由2-甲基-4-噻唑甲酸构筑的过渡金属配合物的合成、晶体结构及与DNA作用
2018-12-10张敏芝武大令赵国良
张敏芝 武大令 沈 伟 赵国良*,,2
(1浙江师范大学化学与生命科学学院,金华 321004)
(2浙江师范大学行知学院,金华 321004)
0 Introduction
In recent year,the rational design and synthesis of coordination polymers based on metals and organic ligands have attracted remarkable attention due to their structural diversities[1-2]and potential applications in gas adsorption[3-4], catalysis[5],luminescence[6-7],bioactivity[8],electronic and magnetic[9]fields.Extensive investigations show that the formation,molecular structures and properties of coordination polymers are influenced by many factors,such as the nature of the organic ligands,metal-ligand ratio,crystallization conditions.So,it is possible to develop a novel targeted structure with tunable properties through the proper choice of organic ligands and metal ions.
In the previous reports,extensive works have been carried out by using heterocyclic carboxylate ligands[10-13],because heterocyclic systems are rich in electrons that can coordinate with metal ions,and easily form non-covalent bonds such as hydrogen bonds,aromatic packing,electrostatic and hydrophobic interactions.Carboxylate groups play important roles in many organic ligands,having many different coordinating modes.What is more,deprotonated carboxylate groups could form hydrogen bonds to participate in supramolecular self-assembly with coordination bonds as acceptors.Therefore,heterocyclic carboxylate ligands are potential candidates for novel functional coordination polymers,which offer a self-assembly solution that can be expected and controlled in certain extent.
Thiazole and its derivatives are an important class of heterocyclic compounds containing N,S atoms,exhibiting a variety of biological activities[14]such as good antibacterial[15-16],anti-fungal[17-18],antitumor[19-21],herbicidal[22-23]properties.Therefore,it is worth carrying out the research concerning about mechanisms and bonding abilities between transition metal complexes with thiazole carboxylate system and DNA.The further study could help us to design and synthesize DNA secondary structure probes,nucleic acid location reagents and anti-cancer drugs[24].
In this paper,a novel ligand 2-methyl-4-thiazolecarboxylic acid (HMTZA)were designed and synthesized,thereby three novel transition metal complexes were synthesized and structurally characterized by some measurements.The interactions of ligand and complexes with ct-DNA were also studied by EtBr fluorescence probe.
1 Experimental
1.1 Materials and measurements
All of the reagents and solvents employed were analytical grade and used without further purification.Elemental analyses of C,H,N were performed on elemental analyzer,Elementar Vario ELⅢ.Crystallographic data of the polymers were collected on a Bruker Smart ApexⅡCCD diffractometer.FTIR spectra were recorded on a Nicolet NEXUS 670 FTIR spectrophotometer using KBr discs in the range of 4 000~400 cm-1.A Mettler Toledo thermal analyzer TGA/SDTA 851ewas used to carry out the thermos analysis with a heating rate of 10℃·min-1from 30 to 800℃in air atmosphere.Fluorescence spectra were measured at room temperature with an Edinburgh FLFS920 TCSPCsystem.1H NMR spectra of ligand were acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMSas internal standard.
1.2 Synthesis of the ligand
2-Methyl-4-thiazolecarboxylate and 2-methyl-4-thiazolecarboxylic acid (HMTZA)were synthesized according to literature (Fig.1)[25].
1.2.1 Synthesis of 2-methyl-4-thiazolecarboxylate

Fig.1 Synthesis of HMTZA
Ethyl bromopyruvate (2.6 mL,20 mmol)was dissolved in absolute ethanol (15 mL).Meanwhile,thioacetamide (1.50 g,20 mmol)and absolute ethanol(15 mL)were added to a flask,placed in a water bath at 60℃and stirred for about 10 min to dissolve completely.Then,the above bromopyruvic acid solution was added dropwise to the flask.After the dropwise addition of it,the temperature of the water bath was raised to 80℃,and the mixture was further stirred under reflux for 6 hours to obtain a pale yellow transparent liquid,then,cooled to room temperature.The solution was neutralized with NaHCO3(3.90 g).The aqueous layer was extracted with Et2O,and the combined organic layer was dried over anhydrous Na2SO4,filtered,and concentrated under reduced pressure.The crude residue was purified by flash chromatographywith petroleum ether/ethyl acetate (2∶1,V/V).Yield:85%.
