On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland
2018-12-22BrianTIMMS
Brian V. TIMMS
Centre for Ecosystem Science, School of Biological, Earth and Environmental Science, University of New South Wales,Kensington, NSW, Australia, 2052
Abstract While the fauna of Australian salt lakes is now well-known, seasonal phenological patterns of invertebrates are not. Two studies on saline lakes in southern Australia suggest the lakes fill in early winter and remain at salinities characteristic for each lake during winter-spring before elevating and drying in summer. The fauna is dominated by crustaceans with few insects and all component species are present most of the time and randomly fluctuating in numbers. Lakes in the southern inland (mainly Lake Eyre) fill in summer, change little in salinity until near drying, and are dominated by crustaceans but have some insects.By contrast temporary salinas in the central inland fill episodically mainly in summer and then their salinity increases steadily as they dry without further rain. Their fauna is also dominated by crustaceans, but with a significant insect component and composition varies though the hydrological cycle. This study reports on an unusual winter fill in two central Paroo lakes and two pools, in which the crustacean fauna is similar to that in summer but insects are delayed till late spring and are not as common as in summer fills. It seems therefore that while insects are more restricted by medium and high salinities than crustaceans, they are also more restricted by cooler temperatures than crustaceans.
Keyword: crustaceans; aquatic insects; rotifers; regional fauna; biogeography; habitat heterogeneity
1 INTRODUCTION
The fauna of Australian salt lakes is relatively well known due to numerous studies by many authors over the last 50 years. Following the pioneering work by Bayly and Williams (1966) on western Victorian lakes,there were major biocenotic studies on the same area by Geddes (1976), Timms (1981, 1983), Walker(1973), and Williams (1981,1995), on southeastern South Australia by Bayly (1970) and De Deckker and Geddes (1980), on Eyre Peninsula, South Australia by Williams (1984) and Timms (2009a), on central South Australia by Bayly (1976), Williams and Kokkinn(1988) and Williams et al. (1998), on the wheatbelt of Western Australia by Geddes et al. (1981), Halse(1981), Pinder et al. (2002), and Timms (2009b), on the Paroo in northwestern NSW and Queensland(Timms, 1993, 1998a, b, 2007) and Lake Buchanan in North Queensland (Timms, 1987). These studies show a diverse and regionalised fauna dominated by crustaceans. Only five studies considered development of the fauna through a filling/drying sequence, Geddes(1976) and De Deckker and Geddes (1980) on temporary lakes in southern Australia which fill seasonally in winter and Bayly (1976), Williams and Kokkinn (1988) and Williams et al. (1998) on the large Lakes Eyre and Torrens which fill episodically often in late summer.
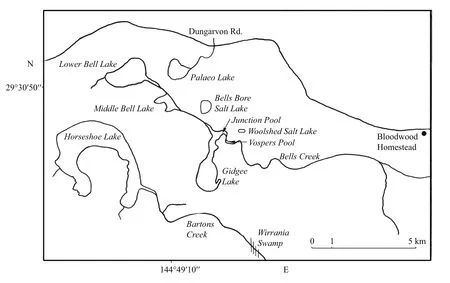
Fig.1 Map of the salt lakes on Bloodwood Station, 130 km northwest of Bourke, NSW
Lakes in central and northern Australia tend to fill episodically in summer while those in southern Australia fill seasonally every winter-spring.Occasionally, roughly at decadal intervals, some salinas in southern Western Australia fill in summer and either support a similar fauna as in winter, but should they dilute significantly, an entirely different fauna (Timms, 2009b). Only the detailed phenology of component invertebrates is known for two sets of southern lakes (Geddes, 1976; De Deckker and Geddes, 1980). The Paroo saline lakes fill episodically and almost always in summer: for instance Lake Wyara experienced significant inflows during 17 periods over a 110-year period and these were correlated with La Niña summer rains (Bridgman and Timms, 2012). Only a coarse phenology of the invertebrates over a 10-year period is available for Lake Wyara and also the nearby Rockwell-Wombah lakes (Timms, 1998a, 2008).
Further south in the Paroo catchment there are many small saline lakes which fill mainly in summer,but also rarely in winter. Those on Bloodwood Station have filled significantly 10 times over a 52-year period (1965–2016), eight of these in summer and twice in winter (based on continuous rainfall records 1965–2016 at nearby Dungarvon Station and records of David Leigo (pers. comm.) and the author’s records since 1987). The 1995 summer filling was studied over subsequent months but only the broad pattern was reported (Timms, 1996), though unpublished data exists on phenologies of individual species. The Bloodwood lakes filled in the winter of 1981 and again in the winter of 2016. This later filling has been studied monthly.
