Effects of dry/wet ratio and pre-immersion on stress corrosion cracking of 7050-T7451 aluminum alloy under wet-dry cyclic conditions
2018-12-15MeiYUHangZUKuoZHAOJianhuaLIUSongmeiLI
Mei YU,Hang ZU,Kuo ZHAO,Jianhua LIU,Songmei LI
School of Materials Science and Engineering,Beihang University,Beijing 100083,China
KEYWORDS 7050-T7451 aluminum alloy;Electrochemical test;Pre-immersion;Stress corrosion cracking;Wet-dry cycle
Abstract The stress corrosion cracking(SCC)behavior and mechanism of 7050-T7451 aluminum alloy under wet-dry cyclic conditions were investigated.Slow strain rate tests(SSRTs)and electrochemical tests were used to study the effects of dry/wet ratio(DWR)and pre-immersion on SCC.Fracture and side surface characterizations were observed by scanning electron microscopy(SEM).The results demonstrate that SCC susceptibility decreases with an increase of the DWR.With an increase of the pre-immersion time,both continuous pre-immersion(CP)and wet-dry cyclic preimmersion(WDP)samples are more sensitive to SCC,and the cracking mode in the SCC fracture region is intergranular.Furthermore,the effect of WDP on SCC is greater than that of CP when the total time immersed in solution before an SSRT is the same with each other.In fact,each single wetdry cycle can be divided into three processes with respect to the change of solution on samples’surface.Volatilization of water on the surface results in an increase in solute concentration,thus accelerating corrosion.
1.Introduction
7xxx aluminum alloys are widely used in the aerospace industry due to their high specific strength and good mechanical properties.1–3The high strength is ascribed to the fine and uniformly distributed precipitates η′(MgZn2)in the matrix,which precipitate during artificial aging.4–6However,it is well known that high-strength 7xxx aluminum alloys are susceptible to stress corrosion cracking(SCC)which may cause a serious service failure particularly in the aerospace industry.7,8
The complexity of the environment faced by the aviation industry determines the complex conditions in which aluminum alloys are serviced.In recent years,the effects of environmental factors on the SCC susceptibility of aluminum alloys have been studied by many scholars.9–15For example,Ricker et al.have studied the influence of chloride ion activity on the SCC susceptibility of aluminum alloy 7075-T6,and deduced that chloride ions interacted chemically with the passivated surface in the potential gradient at the crack tip causing SCC.16The stress corrosion mechanism of 7150 alloy in NaCl solution has been investigated by Kumar et al.It was believed that local anodic dissolution(LAD)of grain boundary precipitates exacerbated by stress and accompanied by hydrogen induced cracking(HIC)was the probable mechanism to induce SCC in the 7150 alloy of T4 and T6 tempers.13All of these experiments were carried out under continuous immersion conditions.
However,it is inevitable for an airframe to suffer from changing environment such as wet-dry cyclic condition in service environment,so studying the effect of wet-dry cyclic condition on corrosion of aluminum alloys is of great interest.Shi et al.have studied the corrosion of 2024-T3 aluminum alloy in simulated acid rain under cyclic wet-dry condition,and revealed that each single wet-dry cycle could be divided into three regions with respect to the change of the cathodic reaction rate.17,18El-Mahdy et al.have investigated the influences of temperature,surface inclination,and relative humidity(RH)on the atmospheric corrosion of aluminum in a wetdry alternating process.An increase in temperature leads to an acceleration of the corrosion process.The corrosion rate of aluminum increases with increasing RH,and increases with a decrease of surface inclination.The results can be explained on the basis of increasing time of wetness.19However,to our best knowledge,there are few scholars who have investigated the SCC behavior and mechanism of aluminum alloys under wet-dry cyclic conditions.
This work aims to detect the stress corrosion behavior and mechanism of 7050-T7451 aluminum alloy under wet-dry cyclic condition with/without pre-immersion and with different ratios of D/W.In this paper,a slow strain rate test(SSRT),a electrochemical test,a scanning electron microscope(SEM),and a transmission electron microscope(TEM)are employed to obtain useful information on the effects of these factors.
2.Experimental
2.1.Material
The material used in this study was rolled 7050-T7451 aluminum alloy with a thickness of 15 mm.According to GB/T 16475-2008,T7451 treatment was developed to obtain a good combination of high strength,high resistance to SCC,and good fracture toughness.20,21The chemical composition of this alloy is listed in Table 1.The initial optical microstructure of 7050-T7451 alloy is shown in Fig.1,where L:longitudinal(rolling)direction;T:transverse direction.Samples were cut from a 7050 Aluminum alloy plate with the tensile axis parallel to the transverse direction.The sampling position was 1/5 at the thickness direction.The schematic diagram of SSRT specimens is shown in Fig.2.All specimens were abraded along the tensile direction using 600 grit,800 grit,1200 grit,and finally 1500 grit SiC abrasive papers,degreased in ethanol,and then dried by cool air prior to testing.

