Voltage-gated K+ channels promote BT-474 breast cancer cell migration
2019-01-18LouisWCChowKaShunChengKarLokWongYukManLeung
Louis WC Chow, Ka-Shun Cheng, Kar-Lok Wong, Yuk-Man Leung
1State Key Laboratory of Quality Research in Chinese Medicines, Macau University of Science and Technology, Taipa, Macau 999078, China;2UNIMED Medical Institute, Hong Kong 999077, China; 3Organisation for Oncology and Translational Research, Hong Kong 999077, China;4Department of Anesthesiology, China Medical University Hospital, Taichung 40447, Taiwan, China; 5Department of Anesthesiology, the Qingdao University Yuhuangding Hospital, Yantai 264000, China; 6Department of Physiology, China Medical University, Taichung 40402, Taiwan, China
Abstract Objective: A variety of ion channels have been implicated in breast cancer proliferation and metastasis. Voltagegated K+ (Kv) channels not only cause repolarization in excitable cells, but are also involved in multiple cellular functions in non-excitable cells. In this study we investigated the role of Kv channels in migration of BT474 breast cancer cells.Methods: Transwell technique was used to separate migratory cells from non-migratory ones and these two groups of cells were subject to electrophysiological examinations and microfluorimetric measurements for cytosolic Ca2+. Cell migration was examined in the absence or presence of Kv channel blockers.Results: When compared with non-migratory cells, migratory cells had much higher Kv current densities, but rather unexpectedly, more depolarized membrane potential and reduced Ca2+ influx. Reverse transcriptasepolymerase chain reaction (RT-PCR) analysis revealed the presence of Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv3.3, Kv3.4 and Kv4.3 channels. Cell migration was markedly inhibited by tetraethylammonium (TEA), a delayed rectifier Kv channel blocker, but not by 4-aminopyridine, an A-type Kv channel blocker.Conclusions: Taken together, our results show that increased Kv channel expression played a role in BT474 cell migration, and Kv channels could be considered as biomarkers or potential therapeutic targets for breast cancer metastasis. The mechanism(s) by which Kv channels enhanced migration appeared unrelated to membrane hyperpolarization and Ca2+ influx.
Keywords: Breast cancer; Kv channels; migration; biomarkers
Introduction
A number of ion channels have been proposed as biomarkers or functional molecules in breast cancer metastasis. For instance, voltage-gated Na+channel(VGSC) Nav1.5 is up-regulated in metastatic breast cancer tissue, and has been postulated as a biomarker and therapeutic targets for breast cancer metastasis (1). How exactly Na+influx leads to metastasis is unclear, but a report has described that the enhanced invasiveness is due to H+efflux via Na+-H+exchanger, which is in turn stimulated by Na+influx via Nav1.5 (2). Another candidate attracting recent attention is the voltage-gated proton channel (Hv1). The latter allows H+to flow out of the cells,and thus an acidic environment is created to facilitate the actions of several enzymes important in cancer cell invasiveness and migration, such as matrix metalloproteinases (3,4). Novel blockers of Hv1 may be useful in inhibiting cancer metastasis (5).
As Ca2+is an important stimulator for cell mobility, Ca2+influx is believed to be one of the essential motor forces in breast cancer migration. Of note, stromal interaction molecule 1 (STIM1) and Orai1, two protein components of store-operated Ca2+channel, have been implicated in breast cancer migration (6). In addition, transient receptor potential channel M7 (TRPM7), a Ca2+-permeable nonselective cation channel-kinase, has also been implicated in cancer cell adhesion and migration, as high TRPM7 expression is associated with poor prognosis in breast cancer patients (7,8). Further, this molecule is required for metastasis formation in a mouse xenograft model of human breast cancer. Findings suggest that TRPM7 is a component of a mechanosensory complex to drive breast cancer cell metastasis (9,10).