These Bloodwood data provide an opportunity to compare phenologies in episodic central and seasonal southern Australian lakes and also to examine the role of seasonality of filling in the central lakes. In that southern lakes and the two large central Australian lakes vary little in salinity during much of the season(Geddes, 1976; De Deckker and Geddes, 1980)compared to steady increasing salinity in the small Paroo lakes in a filling/drying cycle (Timms, 1996)the question arises of how this difference influences diversity and phenological patterns. Another question to address is the possibility of the influence of season on diversity and phenology in the large central episodic lakes where there is a long period of stable salinities due to the large volumes to evaporate, then increasing salinities as they dry (Bayly, 1976;Williams and Kokkinn, 1988; Williams et al., 1998).Finally, diversity in the episodic Paroo lakes is already known to be reduced in drier years compared to wetter years (Timms, 1998b), so is there a difference between summer and winter as well?
2 METHOD
Bloodwood Station lies ca 130 km NW of Bourke within the Paroo Basin, with a dune mass blocking Bells and Bartons Creeks which drain much of Bloodwood and Wampra stations and parts of adjacent stations. Indeed, this damming has resulted in ponded water which over the millennia has salinized to form such lakes as Gidgee, Horseshoe, Bells Bore and Lower Bell (Timms, 2006) (Fig.1). While these and others filled for many months in 1995 (Timms, 1996),only Gidgee, associated pools and Horseshoe filled sufficiently in 2016 for an extended phenology to be followed.
In both years Gidgee Lake was sampled at the same southern shore site and Horseshoe Lake at the southeast bay near the Barton’s Creek inflow, but in 2016 further south by about 200 m.
In 1995 lakes were sampled every six-seven weeks for almost the whole year, commencing late January,just a week after filling. In 2016 the lakes filled in mid-May and were visited in late May and then late in every month till November when dry or almost so.TDS was determined gravimetrically in 1995 but by conductivity with a Hanna H18733 meter and converted to mg/L by the formula in Williams (1966)in 2016. Surface water temperature was measured with a mercury thermometer, but not always at the same time of day in both years.
The presence of aquatic plants in each site was noted but no detailed measurements were made.
Zooplankton was collected with a net of mesh size 159 μm mounted on a pole and with a rectangular aperture of 30 cm×15 cm. It was trawled from the lake edge towards the middle for one minute in 1995 and 1–2 min in 2016 and the contents preserved in formalin. Species present were identified in the laboratory and relative abundance estimated on a four-point scale (< 10 individuals in a collection=+;11–50 individuals=++; 51–500 individuals=+++ and>501 individuals=++++).
A similarly constructed net, but of mesh size 1 mm in 1995 and 2 mm in 2016, was used to catch littoral invertebrates. It was trawled from the shore towards the lake middle for 0.5 to 2 min twice in 1995 and from 2 to 5 min twice in 2016. In 1995 collections were preserved in alcohol and later examined in the laboratory, identified and absolute abundance estimated on a four-point scale (<2 individuals in a collection=+;3–10 individuals=++; 11–50 individuals=+++; >51 individuals=++++). In 2016 collections were examined lakeside in a tray and while tiny individuals (<2 mm)were ignored, having almost certainly been caught in the plankton net, species >2 mm were identified and relative abundance estimated on a four-point scale. In both years, rare species may have been missed. Also‘littoral’ species were caught in the plankton net as well—abundance records of such species were based on which ever estimate was larger.
While sampling was slightly inconsistent between the two periods, the study is broad enough for this not to influence the results (Timms, 1993, 1998a, 2008).Also the benthic community was not specifically sampled in any lake or pool, but this is not a major omission. The littoral net scraped the bottom and as such should have encountered at least epibenthic invertebrates, which it did from time to time. Any eubenthic species in the sediments deeper than 0.5 cm were missed, but sampling with a Birge-Ekman grab caught almost nothing in these lakes in 1995, so was not repeated in 2016. Any zooplanktonic individuals temporarily benthic would have been missed and may account for perceived population fluctuations, but 30 years of collecting from these lakes have not recorded species other than those caught in 1995 and 2016.
3 RESULT
The hydroperiod for Gidgee Lake in 1995 was when it contained water from late January to about mid-October when salinity changed from 3.7 to 85 g/L and in 2016 the hydroperiod was from late May to about December, and salinity change was from 2.2 to beyond 40 g/L (Fig.2). In both years there were minor diluting inflows during the season, in April, May and September in 1995 and in September 2006. Of the two creek pools just upstream of Gidgee Lake, Junction Pool held water late May to about early December, salinity range 2.2 to 60 g/L, and Vospers Pool had water late May to early November,salinity range 1 to 6 g/L (Fig.2). A major creek flow in September diluted both pools. In 1995 Horseshoe Lake contained water from late January to about mid-August, changing in salinity from 5 to 220 g/L and in 2016 water was present late May to about early November changing in salinity from 15 to 183 g/L.Inflows from rainfall during both periods hardly affected water levels as Bartons Creek’s minor to moderate flows are largely trapped in Wirrania Swamp upstream.