1.2.2 Synthesis of 2-methyl-4-thiazolecarboxylic acid(HMTZA)
2-Methyl-4-thiazolecarboxylate (3.15 mmol,0.54 g)was added to the mixture of THF (10 mL)and 10%KOH solution (3.0 mL)at room temperature and stirred continuously for 2 h.HCl (5 mol·L-1)was added until pH=3.The aqueous phase was extracted with ethyl acetate,and dried over anhydrous Na2SO4,and evaporated under reduced pressure.Yield:80%.Anal.Calcd.for C5H5NO2S(%):C,41.95;H,3.52;N,9.78.Found(%):C,41.87;H,3.56;N,9.71.IR(KBr,cm-1):3 101(s),1 677(s),1 500(m),1 485(m),1 447(w),1 411(m),1 229(s),1 178(m),1 109(m),938(m),734(w).1H NMR:δ2.82 (3H,s,CH3);8.19 (1H,s,H-5);10.51(1H,s,COOH).
1.3 Syntheses of the complexes
1.3.1 Synthesis of[Co(MTZA)2(H2O)2]·3H2O (1)
A mixture of HMTZA ligand (0.148 g,1.0 mmol),Co(CH3COO)2·4H2O (0.125 g,0.5 mmol)and H2O (10 mL)/EtOH (10 mL)was put into a 50 mL vessel.Then,KOH solution (10%)was added dropwise until pH=5.And the mixture was stirred and heated in a water bath at 60℃for about 30 mins.The resulted solution was filtered while hot and cooled to room temperature.After a week,red crystals suitable for single-crystal analysis and physical measurements were obtained.Yield:40% (based on HMTZA).Anal.Calcd.for C10H18N2O9S2Co(%):C,27.71;H,4.16;N,6.47.Found(%):C,27.59;H,4.20;N,6.42.IR(KBr,cm-1):3 257(s),3 121(s),1 605(s),1 539(m),1 489(m),1 357(s),1 284(m),1 197(s),961(w),774(w),752(w),628(w).
1.3.2 Synthesis of[Cu(MTZA)2(H2O)]·2H2O (2)
The preparation method of complex 2 was similar to complex 1 using CuCl2·2H2O (0.085 g,0.5 mmol)instead of Co(CH3COO)2·4H2O,and concentrated ammonia instead of KOH solution was added dropwise until pH=9.After volatilizing naturally for a week,blue crystals suitable for single-crystal analysis and physical measurements were obtained.Yield:56%(based on HMTZA).Anal.Calcd.for C10H14N2O7S2Cu(%):C,29.88;H,3.49;N,6.97;Found(%):C,29.79;H,3.45;N,6.92.IR (KBr,cm-1):3 386 (s),1 638(s),1 580(s),1 508(m),1 444(s),1 337(m),1 288(m),1 185(s),1 056(w),853(w),782(w).
1.3.3 Synthesis of[Zn(MTZA)2(H2O)2]·3H2O (3)
The synthetic method was the same as complex 1,justreplacing Co(CH3COO)2·4H2Owith Zn(CH3COO)2·2H2O (0.110 g,0.5 mmol).After volatilizing naturally for a week,colorless crystals suitable for single-crystal analysis and physical measurements were obtained.Yield:49% (based on HMTZA).Anal.Calcd.for C10H18N2O9S2Zn(%):C,27.31;H,4.10;N,6.37.Found(%):C,27.39;H,4.13;N,6.31.IR(KBr,cm-1):3 395(s),3 121(s),1 630(s),1 527(m),1 489(s),1 357(s),1 286(s),1 197(s),1 109(w),1 006(w),961(m),774(m),753(m),627(m).