Fig.1 Microstructure of 7050-T7451 aluminum alloy.
2.2.Slow strain rate test(SSRT)
The SSRT method was used to investigate the SCC behavior of 7050 Aluminum alloy under wet-dry cyclic conditions.The aggressive medium used in this test was 1 M NaCl+0.3 M Na2SO4+0.15wt%H2O2.All the specimens were tested with a crosshead travel speed of 0.0012 mm·min-1,which was equivalent to a strain rate of 10-6s-1.Except for the gauge section,the test specimens were sealed with epoxy resin.Each test was repeated at least three times in order to ensure reproducibility of the measurements.After failure,the percentage of area reduction of each specimen was calculated after removing corrosion products in the solution(a mixture of 200 mL H3PO4(1.7 g/mL),80 g CrO3,and 800 mL deionized water)and cleaning with ethanol.Both the fracture and side surfaces of the specimens were observed by SEM.
Fig.3 is the schematic diagram of the set-up for the SCC test in wet-dry cyclic conditions.22A tensile specimen was installed in a plastic testing chamber connected to plastic pipes at the bottom and top of the chamber.During the tests,solution was stored in a reservoir and continually circulated through the testing chamber by a pump controlled with a timer.When the pump is suspended,the solution will flow out along the inlet pipe under the action of gravity.SCC tests were conducted for various conditions with four DWRs,with each wet-dry cycle conducted over a course of 1 h.For a DWR of 1(DW1),a specimen was immersed in solution for 30 min followed by air-drying for 30 min at room temperature.The other three ratios are 2,3,and 5(DW2,DW3,and DW5).In order to minimize the effect of chemical changes of solution on the test,a large ratio of the solution volume to the specimen surface(about 2000:1)was used.
2.3.Pre-immersion test
The pre-immersion test was divided into two types:CP and WDP.The time of CP was 10 h(CP10),20 h(CP20),and 50 h(CP50),while WDP was carried out for 20 h(WDP20),40 h(WDP40),and 100 h(WDP100)under the condition of DWR equal to 1,respectively.Therefore,the total time immersed in solution before an SSRT of the two kinds of samples was the same,which was critical to the qualitative analysis about the effect of the wet-dry alternating process on SCC.Then the SSRT was immediately carried out in the wet-dry alternating environment at the same DWR.Solution used in pre-immersion tests was the same as that of SSRTs in Section 2.2.