Water temperatures in summer (January and February) ranged from 23 to 36°C (mean in January 29.8±4.5°C) and in winter (June to August) from 7 to 19°C (mean in July 11.2±1.8°C) depending on lake and time of day when measured. Lake waters were clear to the bottom except on initial filling when it took a few weeks for suspended clays to settle.
As in the 1995 fill (Timms, 1996),Lepileana bilocularisT. Kirk.,Lamprothamnionpapulosum(Wallr.) J. Gr. andCharasp., formed an almost complete sward on the bottom of Gidgee, and in Horseshoe the sward was dominated byLepileana bilocularisandRuppiamegacarpaMason. The two creek pools were dominated by drowned terrestrial plants together withLepileanabilocularisand particularlyCharasp. These plants grew far more luxuriantly in the pools than in the lakes.
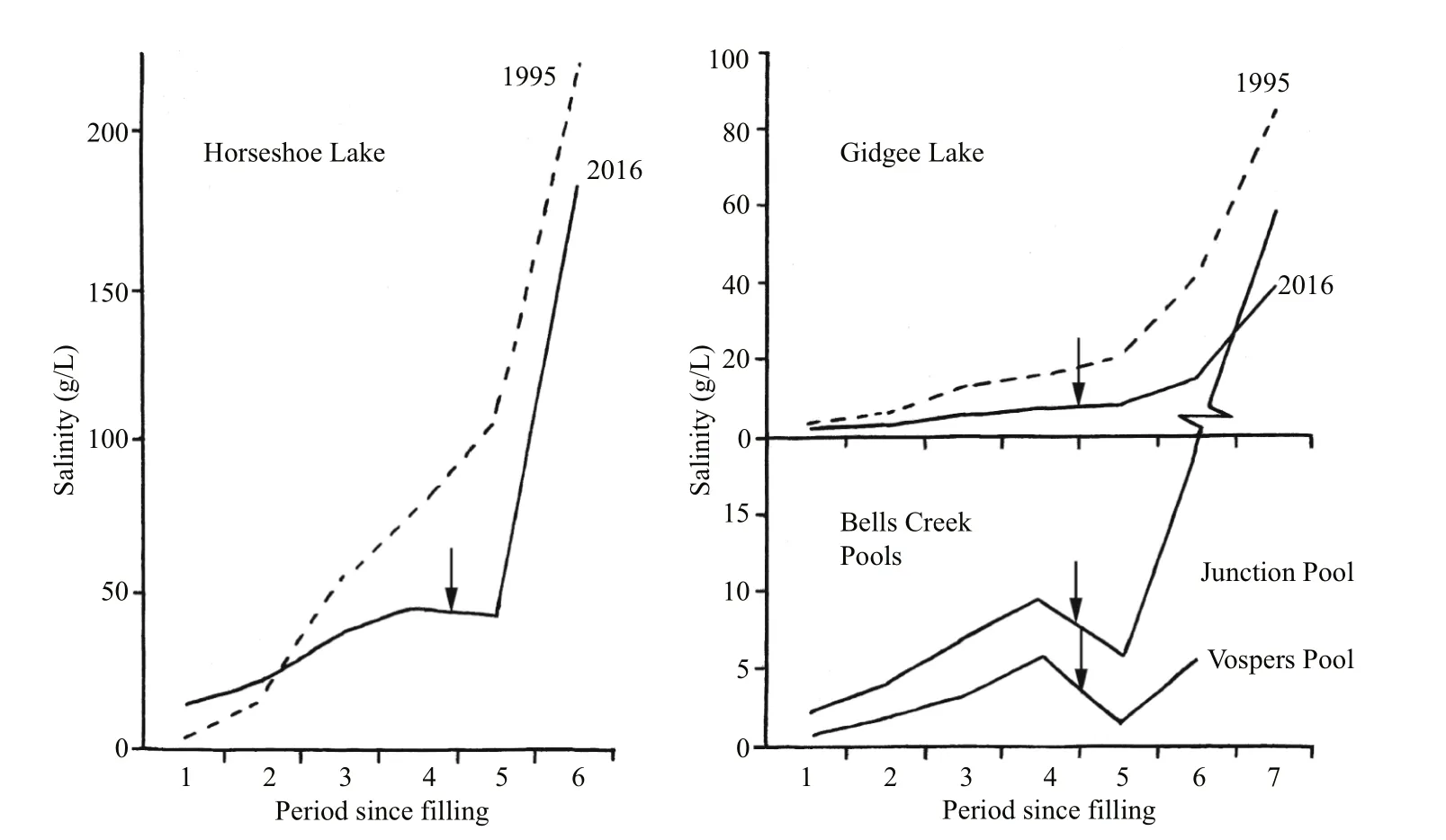
Fig.2 Salinity variations in some Bloodwood salt lakes
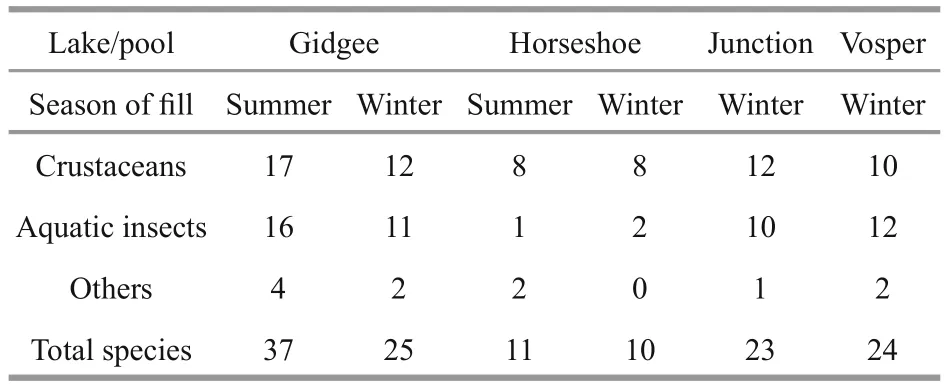
Table 1 Species richness in the saline lakes and pools at Bloodwood
Species richness in Gidgee was greater in the summer fill at 37 species than in the winter fill at 25 species, all three subgroups of crustaceans, insects and others contributing (Table 1, Fig.3). The lower figure in the winter fill is confirmed by the values (23 and 24) for the two creek pools (Table 1, Fig.4).However, in the more saline Horseshoe there was no difference between the two seasonal fills—11 in summer and ten in winter, and both dominated by crustaceans (Table 1, Fig.5). In Gidgee and Horseshoe,which both increased significantly in salinity during their filling/drying cycle, there were species occurring only when the lakes filled initially and salinity was low, many species during the middle phases and some persisting to the final hypersaline stages. Examples of the early group includeBranchinellabuchananensis,Eocyzicusparooensis, andDaphniacarinata, the middle groupDaphnia(Daphniopsis)queenslandensis,Trigonocyprisglobulosa, andApocyclopsdengizusin Horseshoe and of the latter groupDiacyprisspp. andApocyclopsdengizicusin Gidgee. It was not as simple as this and salinity plays a role, with differences between the lakes and between the two seasons. An example is Gidgee which had much lower final salinity in the summer fill than in the winter fill, so thatTrigonocyprisglobulosapersisted to the end in 2016 but not in 1995 in Gidgee. In Horseshoe differences inApocyclopsdengizicushas already been noted, in 1995 its upper salinity limit of about 70 g/L in the Paroo was reached earlier in the filling/drying cycle in Horseshoe than in Gidgee.