Voltage-gated K+(Kv) channels open upon depolarization and allow K+efflux to repolarize excitable cells (11,12). Depending on sequence homology, Kv channels are classified into Kv1 to Kv12 subfamilies with each Kv subfamily having multiple subtype members (13).Kv1.3, Kv4.1, Kv10.1 and Kv11.1 are present in breast cancer cells (14). Of interest, Kv channels have been implicated in breast cancer migration (15). The mechanism is unknown until Hammadi et al. obtained data showing that blocking or silencing hEag1 (Kv10.1) depolarized breast cancer MDA-MB-231 cells, reducing Ca2+entry (via Orai1-associated channel), and eventually inhibiting cell migration without affecting cell proliferation (16). Thus,hEag1 is essential in maintaining a negative potential favorable for Ca2+entry, which is important in cell motility.In this report, Kv channel currents were found to be much higher in migratory than non-migratory breast cancer BT474 cells; blockade of Kv currents by tetraethylammonium (TEA) suppressed cell migration. In contrast to the reported case in MDA-MB-231 cells, migratory BT474 cells had more depolarized membrane potential and reduced Ca2+entry. Alternative models to explain the roles of Kv channels in migration will be discussed.
Materials and methods
Cell culture
BT474 cells were cultured at 37 °C in 5% CO2in Dulbecco’s modified Eagle’s medium (DMEM)supplemented with 10% fetal bovine serum (FBS;Invitrogen, Carlsbad, CA, USA) and penicillinstreptomycin (100 U/mL, 100 μg/mL) (Invitrogen).
Separation of migratory cells from non-migratory cells
BT474 cells (3×105) were seeded in the upper chambers of the Transwell (Corning 3428, 24 mm) and allowed to migrate through the porous (8 μm) membrane for 3 d. The upper chamber medium was serum-free whilst the lower chambers contained 10% FBS as a chemoattractant (17,18).We then separated the non-migratory cells from the migratory cells in the following manner: non-migratory cells in the upper chamber were trypsinized and seeded on culture plates, while migratory cells trapped in the membrane were trypsinized, detached and seeded on separate cultures plates. The non-migratory cells and migratory cells were then allowed to settle on their culture plates for 5 h and then subject to electrophysiological recording for Kv currents or to microfluorimetric measurements.
Migration assay
BT474 cells (3×105) were seeded on the upper chamber of the Transwell (Corning 3428, 24 mm) and incubated for 3 d in the absence or presence of pharmacological agents.The cells were allowed to migrate through the porous(8 μm) membrane for 3 d. The upper chamber medium was serum-free whilst the lower chambers contained 10% FBS as a chemoattractant (17,18). After 3 d, the upper chambers were washed thoroughly and the cells in the porous membrane were stained with crystal violet. Five random views of each sample were photographed and the number of cells was counted. The number of cells in treatment groups was normalized with those in the control group and expressed as % control.
Electrophysiology
Electrophysiological experiments were performed as previously reported (19). Cells were voltage-clamped in the whole-cell configuration. Thin-walled borosilicate glass tubes (o.d. 1.5 mm, i.d. 1.10 mm, Sutter Instrument,Novato, CA) were pulled with a micropipette puller (P-87,Sutter Instrument), and then heat polished by a microforge(Narishige Instruments, Inc., Sarasota, FL, USA). The pipettes, filled with intracellular solution, containing(mmol/L): 140 KCl, 1 MgCl2, 1 EGTA, 10 HEPES, and 5 MgATP (pH 7.25 adjusted with KOH), had typical resistance of 4-7 MΩ. The bath solution contained(mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2,10 HEPES (pH 7.4 adjusted with NaOH). The currents were recorded using an EPC-10 amplifier with Pulse 8.60 acquisition software and analyzed by Pulsefit 8.60 software (HEKA Electronik, Lambrecht, Germany).Data were filtered at 2 kHz and sampled at 10 kHz. After a whole-cell configuration was established, the cells were held at -70 mV and subject to various protocols as detailed in the Protocol. All experiments were performed at room temperature (25 °C).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA of BT474 cells was isolated by RNA Isolater(Vazyme, Nanjing, China), precipitated with iso-propanol,washed with 70% ethanol and finally dissolved with nucleic acid stabilized solution (Topgen Biotech., Co., Ltd,Taiwan, China). Total RNA was determined by EpochTMSpectrophotometer System (Bio Tek Instruments,Winooski, VT, USA). Genomic DNA was removed using 4× gDNA wiper Mix at 42 °C for 15 min. Reverse transcription of 1 μg of total RNA to cDNA was performed using HiScript II 1st Strand cDNA Synthesis Kit (Vazyme),and cDNA synthesis was performed at 25 °C for 5 min,50 °C for 15 min and 85 °C for 2 min on ABI PCR system VeritiTM96-Well Thermal Cycler (Applied Biosystems,Waltham, USA). Qualitative analysis of 12 target genes,KCNA1 (Kv1.1), KCNA2 (Kv1.2), KCNA3 (Kv1.3),KCNA4 (Kv1.4), KCNA5 (Kv1.5), KCNA6 (Kv1.6),KCNA7 (Kv1.7), KCNB1 (Kv2.1), KCNC1 (Kv3.1),KCNC3 (Kv3.3), KCNC4 (Kv3.4), KCND3 (Kv4.3) and internal control GAPDH with custom designed primers(see below; Topgen Biotech., Co., Ltd, Taiwan, China) was performed by 2X Taq Master Mix (Vazyme) using the following program: 95 °C-5 min, 40 cycles for 95 °C-15 s,60 °C-15 s, 72 °C-15 s and 72 °C-1 min on ABI PCR system VeritiTM96-Well Thermal Cycler (Applied Biosystems). Size distribution of target gene PCR products was then examined by an MCE-202 MultiNA Microchip Electrophoresis System with DNA-2500 Kit (Shimadzu,Japan).