Table 1 Chemical composition of the 7050-T7451 aluminum alloy.
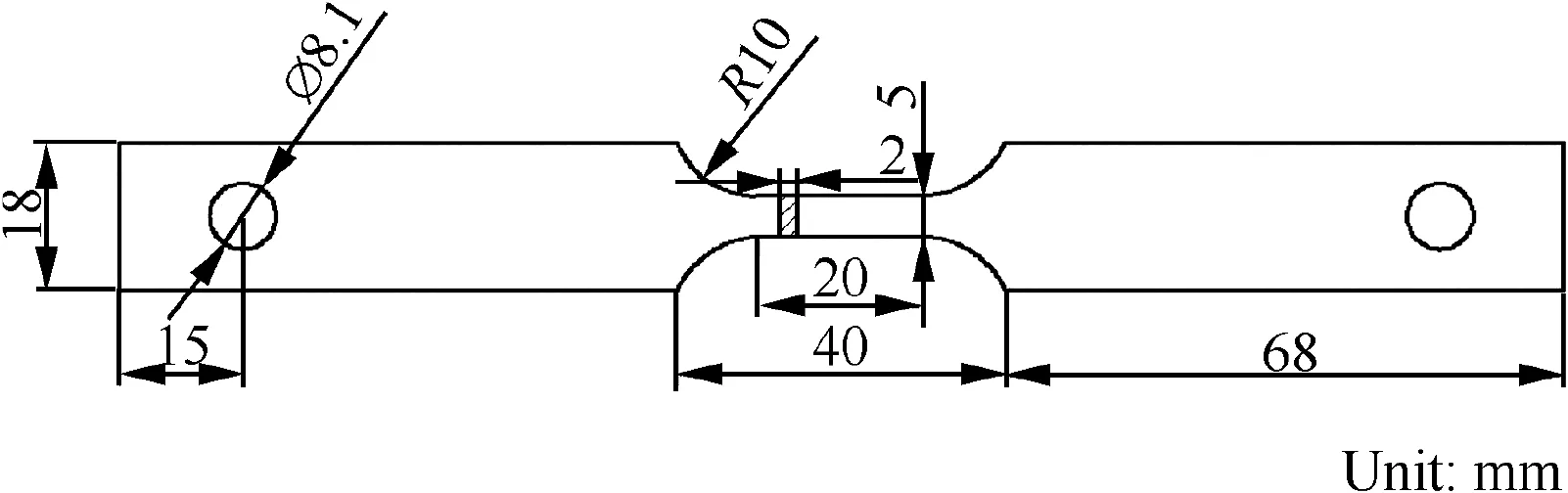
Fig.2 Schematic of SSRT specimens.

Fig.3 Schematic diagram of the set-up for wet-dry cyclic tests.
2.4.Electrochemical test
An electrochemical test was performed to reveal the differences between specimens in various corrosion environments.Before the experiment,specimens were immersed in solution without a load for a period of time.The experiment was performed in a conventional three-electrode cell system,in which a saturated calomel electrode(SCE)was used as a reference electrode and a platinum plate as a counter electrode.Specimens encapsulated in epoxy resin with an exposed surface area of approximately 1.0 cm2were used as working electrodes.All measurements were carried out at room temperature in a solution which was the same as that in Section 2.2,and measurements were started approximately 120 s after immersion.Prior to electrochemical tests,specimens were mechanically polished using a series of SiC abrasive papers up to 1500 grit,and then cleaned with deionized water and ethanol.Potentiodynamic polarization curves were acquired by scanning the potential range from-1000 to 100 mVSCEat a scanning rate of 1 mV/s.In this paper,unless otherwise specified,all potentials quoted in experiments referred to the SCE.
3.Results and discussion
3.1.SCC results
Fig.4 depicts the stress-strain curves for SSRTs in air and wet-dry cyclic conditions under various DWRs.The contrast samples tested in air are the same as those in solution.The drawing of a partial enlargement is shown in the lower right corner.In general,the flow stress increases sharply firstly before the strain reaching about 0.05 and then slowly reaches the maximum stress,followed by a subsequent reduction until final failure.The ductility indexes of specimens show significant decline and stand lower strains to fracture in solution compared to those in air.A stress drop is not obvious before the failure in solution compared with that obtained in air,indicating an obvious brittle fracture of the specimens.Tensile strength(TS)and elongation increase gradually with an increase of the DWR.The specific values are listed in Table 2.
Stress-strain curves of 7050 alloy in wet-dry cyclic condition after two types of pre-immersion are shown in Fig.5.The estimated TS and elongation decrease in the order CP10>CP20>CP50in CP conditions and WDP20>WDP40>WDP100 in WDP conditions.With an increase of the preimmersion time,a penetration of corrosive ions increases the degree of corrosion in the matrix(Fig.8).As a result,the TS of samples decreases during the SSRT,and specimens will break earlier.
A comparison between CP and WDP conditions shows that there is no significant reduction in the TS and elongation when the total immersion time is less than 50 h.However,the TS and elongation are significantly lower under the WDP condition than those under the CP condition when the total time immersed in solution before the SSRT increases to a certain value(50 h),which shows that WDP100 is more sensitive to SCC than CP50.It can be concluded that the wet-dry alternating process can increase the SCC susceptibility,and the SCC resistance reduction caused by the wet-dry alternating process will be more obvious when the number of cycles is further increased.In fact,each single wet-dry cycle can be divided into three processes.In Process I,samples are completely immersed in the solution.In Process II,the solution on the surface is dried naturally after samples are removed from the solution.In this experiment,the time of Process II is about 30 min.In Process III,the samples’surfaces are completely dry.For DWx specimens,there are two processes(I and II)for DW1 during the SSRT.That is,DW1 is immersed in solution for 30 min and then dried naturally for 30 min at room temperature,and the above process is repeated until fracture.For DW2,Process I is 20 min,Process II is 30 min,and Process III is 10 min.The three processes of DW3 are 15 min,30 min,and 15 min.For DW5,the three processes are 10 min,30 min and 20 min.In this part of the experiment,the time of Process II is the same for all DWx specimens,so a smaller DWR means a longer immersion time(Process I)and a more serious corrosion of the sample,leading to higher SCC susceptibility.There is only Process I for CPx specimens in the period of pre-immersion.On the contrary,WDPx specimens have two processes(I and II),and the total time of Process I is the same as that of CPx samples.There is no doubt that the difference between WDPx samples and CPx samples is ascribed to Process II at the same total time immersed in solution.It is noteworthy that hydrogen peroxide in the solution during the wetdrying process rapidly evaporates,leading to the fact that the corrosion in the process drying out of moisture essentially involves oxygen in the air.In summary,the wet-dry process has a significant effect on the occurrence of SCC.