4 DISCUSSION
4.1 Paroo Lakes
Invertebrates in the lakes are a mixture of univoltine and multivoltine species. Among the crustaceans most are multivoltine, includingParartemiaminutain Horseshore and the cladocerans, ostracods and copepods in all sites (Timms, 1993, 1998b) A striking example isDaphniopsisqueenslandensiswhich disappeared in Horseshoe in August 2016 when the salinity spiked to 45 g/L but recovered in September with a new generation when salinity fell to 37 g/L when a minor inflow lowered salinity. Definite univoltine crustaceans included the two large branchiopods,BranchinellabuchananensisandEocyzicusparooensis(Cáceres and Rogers, 2015).For insects the odonatans, trichopterans and hemipterans were univoltine with the boatman and backswimmer populations in Gidgee 1995 a mixture of adults and juveniles (Timms, 1993, 1998b). Two of the beetles (BerosusnutansandSternopriscus multimaculatus) had larvae that took months to develop and so were probably univoltine. Sampling was too widely spaced to indicate if the chironomids with their short life cycles where multivoltine in Gidgee 1995, but multivoltine life cycles would be expected in this group.

Fig.3 Phenology of the invertebrates in Gidgee Lake in the summer fill of 1995 and winter fill of 2016
Some species recorded in Gidgee and the associated Bells Creek pools were washed in from upstream pools (Timms, 2001). These includedOzestheria packardis.l.,Triopsaustraliensiss.l. and possiblyDaphniacarinata, but notBranchinellabuchananensisandEocyzicusparooensis, which hatch from lake sediments. The later species is very common in the littoral and the fairy shrimpB.buchananensiswas probably more common than indicated in Fig.3, as there were many more in the centre of the lake where Marsh Terns (ChlidoniashybridaPallas) were seen diving for them as if they were fish. Further freshwater wash-ins occur in the lower pools of Bells Creek,such asLynceustateiin 2016 and various crustaceans in 1995 and 1998 (Timms, 2001). Such wash-ins were uncommon components of lower lake/pool faunas and soon perished as salinities rose.