1.4 Single X-ray crystallographic study
The single crystals of the complexes with approximate dimensions suitable for single-crystal analysis were selected and mounted on a Bruker Smart ApexⅡCCD diffractometer.A graphite monochromated Mo Kα radiation (λ=0.071 073 nm)wasused to collect the diffraction data at 296(2)K.Absorption corrections were applied using SADABS[26].The correction for Lp factors was applied.The structure was solved by using the SHELXS-97[27]program package and refined with the full-matrix least-squares technique based on F2using the SHELXL-97[28]program package.All non-H atoms were anisotropically refined.Remaining hydrogen atoms were added in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.Hydrogen atoms on water molecules were located in a difference Fourier map and included in the subsequent refinement using restrains(O-H 0.085 nm)with Uiso(H)=1.5Ueq(O).Detailed information about the crystal data is summarized in Table 1,and the selected bond lengths and angles are given in Table 2,3 and 4.All hydrogen bonds are given in Table 5.
CCDC:1830898,1;1830896,2;1830891,3.
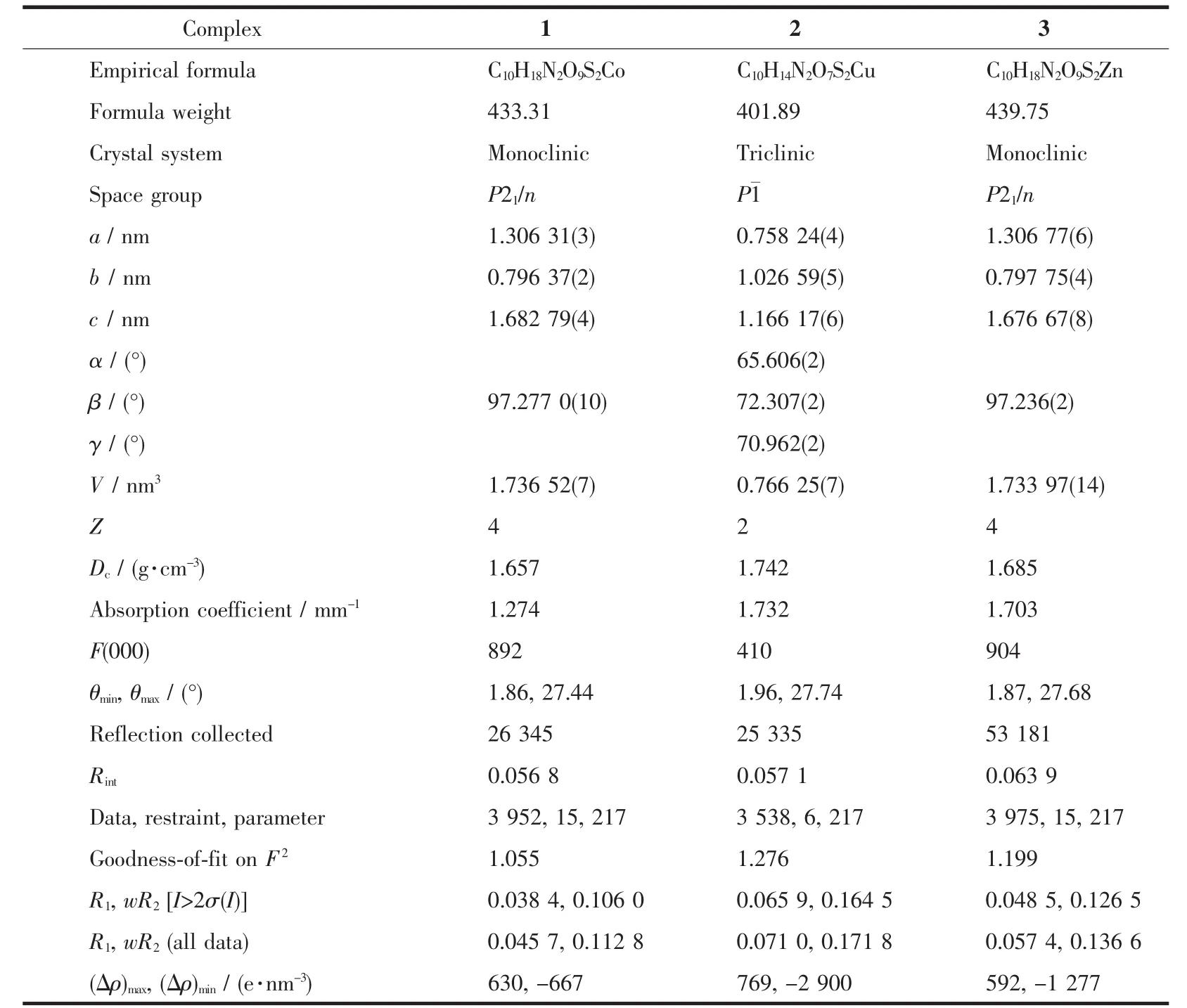
Table 1 Crystallographic data for complexes 1~3

Table 2 Selected bond lengths(nm)and angle(°)of complex 1
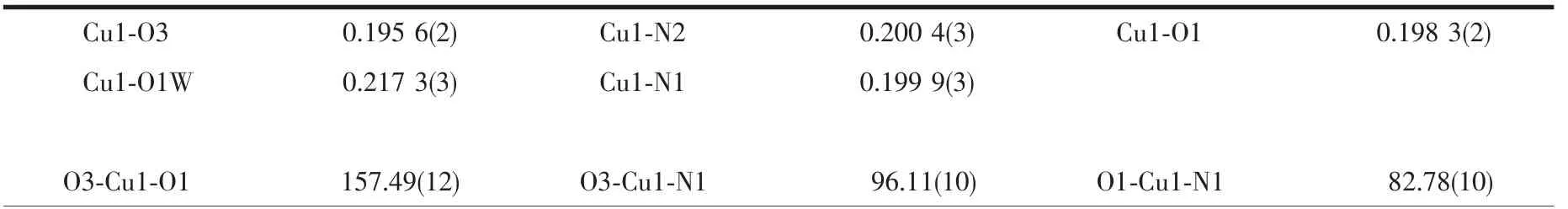
Table 3 Selected bond lengths(nm)and angle(°)of complex 2
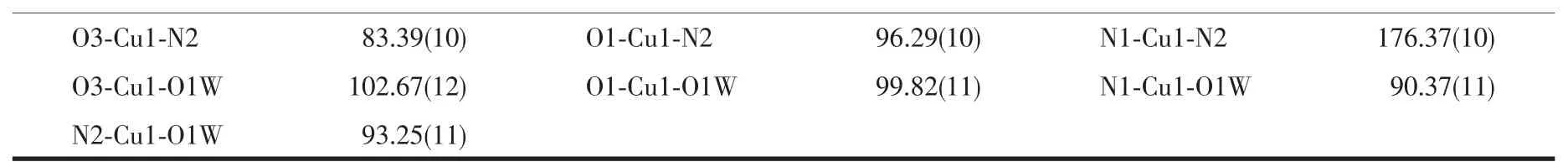
Continued Table 3

Table 4 Selected bond lengths(nm)and angles(°)of complex 3
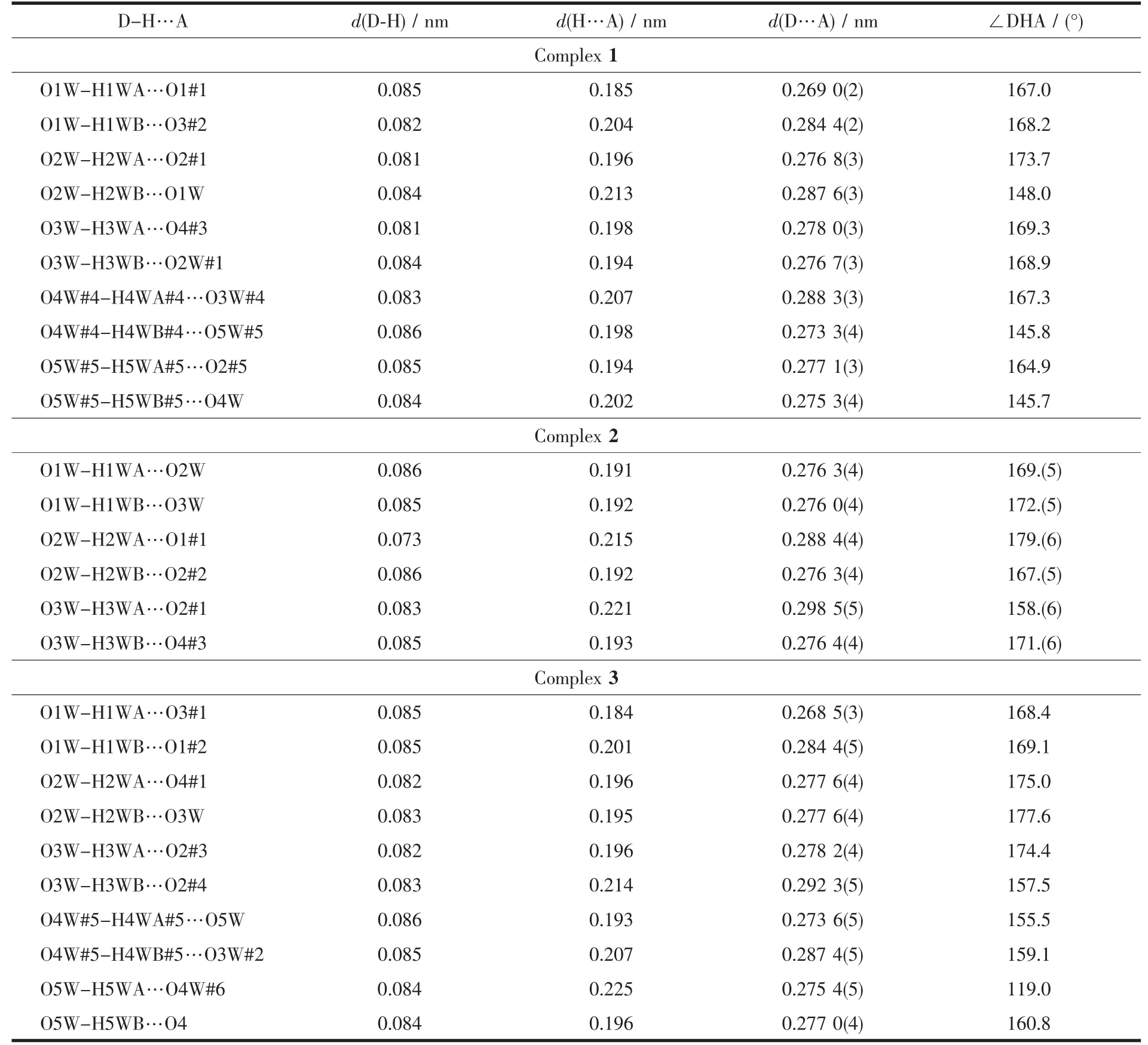
Table 5 Hydrogen bond distances(nm)and bond angles(°)of complexes 1~3
2 Results and discussion
2.1 Crystal structure analysis of the complexes
2.1.1 Structure analysis of complex 1
Single-crystal X-ray analysis reveals that complex 1 crystallizesin themonoclinic systemwith spacegroup P21/n and Z=4.Each asymmetric unit contains one Co2+ion,two independent MTZA-ligands,two coordinated water and three crystal water molecules(Fig.2).The Co2+ion adopts six-coordinated mode with a distorted octahedral coordinated geometry,by two N atoms from two MTZA-ligands (N1,N2)and two carboxyl Oatomsfromtwo MTZA-ligands(O1,O3)and two coordinated water (O1W,O2W).The O1W,O2W,N1,N2 are located in the equatorial plane,and the O1,O3 occupy the axial positions.The distance of Co-O is 0.209 55(17)to 0.211 51(18)nm,Co-N is 0.215 0(2)and 0.212 4(2)nm,respectively,according with the distance of Co-O and Co-N in the literature[29-30].
