Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters
2019-01-18YinanZhangYiqiangLiuJiZhangXiaojiangWuXinJiTaoFuZiyuLiQiWuZhaodeBuJiafuJi
Yinan Zhang, Yiqiang Liu, Ji Zhang, Xiaojiang Wu, Xin Ji, Tao Fu, Ziyu Li, Qi Wu,Zhaode Bu, Jiafu Ji
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), 1Gastrointestinal Cancer Center; 2Department of Pathology; 3Endoscopy Center, Peking University Cancer Hospital & Institute, Beijing 100142, China
Abstract Objective: To create a nomogram to predict the incidence of lymph node metastasis (LNM) in early gastric cancer (EGC) patients and to externally validate the nomogram.Methods: To construct the nomogram, we retrospectively analyzed a primary cohort of 272 EGC patients.Univariate analysis and a binary logistic regression were performed. A nomogram predicting the incidence of LNM in EGC patients was created. The discrimination ability of the nomogram was measured using the concordance index (c-index), and the nomogram was also calibrated. Then, another prospective cohort of 81 patients was analyzed to validate the nomogram.Results: In the primary cohort, LNM was pathologically confirmed in 37 (13.6%) patients. In multivariate analysis, the presence of an ulcer, the maximum lesion diameter observed via gastroscopy, the thickness of the lesion observed via endoscopic ultrasonography, and the presence of enlarged lymph nodes on computed tomography (CT) were independent risk factors for LNM. A nomogram was then created based on the regression model with the c-index of 0.905, and the calibration curve of the nomogram fell approximately on the ideal 45-degree line. The cut-off score of the nomogram was 110, and the sensitivity, specificity, positive predictive and negative predictive values of the nomogram in the primary cohort were 81.1%, 86.0%, 47.6% and 96.7%,respectively, and in the prospective validation cohort were 75.0%, 91.0%, 60.0% and 95.5%, respectively. The calibration curve of the external validation cohort was almost on the 45-degree line.Conclusions: We developed an effective nomogram predicting the incidence of LNM for EGC patients.
Keywords: Early gastric cancer; lymph node metastasis; nomogram; validation
Introduction
According to the Japanese Gastric Cancer Association,early gastric cancer (EGC) is defined by lesions in the stomach that are confined to the mucosa and/or submucosa, regardless of size or lymph node metastasis(LNM) status (1). The curative treatment is radical gastrectomy with regional lymph node dissection because lymph node metastases are found in 2% to 18% of EGC patients (2,3). EGC has a much better prognosis than advanced stages of gastric cancer, and the 5-year survival rate exceeds 95% with proper treatment (4). Previous studies have shown that regional LNM is the most significant prognostic factor in EGC (5); thus, it is the most important consideration when treating EGC patients.
Tumor size, histologic type, the depth of invasion, the presence of an ulcer, and lymphatic invasion are risk factors for LNM in EGC as reported in previous studies (6).However, most of these studies were based on postoperative findings, especially pathology results, which are not available when attempting to predict the likelihood of LNM before surgery. With the development and universal use of preoperative examination methods, such as gastroscopy, endoscopic ultrasonography (EUS) and computed tomography (CT), surgeons may be able to obtain additional data on the lesion and regional lymph node status before surgery. A nomogram is a device or model that uses an algorithm or mathematical formula composed of several variables to predict the probability of an event or outcome (7), and such tools have been used in several types of cancer, such as breast cancer and prostate cancer (8,9). This study focuses on preoperative clinical data and examination results and their relationship to LNM in EGC to develop a predictive nomogram.
Materials and methods
Patients
We retrospectively analyzed 272 patients at Peking University Cancer Hospital between November 2010 and November 2015 as the primary cohort for the construction of a nomogram. Eligible patients were: being treated for the first time, underwent radical gastrectomy plus standard D1+/D2 lymph node dissection, had pathologically confirmed adenocarcinoma and EGC, and underwent complete preoperative examinations, including gastroscopy,EUS, CT and biopsy during gastroscopy. The exclusion criteria were as follows: 1) patients with neoadjuvant therapy; 2) previous history of cancer; 3) two or more sites of primary gastric cancer; 4) Siewert type I gastroesophageal junction carcinoma; or 5) distant metastasis. A cohort of 81 patients was recruited from December 2015 to July 2016 using the same inclusion and exclusion criteria as the primary cohort and prospectively analyzed to validate the nomogram. This study was approved by the Institutional Review Board of the Beijing Cancer Hospital,and informed consent was obtained from all individuals.