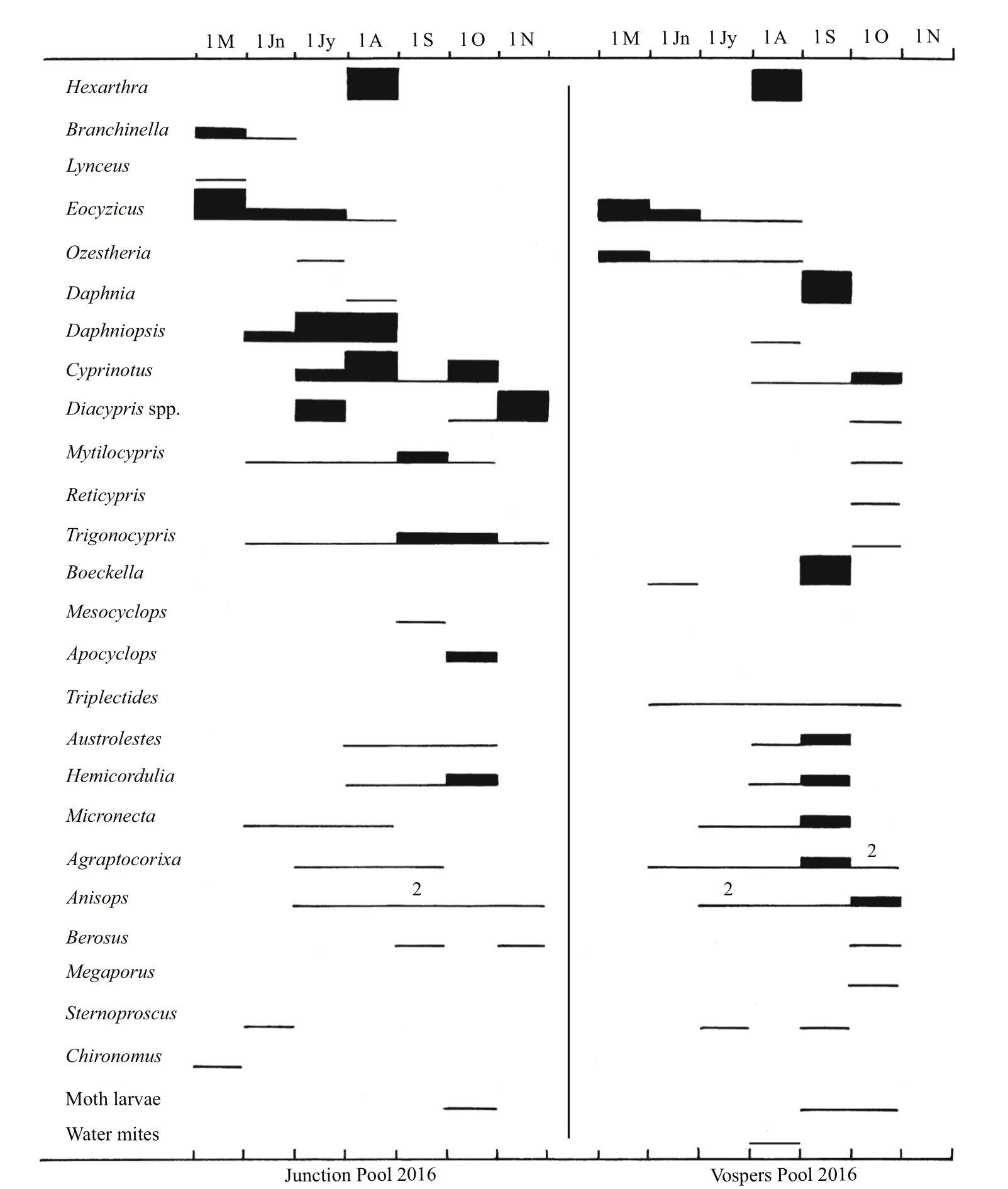
Fig.4 Phenology of the invertebrates in Junction and Vospers Pools in the winter fill of 2016
Among the crustaceans there are many unexplainable differences between the summer and winter fills. Examples includeCyprinotussp. andDaphnia(Daphniopsis)queenslandensisnot appearing in the Horseshoe summer fill but in the winter fill andReticyprissp. missing the winter fill in both lakes (Figs.3, 5). Possibly hatching conditions were not right at times when they were absent when otherwise they would be expected to occur (De Deckker and Geddes, 1980). However, there are stark differences among insects between seasons in Gidgee which had lower salinities than Horseshoe in both seasons (Figs.2, 3). Insects were diverse and abundant the early months of the summer fill, but sparse in the winter fill, but with a few appearing as that fill extended into summer. Their absence as the summer fill extended into winter could be explained by high salinity, not low temperatures, but their delayed seasonality in the winter fill accords with low initial temperatures ameliorating enough beyond August for a few insects to appear. In the two creek pools in 2016, where salinities were always low, there are few insects in winter, but numbers increased from September onwards as water temperatures rose(Fig.4). In Horseshoe salinities were too high much of the time during both fills for insects to colonize,except for the tolerantTanytarsisbarbitarsis(Fig.5).4.2 Comparison with other lakes
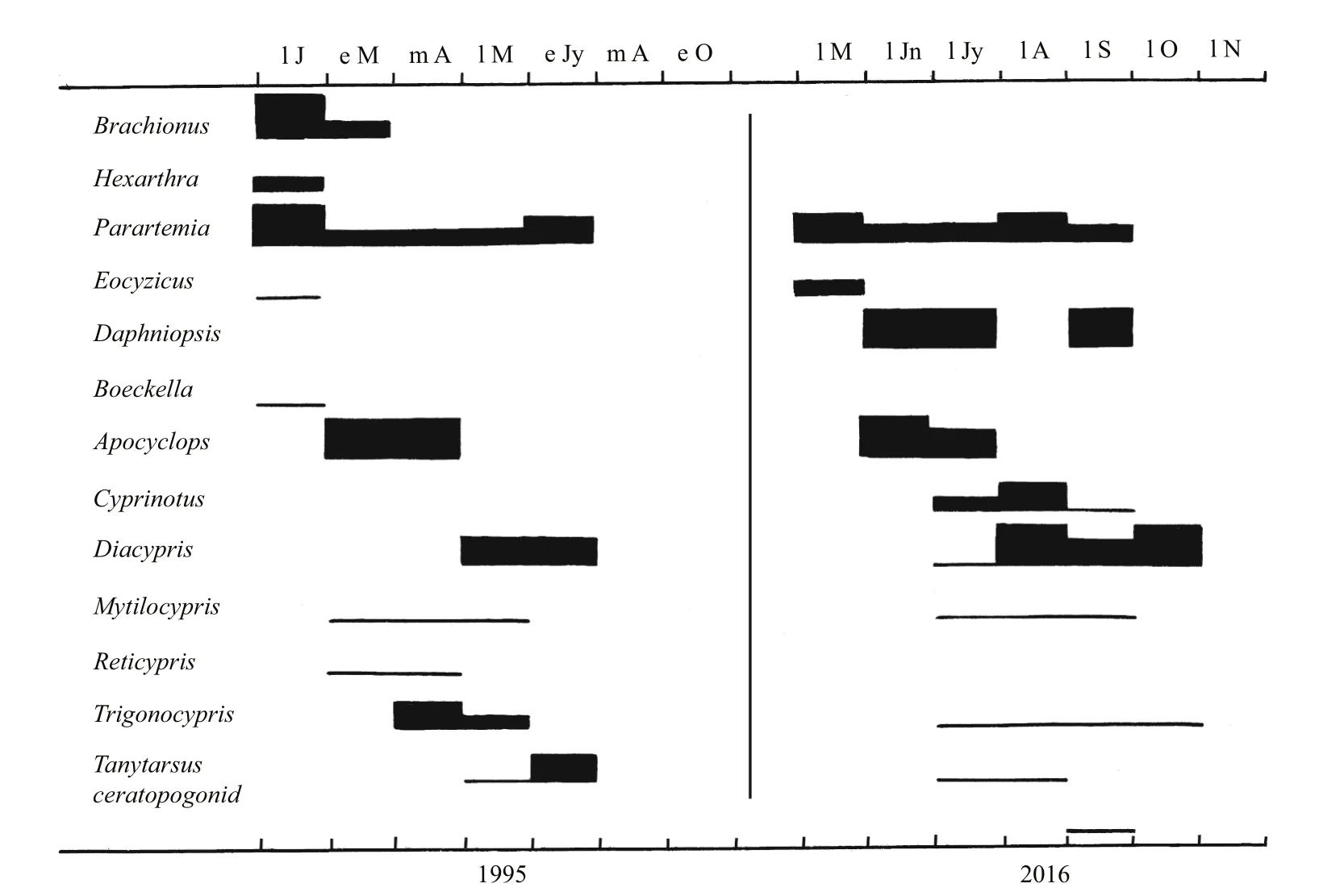
Fig.5 Phenology of the invertebrates in Horseshoe Lake in the summer fill of 1995 and the winter fill of 2016