Fig.4 Stress-strain curves of 7050 alloy in air and in wet-dry cyclic conditions under various DWRs.

Table 2 SSRT results for the 7050 specimens with and without pre-immersion at OCP in air and solution.
The SCC susceptibility index(ISCC(ψ))is defined as the loss of reduction of area after the SSRT as follows:

where ψ0and ψSCCare the values of reduction of area in the air and in the corrosive solution,respectively.The greater the ISCC(ψ)values are,the more susceptible the SCC is.
According to Eq.(1),the variations of SCC susceptibility obtained using SSRTs at various DWR conditions and at the DWR of 1 after pre-immersion are shown in Fig.6.Each data point is the average of tests conducted on at least three samples.It is apparent in Fig.6(a)that SCC susceptibility decreases in the following order:DW1(84.80%)>DW2(82.23%)> DW3(62.35%)> DW5(54.90%),which is consistent with its performance on stress-strain curves shown in Fig.4.As shown in Fig.6(b),SCC susceptibility increases gradually with a prolongation of the pre-immersion time for both CP and WDP specimens.The effect of wet-dry cycles on SCC susceptibility is observed for WDP specimens,which is more sensitive to SCC compared with CP samples as illustrated in Fig.5 and Table 2.
3.2.SEM micrographs
Fig.7 presents the typical tensile fracture surfaces of 7050 alloy at various DWRs.As demonstrated in Fig.7(a),the fractograph of DW1 shows mostly intergranular brittle fracture surface.Elongated pancake shape grains are revealed,which are well separated along the grain boundary.The fractograph of DW2 shown in Fig.7(b)has a relatively small portion of an intergranular crack region with a little portion containing cleavage-like fracture.In both of the fractographs(Fig.7(a)and(b)), dimples are absent,and some secondary cracks are visible(indicated by white arrows).The micrograph of DW3(Fig.7(c))exhibits some dimples,and such intergranular cracks are also observed.The presence of dimples proves that SCC resistance is increased in this situation.With an increase of the DWR,large area fractions of dimples and small areas with cleavage-like fracture are present on the fracture surface of DW5(Fig.7(d)).In fact,the area of intergranular stress corrosion cracking(IGSCC)is clearly observed on the macroscopic fracture morphology.The percentage of the IGSCC region decreases in the following order:DW1>DW2>DW3>DW5.The numerical values of samples listed in Table 3 are calculated according to the ratio of the IGSCC area(marked with red line)to the fracture area in the picture.

Fig.6 SCC susceptibility index(ISCC(ψ))under different conditions.