Specific primer sequences of each target gene are shown below: Kv1.1-F, GGAGAGGAACAGGCCCAATAC;
Kv1.1-R, CCCTCCTGGATCTCCATGTAATC;
Kv1.2-F,TCTGCAAAGCCAGAAGAGTAAGC;
Kv1.2-R, AGCTGGTGTCTGCCTGTCATC;
Kv1.3-F, TTCTCCAGCGCGGTCTACTT;
Kv1.3-R,TGGTCACTGGGTGCATATCG;
Kv1.4-F, CATTGCGGGTGTCTTAACCA;
Kv1.4-R,CTGACTGCATTCTGCGTTAGCT;
Kv1.5-F,GACCCTGGAGAATGCAGACAGT;
Kv1.5-R, ATAAAGGGACCTCCGCAAGTC;
Kv1.6-F, TCGTCTCCGTGTTGGTCATTC;
Kv1.6-R, GACTCGACTCACACCACCATTG;
Kv1.7-F, GGAGACGCTGTGTATTTGTTGGT;
Kv1.7-R, AGTGCCACAAAGTAGGGAAGGA;
Kv2.1-F,CTGTGGCTGCCAAGATCCTT;
Kv2.1-R,GTCTGTGGACTGGCCGAACT;
Kv3.1-F, CGAGAGATACGGACCCTGCTT;
Kv3.1-R, CCTCTGTCGGCATATACTTAGCAA;
Kv3.3-F, GCTCTTCGAGGACCCCTACTC;
Kv3.3-R,CACCGTCTTGTTGCTAATATGGAT;
Kv3.4-F, CGCTCTTCGAGGATCCCTACT;
Kv3.4-R,GGGTCTCCAGGCAGAAAGTG;
Kv4.3-F,ATCCCTGCCTCGTTTTGGTA;
Kv4.3-R, ACGCCACTCAAGGAGCAGAT;
GAPDH-F, GCACCACCAACTGCTTAGCA;
GAPDH-R, TCTTCTGGGTGGCAGTGATG.
Microfluorimetric measurement of cytosolic Ca2+
Microfluorimetric measurement of cytosolic Ca2+concentration was performed using fura-2 as a Ca2+-sensitive fluorescent dye as previously reported (20). In brief, cells were incubated with 5 μmol/L fura-2 AM(Invitrogen) for 1 h at 37 °C and then washed in extracellular bath solution which contained (mmol/L):140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES (pH 7.4 adjusted with NaOH). When intracellular Ca2+release was assayed, Ca2+-free solution was used. This Ca2+-free solution was the same as the extracellular bath solution mentioned above except that Ca2+was omitted and 100 μmol/L EGTA was supplemented. Cells were alternately excited with 340 nm and 380 nm using an optical filter changer (Lambda 10-2, Sutter Instruments, Novato, CA,USA). Emission was collected at 500 nm and images were captured using a CCD camera (CoolSnap HQ2,Photometrics, Tucson, AZ, USA) linked to an inverted Nikon TE 2000-U microscope. Images were analyzed with an MAG Biosystems Software (Sante Fe, MN). All imaging experiments were performed at room temperature (25 °C).