Most studies on invertebrates in saline lakes focus on distribution and abundance across the salinity spectrum, but a few have analysed seasonal phenologies of zooplankton, and aquatic insects or of individual species or taxonomic group (Hammer,1986). The overall conclusion of these studies is that diversity is low and abundances vary widely, often erratically. In Australia there have been seasonal studies of the zooplankton of Lake Werowrap and other Red Rock Lakes, Victoria (Walker, 1973;Hammer, 1981), the zooplankton and littoral of some Western District lakes, Victoria and in adjacent southeastern South Australia (Geddes, 1976; De Deckker and Geddes, 1980), the benthos of Lake Werowrap (Paterson and Walker, 1974) and the fauna of Lakes Eyre and Torrens northern South Australia(Bayly, 1976; Williams and Kokkinn, 1988; Williams et al., 1998). The southern Australia lakes fill with relatively steady winter-spring rainfalls so that salinity varied only a little over the hydrologic cycle except at the very beginning and/or at the end. The fauna of these southern lakes was dominated by crustaceans with insects virtually absent, except in the benthic study. While numbers fluctuated though the season,most species were present throughout and were multivoltine. In the large Lakes Eyre and Torrens,salinities varied only a little for the first 7–8 months then rose sharply as they dried, a scenario not very different from the small southern lakes, but while most species were present much of the time, there was some differentiation into early, middle and late colonizers.
