and more powerful anti-fibrosis agents are on the horizon, but complete reversion from a cirrhotic liver to a soft one may prove difficult.Thus, strategies to detect early-stage NASH with mild-to-moderate fibrosis andprevent fibrosis progression are required as well, leading to preemptive and precise medicine.

Table 4 Dysbiosis in human non-alcoholic fatty liver disease/ non-alcoholic steatohepatitis
Who diagnoses and follows NAFLD/NASH?NAFLD/NASH has become a common liver disease worldwide. Many NAFLD/NASH patients are followed by clinicians other than hepatologists, such as by cardiologists, endocrinologists, and primary care doctors. Therefore, hepatologists should aim to establish clear and simple guidelines on strategies to find, diagnose, follow/assess, and treat NAFLD/NASH for nonspecialists.
Treatment
What is the goal of NAFLD/NASH treatment?The ultimate goal in NAFLD/NASH is the extension of overall survival and improvement of quality of life. Since the disease is frequently accompanied by obesity, atherosclerosis, and diabetes, not only hepatic complications (HCC, portal hypertension, and liver decompensation), but also extrahepatic conditions (cerebrocardiovascular disease, renal failure, and cancer)should be considered. Therapies will require careful adjustment according to underlying risk and NAFLD/NASH stage, and multi-disciplinary cooperation among caregivers will be key. Indicators of adequate disease control are currently lacking in NAFLD/NASH. In diabetic patients, the correction of hemoglobin A1c values prevents diabetic complications and improves outcome. Hepatologists require more of such indicators of NAFLD/NASH control[99].
Who should be treated intensively?It is important that NAFLD/NASH patients with advanced fibrosis should be intensively followed and treated due to the high risk of liver failure and HCC. The speed of fibrosis progression may differ among NAFLD/NASH patients, even in the early stage of fibrosis. Finding clinical determinants to detect rapid fibrosis candidates and high-risk HCC group at fibrosis stage 0-1 NAFLD/NASH will enable clinicians to better identify patients requiring careful monitoring and early intensive treatment[100-102].
How is the efficacy of pharmacotherapies improving?Although several new agents will be available in a near future[83,103-105], the efficacy of any single drug may vary widely for each patients. NAFLD/NASH is a complex syndrome, and its main pathogenesis likely differs among individuals; for example, hepatic lipid metabolism might depend on the underlying diseases (diabetes vs hypercholesterolemia) or fibrosis stage (stage 0-1 vs stage 3-4)[106]. Accordingly, patient stratification and careful selection of therapies, such as dual/triple agonists and combinations of several agents, may improve efficacy. In addition, enhancing target tissue/cell specificity (e.g.,adipose-specific PPARγ activators and intestine-specific FXR agonists/antagonists)will help achieve higher efficacy and reduced adverse effects[18].
CONCLUSION
Recent trends in diet and lifestyle have increased the prevalence of NAFLD/NASH worldwide. Although advances in non-invasive biomarkers and imaging modalities have improved disease detection and follow-up, considerable work is needed to identify individuals with low fibrosis stages or at risk of rapid disease progression. In the future, earlier detection will enable prompt single or combination treatment with new-line drugs that have been optimized for maximum benefit and fewer adverse events. Only with a concerted effort across multi-disciplinary fields can clinicians begin to halt the rapid spread of NAFLD/NASH.
ACKNOWLEDGMENTS
The authors thank the following collaborators for their invaluable helps, advice,instruction, and encouragement throughout our fatty liver disease studies: Dr. Kenji Sano, Dr. Wataru Okiyama, Dr. Goro Tsuruta, Dr. Hiroyuki Kitabatake, Prof.Masahide Yazaki, Dr. Yasunari Fujinaga, Dr. Akira Kobayashi, Dr. Takahiro Yamaura,Dr. Ayumi Sugiura, Dr. Tomoo Yamazaki, Dr. Satoru Joshita, Dr. Takeji Umemura,Dr. Tetsuya Ichijo, Dr. Akihiro Matsumoto, Dr. Kaname Yoshizawa, and Emeritus Prof. Kendo Kiyosawa (Shinshu University School of Medicine); Dr. Akira Horiuchi(Showa Inan General Hospital); Dr. Makoto Nakamuta (Kyushu Medical Center); Dr.Frank J. Gonzalez (National Institutes of Health); and Prof. Etsuko Hashimoto (Tokyo Women's Medical University). The authors also appreciate Mr. Trevor Ralph for his English editorial assistance.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of diet and gut microbiota on colorectal cancer immunomodulation
- Prevention of overuse: A view on upper gastrointestinal endoscopy
- Adverse events related to colonoscopy: Global trends and future challenges
- Human antigen R mediated post-transcriptional regulation of inhibitors of apoptosis proteins in pancreatic cancer
- Six-long non-coding RNA signature predicts recurrence-free survival in hepatocellular carcinoma
- Biomarker identification and trans-regulatory network analyses in esophageal adenocarcinoma and Barrett's esophagus