Parameters
The clinical characteristics of the patients, including gender and age, were collected. Gastroscopy data were gathered from the report. These data included the location of the lesion, the size of the tumor, the macroscopic type and the presence of ulcers. The location of the tumor was described in both the vertical and the horizontal planes. In the vertical plane, tumors were classified according to their location in the esophagogastric junction (EGJ) or upper(U), middle (M), or lower (L) portion of the stomach. In the cross-sectional plane, tumors were classified according to their location in the lesser (Less) or greater (Gre)curvature and the anterior (Ant) or posterior (Post) wall, as outlined in the Japanese classification of gastric carcinoma(3rd edition). The maximum diameter of the lesion measured during gastroscopy was recorded as the size of the tumor on endoscopy. The patients were categorized into two groups based on the macroscopic appearance of their tumors: those with non-depressed tumors and those with depressed and advanced tumors. Non-depressed tumors were those that were classified as protruding (I),superficially elevated (IIa), flat (IIb), or superficially depressed (IIc). The depressed and advanced tumor group included those with depressed-type tumors (III) and advanced gastric cancer; certain EGCs were macroscopically similar to advanced gastric cancer. The presence of an ulcer was defined by lesions with ulceration or scarring from previous ulceration (converging folds or deformity of the muscularis propria or fibrosis in the submucosal or deeper layer). The EUS parameters included the maximum diameter of the lesion, the depth or thickness of the tumor, the presence of metastatic lymph nodes (N stage), and in cases of LNM, the maximum diameter of the lymph nodes. The CT parameters, such as the vertical location of the tumor (EGJ, U, M, L), the thickness of the lesion, the presence and maximum diameter of enlarged lymph nodes, and the presence of ulceration were gathered from the reports and confirmed by two independent radiologists. All the patients had pathologically confirmed gastric cancer based on biopsy,and the cancer was categorized into differentiated (well and moderately differentiated tubular adenocarcinomas and papillary adenocarcinomas) or undifferentiated types(poorly differentiated adenocarcinomas and signet-ring cell carcinomas). All these parameters were defined in accordance with the Japanese classification of gastric carcinoma (3rd edition).
Statistical analysis
The statistical analyses were performed and graphs were constructed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA) and R software (Version 3.2.1; R Foundation for Statistical Computing, Vienna, Austria).For continuous variables, a test of normality was first performed. For normally distributed variables, differences between groups were analyzed using Student’s t test, and for non-normally distributed variables, the rank sum test was used. The Chi-squared test and Fisher’s exact test(when appropriate) were used for comparisons of categorical variables. Significant factors noted on univariate analysis were subsequently entered into a multivariate logistic regression model for analysis. Two-sided P<0.05 were considered statistically significant. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the predictive accuracy of the logistic model. Meaningful predictive factors from the multivariate logistic regression were used to formulate a nomogram to estimate the risk of LNM in EGC. The internal validation of the nomogram included two steps. First, a concordance index (c-index) was calculated using the bootstrap resampling method to assess the discriminative ability of the nomogram; the c-index measures the model’s ability to differentiate patients with different outcomes, which is similar to the AUC of a ROC curve. The second step was calibration, which was performed by reviewing plots of predicted probabilities from the nomogram versus the actual probabilities. The ideal nomogram with 100%accuracy would be a graph in which the observed and predicted probabilities fall along the 45-degree line (10,11).In the external validation of the nomogram, the incidence of LNM for each of the 81 patients in the validation cohort was estimated based on the constructed nomogram, and another calibration curve was plotted to illustrate the actual predictive ability of the nomogram. The cut-off score of the nomogram was determined by the Youden index, and the sensitivity, specificity, positive predictive value, and negative predictive value of the nomogram were calculated for the two cohorts.
Results
A total of 272 patients in the primary cohort were involved in this study. The median age was 58 (range, 18-81) years and 182 (66.9%) of the patients were male. There were 37 (13.6%) patients pathologically confirmed to have LNM, including 22 (8.1%) patients with stage N1, 9(3.3%) with stage N2 and 6 (2.2%) with stage N3 according to the Japanese classification of gastric carcinoma(3rd edition). The mean numbers of metastatic lymph nodes and the number of dissected lymph nodes were 3(range, 1-13) and 25 (range, 8-64), respectively. Tumors were limited to the mucosal layer (T1a) in 128 (47.1%)patients, whereas the tumors invaded the submucosal layer(T1b) in 144 (52.9%) patients, and the percentages of lymph node positivity in these two groups of patients were 4.7% and 21.5%, respectively.