Fig.7 SEM images of fracture surfaces after SSRTs.
The morphologies of the fracture surfaces of various samples in the solution containing hydrogen peroxide after SSRTs are shown in Fig.8.As shown by macro images of the fractures,the percentage of the IGSCC region is gradually increased by increasing the pre-immersion time for both CP and WDP specimens.Compared with the numerical values listed in Table 3,the percentage of the IGSCC region of WDP samples is higher than that of CP samples when the total time immersed in solution is the same.This result agrees with the SSRT and SCC susceptibility,that is,the corrosion of the samples is still under way in Process II.There are obvious intergranular fractures in the micrographs in Fig.8,while some dispersoid particles are observed on intergranular facets in Fig.8(a),which shows that the corrosion degree of CP10 is lower than those of other specimens.In fact,these particles are impurity phases containing Fe,Si,and Mn.23For comparison,there is an obvious dissolution area on the fracture surface of WDP100,while no obvious corrosion trace is observed on CP50.Since the times of these two samples immersed in solution are the same during the process of pre-immersion,the relatively severe corrosion degree of WDP100 is attributed to Process II of wet-dry alternation.
Fig.9 shows the SEM morphology of the side surface for specimen DW1 after the SSRT.There are a number of intergranular cracks on the sample’s surface.The cross sections of the specimen are shown in Figs.10 and 11.Localized corrosion attack is clearly seen on the specimen edge.Such attack is believed to occur along the grain boundary(Figs.7–9).In this experiment,corrosion along the grain boundary is accelerated because of hydrogen peroxide in the solution.When a large number of grains fall off due to grain boundary dissolution,localized corrosion pits(an IGSCC region)are formed(Figs.7,8,and 10).The grain boundary region of 7xxx series alloys contains grain boundary precipitates(GBPs)and a precipitate-free zone(PFZ)next to the grain boundary.All GBPs are steady phase and pretty coarse.Fig.12 is the TEM micrograph of 7050-T7451 aluminum alloy.GBPs and a PFZ can be clearly observed at the grain boundaries.It is well known that GBPs have considerable alloy elements contents like Zn and Mg,which are more active than the aluminum matrix.24,25Birbilis and Buchheit measured the anodic polarization curves of MgZn2in 0.1 M NaCl(pH=6).Their results reveal that MgZn2are active particles with a high self-dissolution rate.26The dissolution of the GBPs affects the microchemistry of the grain boundary region.Incoherent and active GBPs accelerate the local corrosion initiation,since the severe heterogeneity of chemical components in the grain boundary region produces a number of micro galvanic cells.27In this part of the experiment,under applied loading during testing,the galvanically corroded regions(i.e.,dissolved precipitates)acts as crack initiation sites and crack propagation paths as well resulting in IGSCC.Therefore,local anodic dissolution along the grain boundary is the operating model inducing SCC.13,28

Table 3 Percentage of the dissolved area measured according to the fractures after SSRTs.

Fig.8 SEM images of fracture surfaces after SSRTs for pre-immersion specimens.

Fig.9 SEM morphology of the side surface for specimen DW1 after the SSRT.
3.3.Electrochemical characterization