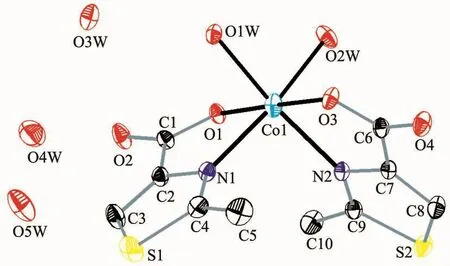
Fig.2 Ellipsoidal structural view of complex 1 with probability level of 30%

Fig.3 Hydrogen bonding interactions of complex 1
Intermolecular hydrogen bonding interactions is listed in Table 5 (Fig.3).Interestingly,the noncoordinated oxygen atom O2 and O2W from coordinated water and O3W,O4W,O5W from water molecules in complex 1 form a five-membered ring structure through hydrogen bonds.The five-membered rings are regularly connected to each other,forming a 1D chain along the c axis.Rich hydrogen bonds(O1W-H1WA…O1#1,O1W-H1WB…O3#2,O2WH2WB…O1W,O3W-H3WA…O4#3)make the molecules further connect into a 3D supramolecular network (Fig.4).
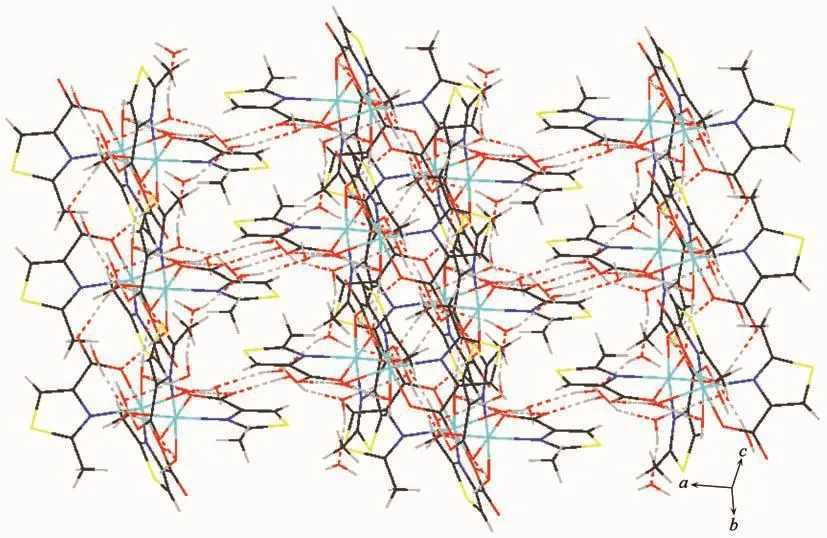
Fig.4 Three-dimensional supramolecular network of complex 1
2.1.2 Structure analysis of complex 2
Single-crystal X-ray analysis shows that complex 2 crystallizes in the triclinic system with space group P1,and Z=2.Each asymmetric unit consists of one Cu2+ion,two independent MTZA-ligands,one coordinated water and two crystal water (Fig.5).Each Cu2+ion adopts five-coordinated mode with a distorted pentahedral geometry,by one coordinated water(O1W)and two N atoms (N1,N2)from two ligands and two carboxyl O atoms (O1,O3)from the two ligands.The O1,O3,N1 and N2 are located in the basal plane,whereas the O1W from the coordinated water occupies the axial position.The distance of Cu-O is in the range of 0.195 6(2)~0.217 3(3)nm,and Cu-N is 0.199 9(3)and 0.200 4(3)nm,respectively,according with the distance of Cu-O and Cu-N in the literature[31-32].

Fig.5 Ellipsoidal structural view of complex 2 with probability level of 30%
As shown in Fig.6,the coordinated water molecule (O1W)and crystal water molecules(O2Wand O3W)in complex 2 act as hydrogen donors,contributing hydrogen atoms to O2W,O3W,O1#1,O2#2,O2#1 and O4#3 forming the hydrogen bonds.Interestingly,the abundant hydrogen bonds (H1WA…O2W 0.191 nm,H1WB…O3W 0.192 nm,H2WB…O2#2 0.192 nm,H3WA…O2#1 0.221 nm)form a more stable eight-membered ring chair structure.The more stable structures further connect the molecules into a 2D network through the intermolecular hydrogen bonding interactions.