Detailed information on the distributions of categorical and continuous variables is shown in Tables 1, 2. The gastroscopy results showed that LNM was associated with circumferential lesions in the horizontal plane, a depressed and advanced macroscopic tumor type, the presence of an ulcer and the maximum diameter of the tumor. For the EUS parameters, the depth of invasion (T1b stage), N stage, maximum diameter, and thickness of the lesion, as well as the maximum diameter of the detected lymph nodes, were associated with LNM. The detection of enlarged lymph nodes on CT, the maximum diameter of these lymph nodes and the thickness of the lesion were risk factors for LNM in univariate analysis. The differentiated biopsy type obtained during gastroscopy was not associated with LNM.
Parameters that were significant in the univariate analysis were included in the multivariate logistic regression analysis except for the horizontal tumor location on gastroscopy (circumferential lesion or not) because circumferential lesions were rarely observed in EGC and because the 3 suspected circumferential lesions in our sample population were eventually confirmed to be much smaller and confined to only one quarter of the stomach wall on postoperative pathology. The results of the multivariate logistic regression analysis are shown in Table 3. The presence of an ulcer (95% CI, 2.422-18.877)and the maximum diameter of the lesion (95% CI,1.363-2.818) on gastroscopy, the thickness of the lesion on EUS (95% CI, 1.116-1.418) and the presence of enlarged lymph nodes