Fig.10 Optical photomicrograph of the cross section for specimen DW1 after the SSRT.
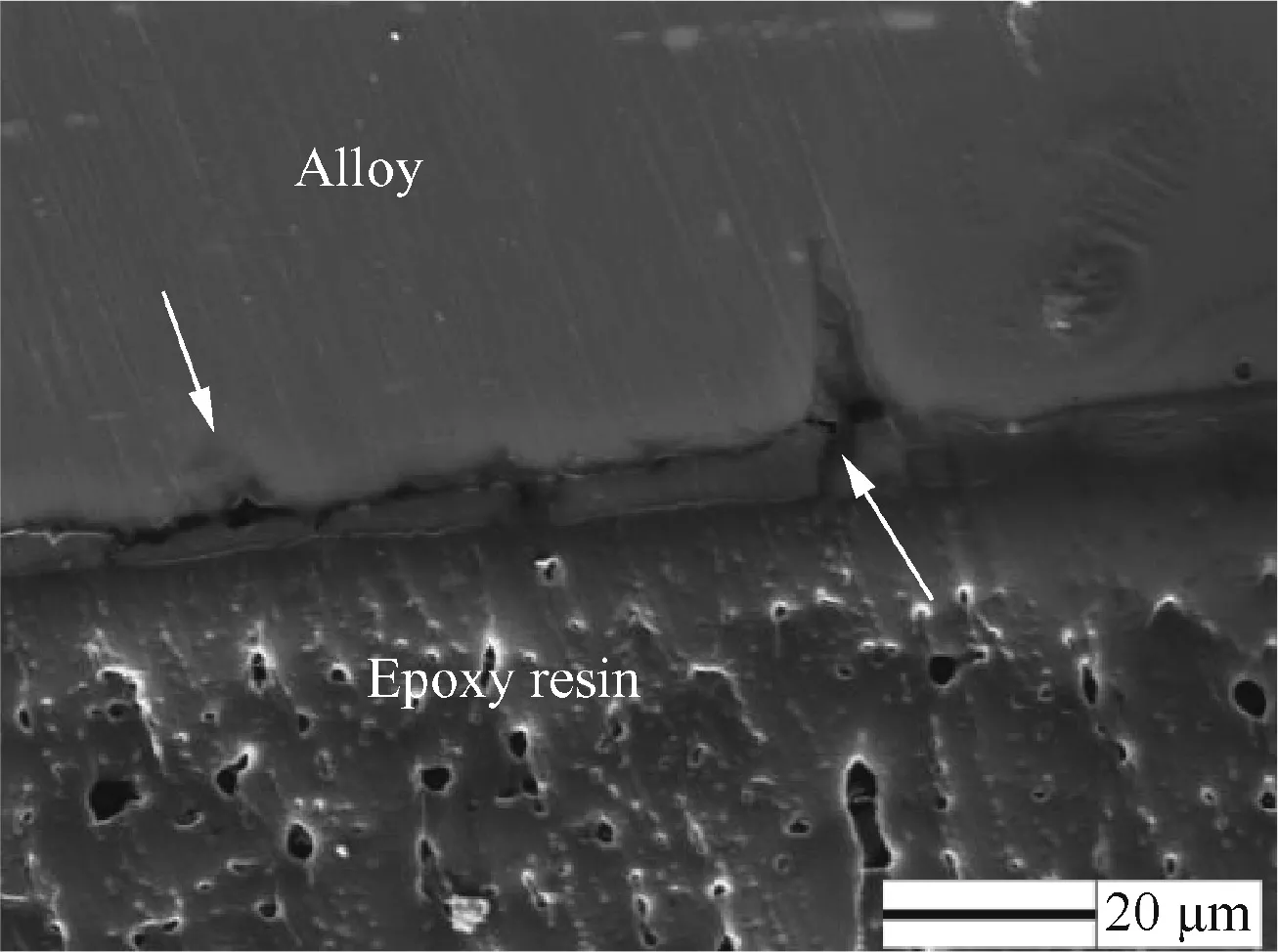
Fig.11 SEM image of the cross section for specimen DW1 after the SSRT.
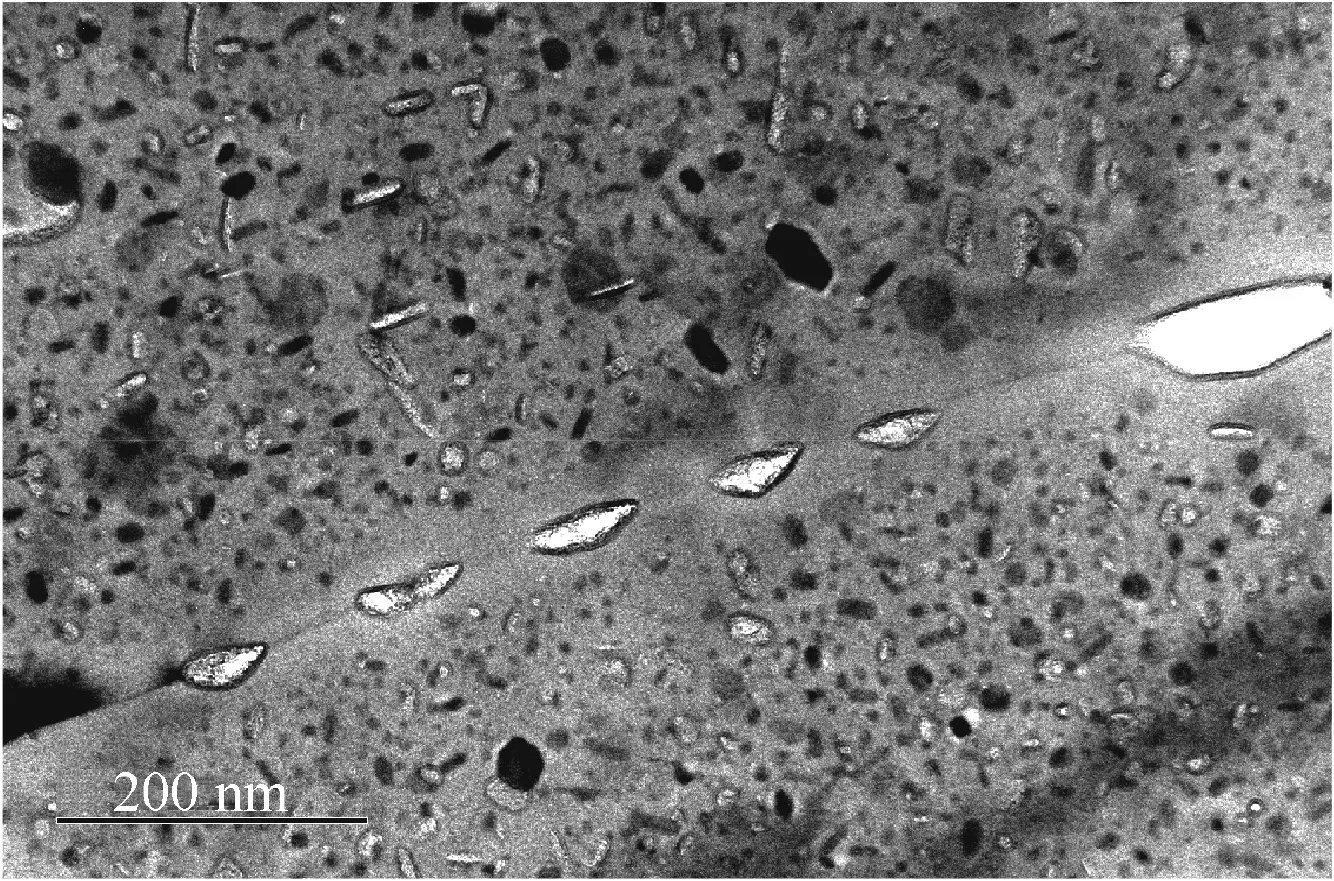
Fig.12 TEM micrograph of 7050-T7451 aluminum alloy.
Fig.13 demonstrates the potentiodynamic polarization curves of DWx specimens after being immersed in the solution for 20 h without load.The cathodic branches have almost no difference in shape,indicating that the cathodic reactions of corrosion performed on all of the DWx specimens are the same.It is noticed that when the applied over-potential is higher than the corrosion potential(Ecorr)between 50 and 250 mV at the anodic branch and above 30 mV at the cathodic branch relative to Ecorr,the log current density increases linearly with the applied potential,but the Tafel range in this test is much smaller than the traditional Tafel range whose over-potential range is generally over 100 mV.2,29This may be attributed to the existence of hydrogen peroxide in solution leading to a rapid cathodic rate below Ecorrand fast anodic dissolution of aluminum above Ecorr,since hydrogen peroxide is usually an intermediate material of oxygen reduction.30,31Therefore,the corrosion current density(Icorr)is still calculated using Tafel extrapolation,as listed in Table 4.Both anodic and cathodic Tafel slopes(baand bc)increase with an increase of the DWR,implying that both the anodic and cathodic reactions of corrosion are retarded.A sudden reduction of bafor DW5 may be attributed to the unstable film.The difference of Ecorrcould be distinguished based on the detailed drawing.Ecorrincreases in the following order:DW1(-748 mV)<DW2(-743 mV)<DW3(-721 mV)<DW5(-714 mV),indicating a gradual decrease in the tendency to corrode with an increasing DWR.Ecorrof DW3 is obviously higher than that of DW2,which is consistent with the trend of SCC susceptibility in Fig.6(a).The SEM diagrams in Fig.7 show that the fractures of DW3 and DW5 specimens have obvious ductile fracture components,while those of DW1 and DW2 are brittle fractures.Therefore,there exists a critical WDR between DW2 and DW3,at which a sample is transformed from brittle fracture to mixed fracture.