By contrast, the present study shows that the two smaller Paroo lakes steadily rise in salinity through the first few months then as in the others, rise sharply in the late stages of drying. As in the southern examples crustaceans dominated but with insects important in the less saline Gidgee Lake. Again numbers varied somewhat erratically, but in the Paroo sites an early hyposaline, middle mesosaline and late hypersaline fauna could usually be distinguished.Surely the progressive increase in salinity though the season due to evaporation and general lack of subsequent inflows must be responsible for this difference.
The case of the insects is an interesting one. Why are insects virtually absent in the small southern lakes,just present in large Lake Eyre and Torrens (3 species each, see Williams and Kokkinn, 1988; Williams et al., 1998) yet diverse and common in Paroo lakes (21 species in the study lakes, 16 in Lake Wyara (Timms,1998a), 58 across 25 Paroo saline lakes (Timms,1993))? Is this due to salinity influences, seasonal influences, or biogeographical reasons? Certainly,insects overall are not as tolerant of high salinities as are many crustaceans (Hart et al., 1991) though beetles are regularly found in Colac lakes to 40 g/L and a few species to even more at 93 g/L (Bayly and Williams, 1966; Timms and Watts, 1987) while odonatans are known to tolerate 37.5 g/L (Timms,1993) and some dipterans are particularly salt tolerant.Almost all of the phenologies in southern salinities were at >40 g/L most of the time so the absence of insects could be explained by high salinities, but insect absence in the few lower salinity lakes is inexplicable on these grounds. Lakes Eyre and Torrens spent 2 and 7 months respectively at <40 g/L yet had only three insects each, so that their relative lack of insects could hardly be explained by high salinities. However, lack of shoreline heteorogenity could adversely influence their presence together with the rarity of filling and lack of other surrounding recruitment waters could contribute to low aquatic insect populations (Timms, 1998a, b). The high number of insects in Paroo saline lakes could be due to most of them (72%) averaging <40 g/L and sheer numbers of salt lakes in the Paroo, so that insects breed in them and expand numbers.
Any effect of seasonal influence on insects is best seen in this study of summer vs. winter fills in the Paroo. In Gidgee no insects were present in winter in either the summer fill as it extended into winter or the winter fill for at least the first half. But insects were particularly diverse and abundant in the summer of the summer fill and also were present as the winter fill passed into late spring. In this study seasonal influences were recorded by the influence of temperature differences, but photoperiods could well be involved too (Speight et al., 2008). Insects were scarce in the mesosaline Horseshoe Lake at all times.Their virtual absence in the phenologies in southern lakes could be explained by their low salinities occurring in winter when insects are not active.
It is hard to see much biogeographical influence on the presence/absence of insects in Australian saline lakes. Most aquatic insects in Australian saline lakes have wide distribution ranges, so that they occur throughout the inland and also in southern regions(Williams, 1984; Timms, 2007). On the other hand,many crustaceans are endemic at the species level to regional areas, though many genera are widespread.So the regionalisation seen in Australian salt lakes(Williams, 1984; Timms, 2007) is mediated mainly through the crustacean component, not the insects.
In conclusion, the abundance of insects in Paroo salt lakes and scarcity in southern lakes is due to many Paroo lakes being <40 g/L and usually with water in summer when insects are active, while their scarcity in southern lakes is a function of their generally holding water at salinities >40 g/L and filling in winter when insects are less active. The situation is less clear in the large Lakes Eyre and Torrens, but their low species richness could be influenced by homogeneity of their littorals, and/or lack of nearby waters as a recruitment source by dispersal and/or in Lake Eyre by being largely at >50 g/L much of the time (Bayly,1976; Williams and Kokkinn, 1988) and hence too saline and in Lake Torrens by severe ephemerality(the 1989 filling is the only filling in living memory)(Williams et al., 1998).