on CT (95% CI, 1.129-6.657) were independent risk factors for LNM in EGC. An ROC curve was constructed to assess the predictive value of the regression model, which showed satisfactory predictive ability with an AUC of 0.905 (Figure 1).
The four factors that were found to be significant in themultivariate analysis were used to construct the nomogram,as shown in Figure 2. The first row (“Points”) is used to assign a score for each variable below the first row by drawing a vertical line from the value for each variable to the “Points” line. The “Total points” are calculated by summing the scores for all the variables, and the final predicted risk of LNM for each patient is obtained by drawing a vertical line from the “Total points” line to the
“Risk” line.
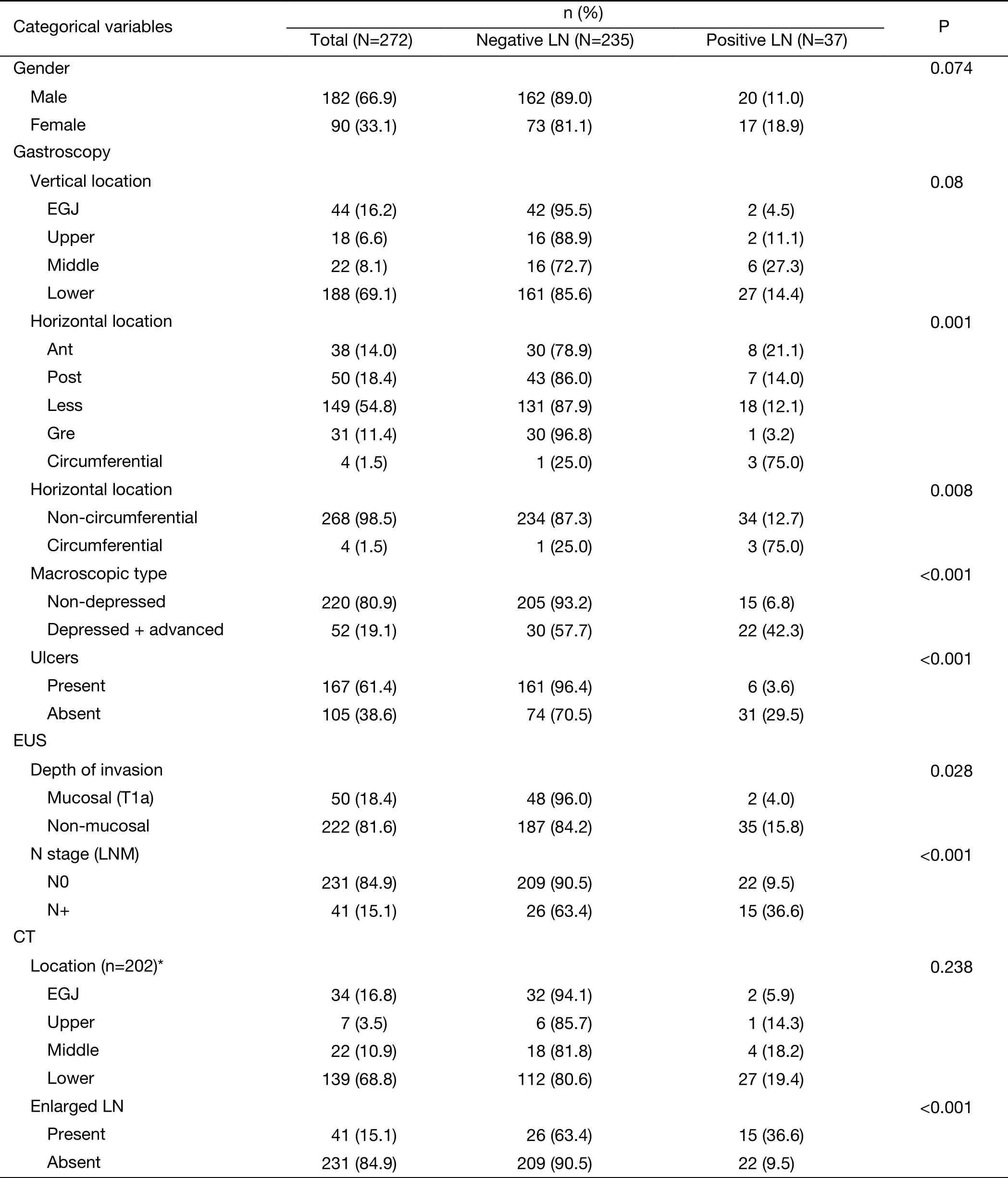
Table 1 Univariate analysis of categorical variables
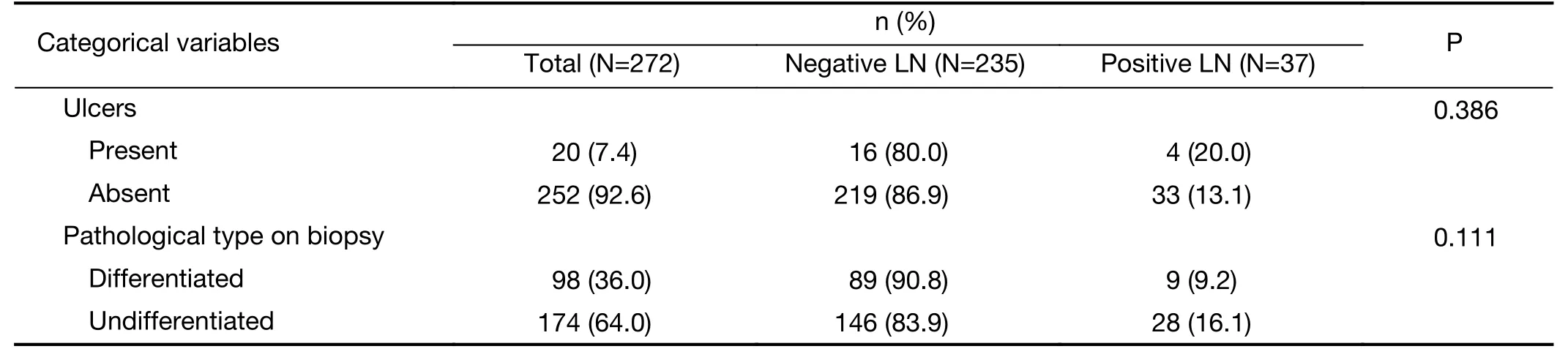
Table 1 (continued)
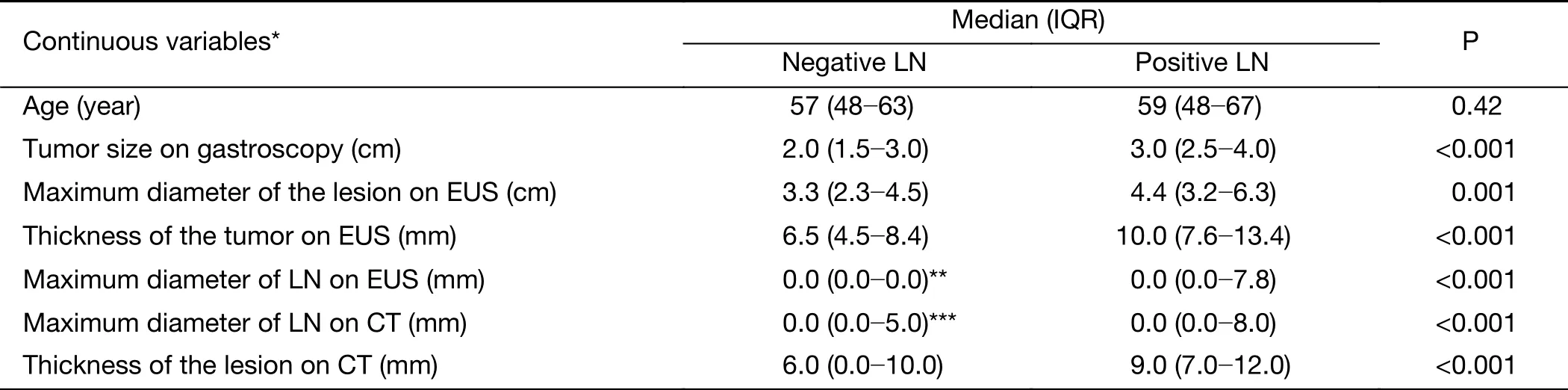
Table 2 Univariate analysis of continuous variables

Table 3 Multivariate analysis of significant factors on univariate analysis by logistic regression model
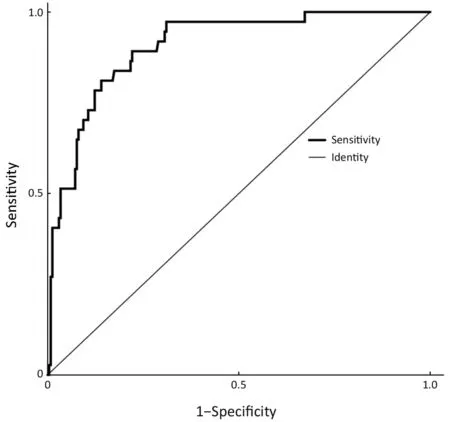
Figure 1 Receiver operating characteristic (ROC) curve. The ROC curve for the prediction of lymph node metastasis based on the logistic regression model had an area under the curve (AUC)value of 0.905, which showed satisfactory accuracy in predicting positive lymph nodes.
The cut-off score of the nomogram was 110, as determined by the Youden index. In the primary cohort,the sensitivity, specificity, positive predictive and negative predictive values of the nomogram were 81.1%, 86.0%,47.6% and 96.7%, respectively.