Fig.13 Potentiodynamic polarization curves of DWx specimens after being immersed in the solution for 20 h.
The potentiodynamic polarization curves of the samples obtained after different times of pre-immersing are presented in Fig.14.In this part of the experiment,when the applied over-potential was above tens to a hundred millivolt relative to Ecorr,the log current density increased linearly with the applied potential and approached a Tafel-type behavior.The relevant results of polarization curves analyzed using Tafel extrapolation are listed in Table 5.P0 is the specimen without pre-immersion.As shown in Fig.14,Ecorrbecomes negative with an advance of the pre-immersion time for both CP and WDP specimens,which may be related to the dissolution of copper and iron-rich intermetallic particles.According to references,the potential of copper and iron-rich intermetallic particles is more than 200 mV higher than that of the surrounding matrix,and thus particles containing copper and iron act as a cathode in a micro-galvanic cell with active phases while the metal matrix as an anode.26,29Wang et al.reported that a change of Cu in the matrix solid solution can alter the pitting potential of 7xxx series alloys.32Therefore,the dissolution of copper and iron results in a decrease in Ecorrand an increase in corrosion sensitivity.In addition,the dissolution of grain boundaries leads to stress concentration,resulting in crack initiation at the grain boundaries.This is consistent with the previous analysis.Comparing two kinds of pre-immersion samples with the same total pre-immersion time(such as WDP100 and CP50 specimens listed in Fig.15),Ecorrof WDP100 is obviously lower than that of CP50,indicating that the sample is still corroded during the transition from a wet state to a dry state,i.e.,Process II mentioned in Section 3.1.bain this work is far bigger than the values of 20–30 mV/dec caused by aluminum oxidation to Al3+,which could be attributed to the product film of alloys.33,34-bcalso shows higher values than the cathodic reactions controlled by oxygen reduction(about 120 mV/dec).34–37It is also noticed that Icorrdecreases with the prolongation of the pre-immersion time,which indicates that the corrosion resistance increases gradually.It seems to be contrary to the previous conclusions.The previous experiment shows that the longer the pre-immersion time is,the more sensitive the SCC is(Figs.5,6(b),and 8).In principle,once the corrosion products are formed on the specimens in the testing environment,the nature of the corrosion products plays a role on SCC-resistance.SCC susceptibility will decrease under this condition.In fact,the thickness of the corrosion product film does become thicker as the preimmersion time increases(as listed in Table 2),resulting in a decrease of the current density on the surface.However,the samples are continuously elongated during SSRT,resulting in a rupture of the corrosion product film and further corrosion of the metal matrix(Fig.11).In addition,OH-produced by the reduction of hydrogen peroxide leads to a breakdown of the passive film and dissolution of the adjacent aluminum matrix.34