Statistical analysis
Results
Migratory cells had remarkably increased Kv currents
We observed that only a minor fraction of non-migratory cells (6 out of 36; 16.7%) had Kv currents, while 58.1% of migratory cells (18 out of 31) had Kv currents (albeit of variable size). The current densities were calculated and migratory cells had remarkably larger Kv current density than non-migratory cells (Figure 1). Large-conductance Ca2+-activated K+(BK) currents are also voltage-activated(12), but we believe the outward K+currents we observed are Kv currents but not BK currents for dual reasons: first,BK currents are activated by Ca2+>1 μmol/L, but our pipette solution (hence intracellular milieu) was Ca2+-free supplemented with 1 mmol/L EGTA. Second, BK currents could be largely inhibited by <1 mmol/L TEA (12), but the currents we observed were only significantly inhibited by>10 mmol/L TEA.
As the lower chambers contained 10% FBS as chemoattractant, we investigated whether the increased expression of Kv current in migratory cells was due to the presence of FBS. Therefore, cells were grown in the absence or presence of 10% FBS for 3 d and then currents were examined. As shown in Figure 2, there was no significant difference in current densities between the two groups, suggesting the up-regulation of Kv currents in migratory cells was not due to stimulation by FBS.
Delayed rectifier type Kv channels were present and were important in cell migration
We next examined what Kv channel members were present in BT474 cells using RT-PCR with a microchip electrophoresis system (Figure 3). Electropherogram shows peaks of Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv3.3, Kv3.4, Kv4.3 and the internal control GAPDH at the expected 100-200 bp range (Figure 3A), which are indicated as major bands in Figure 3B. Very tiny peaks (Figure 3A), indicated as very faint bands in Figure 3B, were non-specific amplifications but were very few. Thus, Kv1.1, Kv1.3,Kv1.5, Kv2.1, Kv3.3, Kv3.4 and Kv4.3 channels were expressed in BT474 cells.
To examine whether the Kv currents played a role in BT474 cell migration, we deployed TEA, a delayed rectifier Kv channel blocker, as a tool to inhibit BT474 cell Kv currents and migration. Significant inhibition of Kv currents required >10 mmol/L TEA (Figure 4A).Consistently, significant inhibition of migration required 20-30 mmol/L TEA (Figure 4B, C). TEA at these concentrations did not affect cell viability when compared to the control which had no TEA (Figure 4D). These results suggest delayed rectifier type Kv currents are important in cell migration. However, 4-aminopyridine(4-AP), an A-type Kv channel blocker, did not inhibit BT474 cell Kv currents and did not significantly affect cell migration (Figure 5). In concordance, BT474 cell Kv currents exhibited very slow inactivation (Figure 1, 4, 5)instead of fast inactivation characteristic of A-type Kv channels.

Figure 1 Migratory BT474 cells had much larger voltage-gated K+ (Kv) current density than non-migratory (control) BT474 cells. (A)BT474 cells from the upper chamber (control) and porous membrane (migratory) of Transwell were separated and subject to electrophysiological examination for Kv currents; (B) Cell currents are normalized with cell size to yield current density and plotted against voltages. Results are from 31-36 cells. *, significantly different (P<0.05) from control.

Figure 2 BT474 cell current density was not affected by fetal bovine serum (FBS). BT474 cells were cultured in the absence or presence of 10% FBS for 3 d and then subject to electrophysiological examination for voltage-gated K+ (Kv) currents. Cell currents are normalized with cell size to yield current density and plotted against voltages. Results are from 9-10 cells.
Migratory cells had more depolarized membrane potential and reduced Ca2+ influx
It has been reported before that Kv channels regulate membrane potential which in turn regulates Ca2+signaling in breast cancer MDA-MB-231 cells (16). More Kv channels may be expected to cause a lower membrane potential (hyperpolarization). Therefore we examined if there was a difference in membrane potential between the non-migratory and migratory BT474 cells. Rather unexpectedly, the migratory cells, having much higher Kv channel density, had slightly but significantly depolarized membrane potential (Figure 6).
We next examined whether there was a difference in Ca2+signaling between non-migratory and migratory BT474 cells. Cyclopiazonic acid (CPA; intracellular Ca2+pump inhibitor) was deployed to cause intracellular Ca2+release, and then replenishment of extracellular Ca2+resulted in Ca2+influx. We observed that while intracellular Ca2+release was similar in the two groups of cells, Ca2+influx was smaller in the migratory cells (Figure 7). This is consistent with the more depolarized membrane potential in the migratory cells: such depolarization decreased the electric driving force for Ca2+influx.