The internal validation of this nomogram involved two components. Discrimination was quantified with the cindex, and the value of 0.905 suggested that the constructed nomogram had a high accuracy in discriminating the patients’ lymph node status. The second component was calibration, which compared the predicted probability of LNM with the actual probability, as shown on the calibration curve in Figure 3. External validation was performed with the validation cohort. Another calibration curve was plotted for the validation cohort, and the sensitivity, specificity, positive predictive and negative predictive values of the nomogram in this cohort were 75.0%, 91.0%, 60.0% and 95.5% respectively.
Discussion
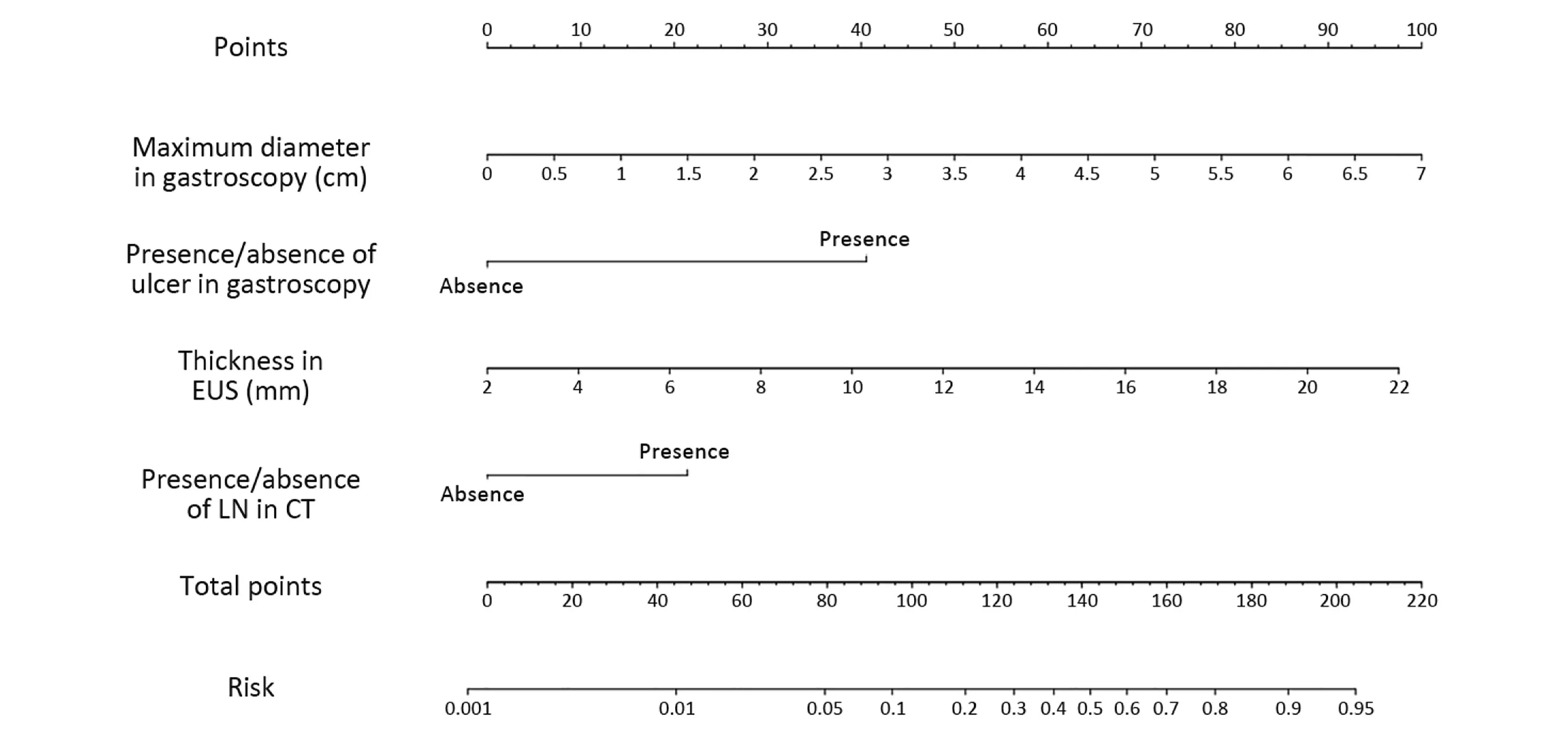
Figure 2 Nomogram predicting lymph node metastasis (LNM) in early gastric cancer (EGC). The nomogram includes four parameters: the maximum diameter of the tumor on gastroscopy, the presence or absence of an ulcer on gastroscopy, the thickness of the lesion measured by endoscopic ultrasonography (EUS), and the presence or absence of LNs on computed tomography (CT); each parameter has its own scale.For any given patient, the four parameters can be obtained from preoperative examinations, and the corresponding value can be found on the scale. By projecting these values vertically to the first line (“Points”) and adding the corresponding point values, physicians can calculate the total number of points and project a vertical line from the “Total points” line to the “Risk” line to obtain the predicted probability of LNM.