5 ACKNOWLEDGEMENT
I thank the various owners through the years of Bloodwood Station for ready access to its wetlands,and especially to the recent owners, the Hansons, for hospitality as well. David Leigo is thanked for sharing his rainfall records from Dungarvon Station. Finally,I am indebted to Ian Bayly for his helpful comments on the manuscript.
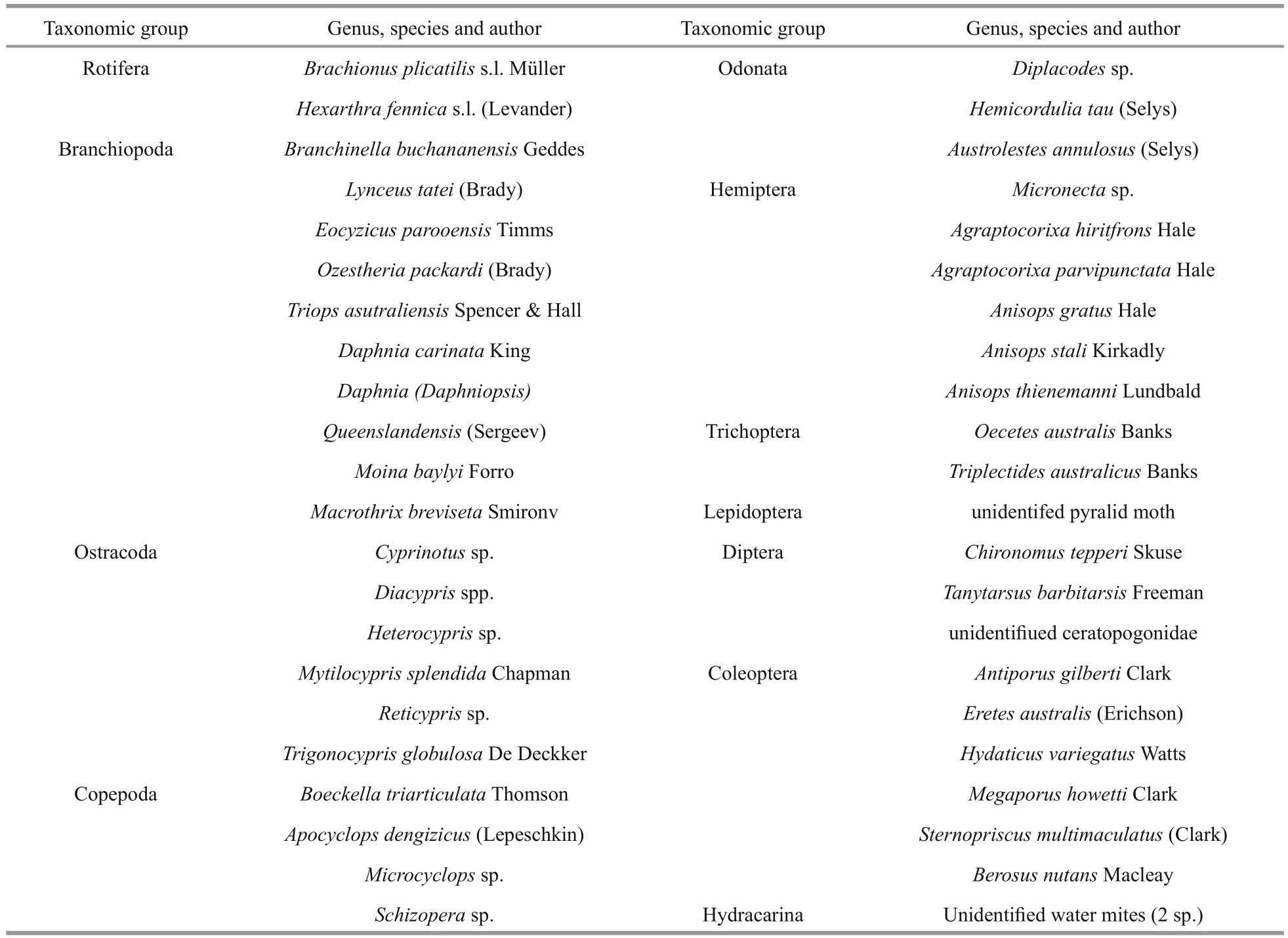
Appendix 1 Invertebrate species in the study lakes
杂志排行
Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Man-made plutonium radioisotopes in the salt lakes of the Crimean peninsula
- Cladophora mats in a Crimean hypersaline lake: structure,dynamics, and inhabiting animals
- Antimony speciation at the sediment-water interface of the Poyang Lake: response to seasonal variation*
- Seasonal variations of phosphorus species in the Tuohe River,China. Part I. Sediments*
- The characteristic pattern of multiple colored layers in coastal stratified lakes in the process of separation from the White Sea*