Discussion
Data in this report show that there was an increased expression of Kv channels in migratory BT474 cells. In both non-migratory and migratory cells, Kv currents show no or very slow inactivation, suggesting most of the members were of delayed rectifier types. Consistently,TEA (delayed rectifier blocker), but not 4-AP (A-type channel blocker), could significantly inhibit currents and cell migration, suggesting that delayed rectifier Kv channels were important in cell migration. An RT-PCR analysis revealed that several members were present,namely, Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv3.3, Kv3.4 and Kv4.3.

Figure 3 Reverse transcriptase-polymerase chain reaction (RT-PCR) revealed presence of different voltage-gated K+ (Kv) members in BT474 cells. (A) Electropherogram shows peaks of Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv3.3, Kv3.4, Kv4.3 and the internal control GAPDH. The horizontal ordinate denotes amplification products length and the vertical ordinate denotes peak intensity; (B) Bands in the 100-200 bp range, Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv3.3, Kv3.4, Kv4.3 and the internal control GAPGH were detected. Same results were obtained in two more separate experiments.

Figure 4 Voltage-gated K+ (Kv) currents and migration were inhibited by tetraethylammonium (TEA). (A) Kv currents were stimulated by+30 mV depolarization pulses in migratory BT474 cells. TEA at 10 and 30 mmol/L was added to suppress currents. Similar results were obtained in 3 other experiments; (B) Cells (after being cultured in different TEA concentrations for 3 d) in Transwell porous membranes were stained with crystal violet; (C) Quantification of results in (B); (D) Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after cells were cultured in different TEA concentrations. Results are from 3 separate experiments. *, significantly different (P<0.05) from control.

Figure 5 Voltage-gated K+ (Kv) currents and migration were not affected by 4-aminopyridine (4-AP). (A) Kv currents were stimulated by+30 mV depolarization pulses in migratory BT474 cells. 4-AP at 1 mmol/L was added to the cell. Similar results were obtained in 3 other experiments; (B) Cells were cultured in absence or presence of 4-AP for 3 d and Transwell porous membranes were stained with crystal violet; (C) Quantification of results in (B). Results are from 3 separate experiments.
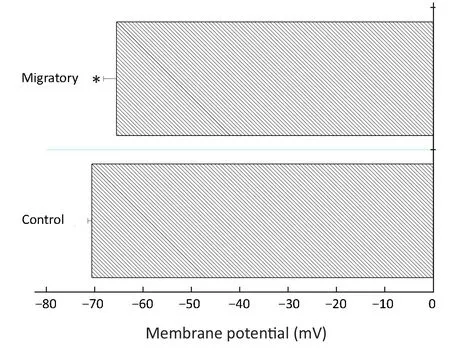
Figure 6 Migratory BT474 cells had more depolarized membrane potential than non-migratory (control) BT474 cells. BT474 cells from the upper chamber (control) and porous membrane(migratory) of Transwell were separated and subject to current clamp to measure membrane potential. Results are from 25-29 cells. *, significantly different (P<0.05) from control.
How do Kv channels promote cell migration? Hammadi et al. obtained data showing that blocking or silencing hEag1 (Kv10.1) depolarized breast cancer MDA-MB-231 cells, reducing Ca2+entry (via Orai1-associated channel),and eventually inhibiting cell migration without affecting cell proliferation (16). Thus, hEag1 is essential in maintaining a negative membrane potential to be favorable for Ca2+entry, which is important in cell motility.However, using BT474 cells, our data do not support such a hyperpolarizing role of Kv channels. In fact, migratory cells, which expressed more Kv channels, were more depolarized (Figure 6). Further, migratory cells had reduced Ca2+entry after store depletion (Figure 7); such reduction is consistent with the more depolarized membrane potential, which decreased the electrical driving force for Ca2+. Therefore, our findings in BT474 cells are novel and significant in showing that Kv channels promote migration via mechanism(s) independent of Ca2+influx.
In a very recent report, Kv1.1 and 1.3 channels are demonstrated to play a role in MDA-MB-231 cell migration (21). In addition, human ether-à-go-go-related gene 1 (hERG1) plays a role in human osteosarcoma cell migration (22). However, in these two works, electrophysiological measurements and Ca2+assays were not performed to show whether migratory cells have enhanced K+currents or altered Ca2+homeostasis.