Table 4 Tafel extrapolation results of potentiodynamic polarization curves of DWx specimens.
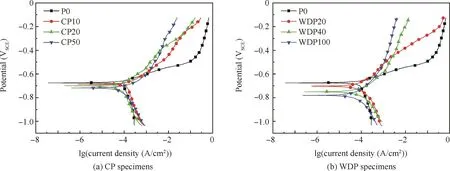
Fig.14 Potentiodynamic polarization curves of working electrodes after various periods of pre-immersion time.

Table 5 Tafel extrapolation results of potentiodynamic polarization curves of CPx and WDPx specimens.

Fig.15 Potentiodynamic polarization curves of CP50 and WDP100.
4.Conclusions
(1)SCC susceptibility decreases with an increase of the D/W ratio.
(2)Whether it is wet-dry pre-immersion or continuous preimmersion,SCC susceptibility increases with an increase of the immersion time.This is attributed to a rupture of the corrosion product film and destruction of OH-produced by reduction of hydrogen peroxide.
(3)When the total time immersed in solution before an SSRT is the same between CP and WDP,the effect of WDP on SCC is greater than that of CP,which indicates that corrosion on the surface of alloy continues to occur in Process II.
(4)Anodic dissolution of active precipitations occurs at grain boundaries,and stress concentration causes crack initiation and propagation along grain boundaries resulting in IGSCC.Therefore,anodic dissolution is the operating model that has induced SCC of 7050-T7451 aluminum alloy in the solution.
Acknowledgements
This study was co-supported by the National Natural Science Foundation of China(No.51671013)and the Beijing Nova Program of China(No.Z161100004916061).
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- Effect of rotor-mounted protrusion on sealing performance and flow structure in rotor-stator cavity
- Application of shear-sensitive liquid crystal coating to visualization of transition and reattachment in compressor cascade
- Experimental and numerical study of chaffcloud kinetic performance under impact of high speed air flow
- Delaunay graph-based moving mesh method with damping functions
- Performance analysis of variable speed tail rotors with Gurney flaps
- Numerical investigation of transitions in flow states and variation in aerodynamic forces for flow around square cylinders arranged inline