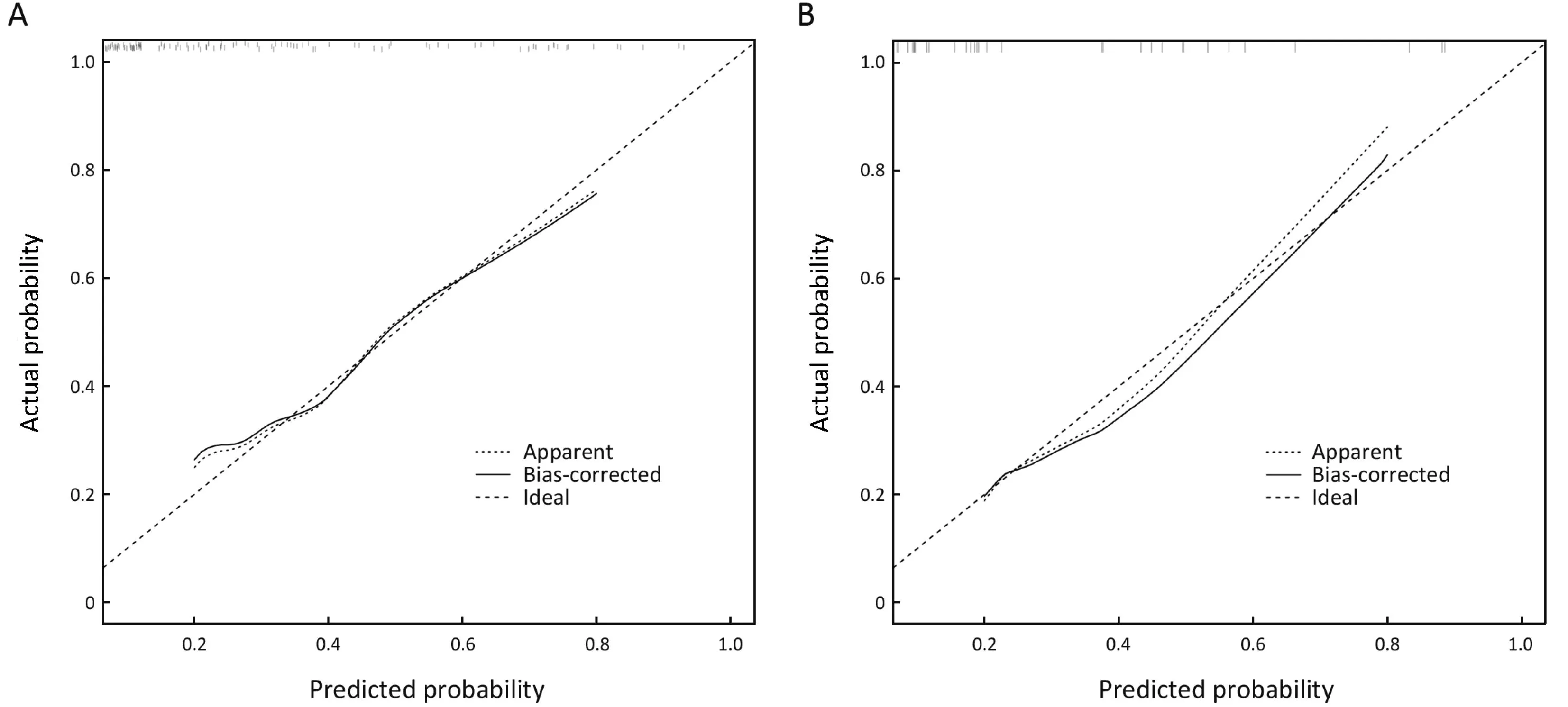
Figure 3 Calibration of the nomogram. (A) Primary cohort; (B) Validation cohort. The X-axis represents the probability of lymph node metastasis (LNM) as predicted by the nomogram, and the Y-axis shows the actual probability. The 45-degree long-dashed line represents an ideal nomogram whose predicted outcome perfectly corresponds to the actual outcome. The solid line represents the bootstrap-corrected performance of our nomogram, and the short-dashed line represents apparent accuracy of the constructed nomogram. The apparent and bias-corrected line fell approximately along the ideal line, which indicates that the probability calculated by the nomogram accurately represents the actual risk of LNM for early gastric cancer (EGC) in both the primary and validation cohorts.
Gastric cancer is one of the most common cancers worldwide, especially in China, where over 423 thousand new cases were observed and nearly 300 thousand deaths were caused by gastric cancer in 2011 (12). EGC is defined as gastric cancer with tumor invasion limited to the mucosa or submucosa, regardless of the presence of LNM (3).Current treatment methods include minimally invasive endoscopic surgery, wedge resection, and gastrectomy with regional lymph node dissection laparoscopically or by open laparotomy (13). Endoscopic procedures, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), allow for the resection of the lesion and preservation of stomach function but leave regional lymph nodes undissected and it should therefore only be conducted when the likelihood of LNM is extremely low and the site and size of the lesion are amenable to en bloc resection (14,15). Patients who underwent curative ESD as an absolute indication had a favorable 5-year survival that was not significantly different from that of patients who had laparoscopic or open surgery;thus, ESD could be employed as a standard treatment for EGC lesions in selected patients (16,17). For EGC patients, LNM is the most important prognostic factor, and predicting the risk of LNM is crucial when selecting treatment methods (18).
By retrospectively reviewing 3,131 ECG patients,Sekiguchi et al. demonstrated that tumor size, histological type, presence of ulcerative finding and presence of lymphovascular involvement were independent risk factors of LNM (19). Other studies also revealed that overexpression of CD44v6, increased tumor markers[carcinoembryonic antigen (CEA), carbohydrate antigen(CA) 19-9, CA125], gender, age at diagnosis were also associated with LNM (20-23). However, most of these risk factors mentioned above were postoperative findings which were not available before treatment. Although some predictive models were constructed based on postoperative findings, the usefulness of these models were limited. In this research, the author only focused on the relationship of preoperative parameters and LNM in order to construct a true predictive model before treatment.
The gastric cancer guidelines of the National Comprehensive Cancer Network (NCCN) recommend upper gastrointestinal endoscopy with biopsy, abdomen and pelvis CT, and EUS as preoperative examinations for gastric cancer patients (24). However, none of these examinations alone could exactly estimate LNM. In this study, 2 of the 37 (5.4%) patients with positive lymph nodes met the criteria for ESD but were pathologically confirmed to have LNM, which indicated that endoscopy features alone were not reliable predicting LNM. As for CT, the presence and size of lymph nodes are measured routinely during CT scanning, but it is still difficult to distinguish metastatic lymph nodes because most metastatic lymph nodes are less than 10 mm, and only a small proportion of the patients with LNM exhibited an metastatic lymph node as the largest (25). In this study, 202 patients presented lymph nodes smaller than 5 mm,including 15 patients (7.4%) who had positive lymph nodes suggesting that preoperative CT scans alone might not be sufficiently accurate to predict lymph node status based on the presence or size of lymph nodes. EUS has been used for T staging of gastric cancer and is considered the best available method for the assessment of invasion depth which is one of the risk factors of LNM (26). However, in a Cochrane review, Mocellin et al. concluded that EUS was not sufficiently accurate to confirm or exclude the presence of LNM (27). Lee et al. also reported that the accuracy of EUS was similar to that of traditional endoscopy in the assessment of the depth of tumor invasion (73.6% and 66.7%, respectively), and proper treatment selection based on EUS occurred in 71.5% of cases, which is even lower than with endoscopy (75.3%) (28). In this study, the accuracy of EUS in the assessments of T1a status was 61.0% (166/272), which could explain the thickness of lesions rather than T1a/T1b was an independent risk factor of LNM. In previous studies, the differentiation type was associated with LNM in EGC, thus making it one of the criteria for ESD/EMR (29). However, Nakagawa et al.reported that preoperative histology was not a significant predictor of LNM for several reasons. One of the characteristics of gastric cancer is histological heterogeneity,and the limited amount of tissue collected by biopsy cannot always represent the dominant histology type of the tumor(30). The frequency of discrepancies in the histology between preoperative biopsy and postoperative pathology ranges from 16.3% to 53.7% (31,32). In this study, the accuracy of preoperative biopsy was 86.0%, which explained why the differentiation type on preoperative biopsy was not a significant predictor of LNM.
Nomogram is a graphic tool for individual probability of a clinical event based on a statistical predictive model. The advantage of nomogram is that it takes several risk factors into consideration when calculating the probability and it is practical because the nomogram directly presents the score and risk of certain clinical outcomes. Our study provides an effective tool for the selection of true lymph node-negative patients because of its high specificity and negative predictive value. Combining the nomogram presented here with other criteria such as the sentinel lymph node technique might represent a promising approach for predicting LNM and could provide the basis for organpreserving gastrectomy, which is the next step of research in our center.
This research has certain limitations. It is an investigational study to propose a potential formula for predicting LNM in EGC. More efforts should be done before clinical application. The nomogram was constructed using a retrospective cohort, and systemic bias was inevitable because many of the patients with mucosal tumors underwent endoscopic surgery and were not included in the study, thus making the proportion of T1a tumors lower than that in the general population.
Conclusions
Our study revealed that the presence of ulceration and the maximum lesion diameter on gastroscopy, the thickness of the lesion on EUS and the presence of enlarged lymph nodes on CT were independent risk factors for LNM in EGC. A nomogram that can estimate the risk of LNM for EGC patients was created based on these risk factors.Patients who had a nomogram score of less than or greater than 110 were considered to have a low or high risk for LNM, respectively. This nomogram should provide more accurate information for surgeons treating EGC patients in their clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- BRAF inhibitor: a novel therapy for ameloblastoma in mandible
- Tumor pyruvate kinase M2: A promising molecular target of gastrointestinal cancer
- Novel circular RNA expression profile of uveal melanoma revealed by microarray
- Limited energy parametrial resection/dissection during modified laparoscopic nerve-sparing radical hysterectomy
- Identification of liver metastasis-associated genes in human colon carcinoma by mRNA profiling
- Voltage-gated K+ channels promote BT-474 breast cancer cell migration