Figure 7 Migratory BT474 cells had reduced store-operated Ca2+ entry than non-migratory (control) BT474 cells. (A) BT474 cells from the upper chamber (control) and porous membrane (migratory) of Transwell were separated and subject to microfluorimetric examination for cytosolic free Ca2+ concentration. Cyclopiazonic acid (CPA), 30 μmol/L; Ca2+, 2 mmol/L; (B) Quantified results. Results are of 26-33 cells from 5 separate experiments. *, significantly different (P<0.05) from control.
Inward rectifier K+channels (Kir2.2, Kir3.1, Kir6.1,Kir6.2) and Ca2+-activated K+channels (KCa1.1, KCa2.3 and KCa3.1) are expressed in breast cancer cells (14).Blocking intermediate- and large-conductance KCa channels could inhibit glioma migration and tumor infiltration (23,24). Small- and large-conductance KCa channels are also implicated in breast cancer migration and metastasis; in case of small-conductance KCa channels,they form a complex with Orai-Ca2+channels so that they could be directly activated by localized Ca2+elevation with lipid rafts (25). However, a model integrating the roles of Kv, Kir and KCa channels in cancer cell migration is hitherto lacking. Mobile lamellipodia are observed at the leading edge of mobile cells, while the contracting cell body is at the trailing edge. A possible mechanism is proposed in which at the posterior of the moving cell, Kv and KCa channels provide K+efflux and subsequently water flows out osmotically via aquaporin; this causes local volume decrease at the cell posterior and culminates in rear retraction (14). At the cell anterior, Kir channels provide an influx K+pathway, osmotically drawing water in via aquaporin; this results in local volume increase and culminates in front protrusion. There is evidence this could provide enough mobile force for cell motility even in the absence of actin polymerization (26). Whether this could occur in breast cancer migration is hitherto unknown and would warrant future investigation.
The non-canonical functions of Kv channels (that is,functions unrelated to ion conduction) in BT474 migration also warrants consideration. In this case, Kv channels may form molecular complexes with other proteins such as integrin to facilitate cell migration. For example, hERG channels have been demonstrated to form complex with vascular endothelial growth factor receptor-1 (VEGFR-1)and β1 integrin to promote the formation of a migratory leukemia phenotype (27).
Besides their most well-known functions in dampening cellular excitability, convincing evidence supports the involvement of Kv channels in cell death, differentiation and functions of a wide variety of cells. For instance, there is an overexpression of Kv channels during apoptosis of neurons, immune cells and muscle cells (28,29). The massive K+efflux through over-expressed Kv channels leads to loss of intracellular K+and the reduced [K+]iresults in relief of inhibition (thus activation) of pro-apoptotic caspases and nucleases (30,31). K+efflux through Kv1.1,Kv1.4 and Kv2.1 is responsible for cAMP-stimulated neuritogenesis in mouse neuroblastoma N2A cells (20).Strong evidence from Ilana Lotan’s lab has suggested that,in a manner independent of K+flow, Kv2.1 directly interacts with syntaxin in facilitating exocytosis, further substantiating the presence of non-canonical functions of Kv channels (32). Kv1.3 has been shown to be involved in breast cancer cell proliferation (33). In this report Kv channels have been further demonstrated to promote breast cancer cell migration. Therefore, there is mounting evidence suggesting Kv channels are versatile molecules which have a variety of functions in addition to provision of K+efflux for repolarization.
Conclusions
Kv channel expression was up-regulated during BT474 cell migration, and hence Kv channels could be considered as biomarkers or potential therapeutic targets for breast cancer cell migration.
Acknowledgements
The study was funded by the Macau Science and Technology Development Fund (FUNDO PARA O DESENVOLVIMENTO DAS CIÊNCIAS E DA TECNOLOGIA) and the reference number was 002/2015/A1.
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- BRAF inhibitor: a novel therapy for ameloblastoma in mandible
- Tumor pyruvate kinase M2: A promising molecular target of gastrointestinal cancer
- Novel circular RNA expression profile of uveal melanoma revealed by microarray
- Limited energy parametrial resection/dissection during modified laparoscopic nerve-sparing radical hysterectomy
- Identification of liver metastasis-associated genes in human colon carcinoma by mRNA profiling
- Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters