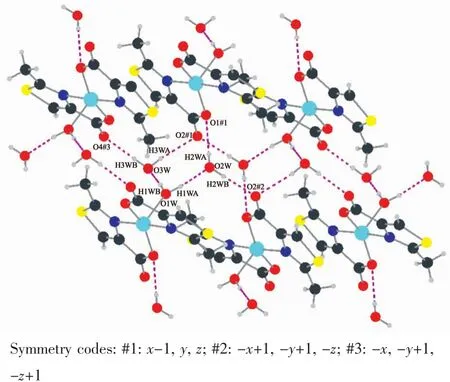
Fig.6 Hydrogen bonding interactions of complex 2
2.1.3 Structure analysis of complex 3
Single-crystal X-ray analysis shows that complex 3 crystalizes in monoclinic,space group P21/n,and Z=4.As shown in Fig.7,each asymmetric unit contains one Zn2+ion,two independent MTZA-ligands,two coordinated water and three crystal water molecules.Each Zn2+ion is six-coordinated with two N atoms from two ligands (N1,N2)and two carboxyl O atoms from the two ligands (O1,O3)and two coordinated water (O1W,O2W).The O1W,O2W,N1,N2 are located in the equatorial plane,whereas the O1,O3 from the ligands occupy the axial positions,forming a distorted octahedral coordinated geometry by considering short-range atomic interactions.The distance of Zn-O ranges from 0.207 2(2)to 0.211 7(2)nm,and Zn-N is 0.216 9(3)and 0.213 0(3)nm,respectively,according with the distance of Zn-O and Zn-N in the literature[33-34].

Fig.7 Ellipsoidal structural view of complex 3 with probability level of 30%
Intermolecular hydrogen bonding interactions is observed in complex 3 (Fig.8).As shown in Fig.9(a)and (b),respectively,a non-coordinated oxygen atom O4 and O2W#2 from coordinated water and O3W#2,O4W#1,and O5W from water molecules in complex 3 form a five-membered ring structure through hydrogen bonding,meanwhile,O2#2,O2#5,O3W#3 and O3W#4 form a four-membered ring structure.It is worth noting that the four-membered ring and the fivemembered ring are connected to each other through abundant hydrogen bonds,eventually forming a two-dimensional network (Fig.9 (c).The two-dimensional network structure further connects the entire structure into a 3D supramolecular network through hydrogen bonding (O1W-H1WA…O3#1,O1W-H1WB…O1#2)(Fig.10).
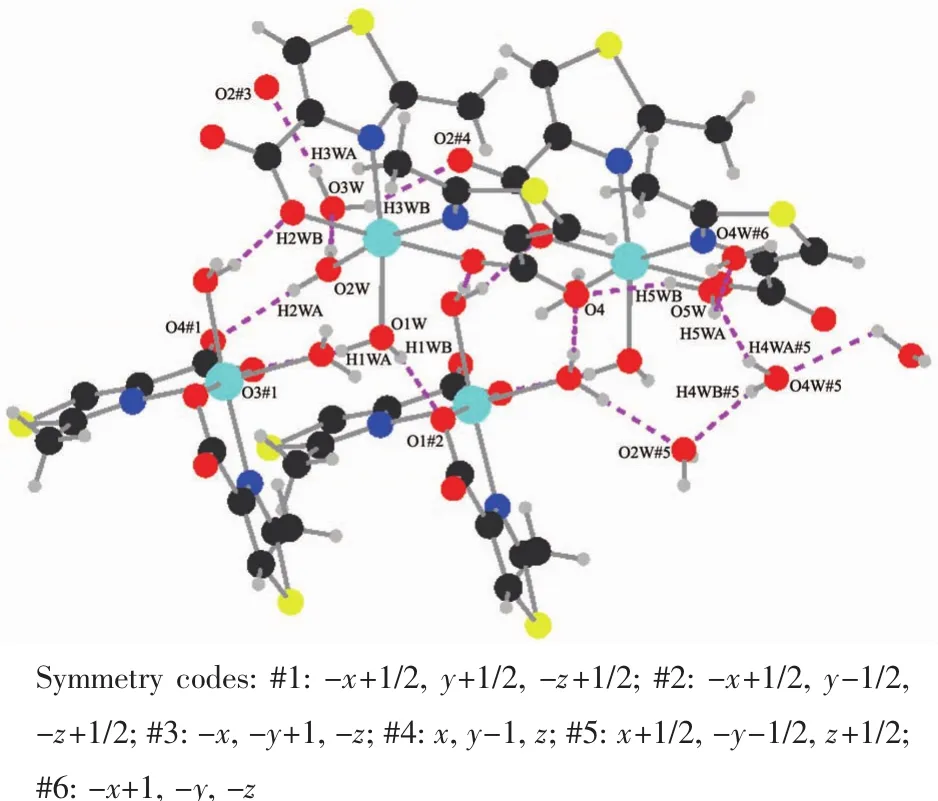
Fig.8 Hydrogen bonding interactions of complex 3
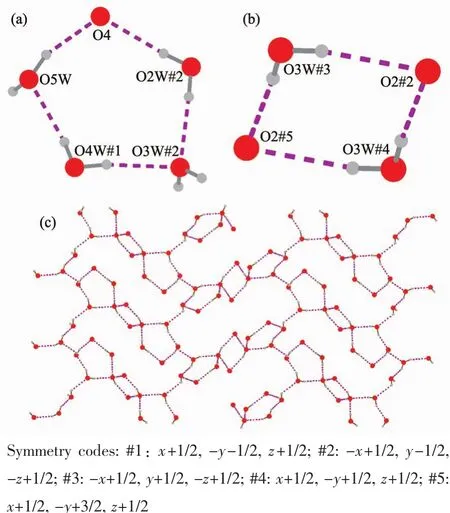
Fig.9 (a)Hydrogen bonding interaction forming a fivemembered ring structure in complex 3;(b)Hydrogen bonding interaction forming a fourmembered ring in complex 3;(c)Twodimensional network of hydrogen bonding in complex 3
2.2 IR spectrum
The IR spectrum of complexes and the ligand(HMTZA)can be seen that all complexes have broad and strong absorption peaks in the range of 3 500~3 200 cm-1.It can be seen that there is a stretching vibration of the water molecule (ν(O-H),indicating the presence of water in the complexes[35].
In the IR spectrum of complexes,the asymmetric and symmetric stretching of COO-appeared at 1 605 cm-1(νasym(OCO)and 1 357 cm-1(νsym(OCO)for 1,1 638 cm-1(νasym(OCO)and 1 444 cm-1(νsym(OCO)for 2,1 630 cm-1(νasym(OCO)and 1 357cm-1(νsym(OCO))for 3,respectively,which shows the presence of monodentate carboxylate linkage.The C=N characteristic stretching vibration appeared at 1 489,1 508,1 489 cm-1,respectively,which shows the coordination of nitrogen atoms from the ligands.Besides,the peak at 750~800 cm-1is the C-H stretching vibration of the thiazole heterocycle,indicating the presence of the thiazole heterocycle.
2.3 Thermal analysis
Thermal gravimetric (TG)analysis was carried out from 30 to 800℃.The TG curves of the title complexes are shown in Fig.11.For 1,the first weight loss of 12.12%up to 145℃can be assigned to the removal of water molecules (Calcd.12.46%).A quick weight loss in the temperature range of 315~375 ℃was observed,indicating the framework of complex 1 starts to collapse.Over 490℃,continuous weight loss was also found.The remaining weight of 19.57%corresponds to CoO (Calcd.19.29%).Complex 2 was stable up to 85℃,and the first weight loss of 8.52%in the range of 85~130 ℃ can be assigned to the removal of water molecules (Calcd.8.96%).The second step in the range of 260~272 ℃ with a quick weight loss corresponds to the decomposition of the ligand.Finally,the remaining weight of 19.64%seems likely to correspond to CuO (Calcd.19.79%).Complex 3 was stable up to about 78℃,and the observed weight loss (12.06%)in the temperature range of 78~125℃can be assigned to the loss of water molecules(Calcd.12.28%).Then,no significant weight loss was observed until the decomposition of complex 3 occured at about 335℃.A quick weight loss in the range of 335~365 ℃ is observed,which means complex 3 begins to decompose with the collapse of the ligand.Over 365 up to 665℃,continuous weight loss was also found.The final residue weight of 18.98%corresponds to ZnO (Calcd.18.42%).
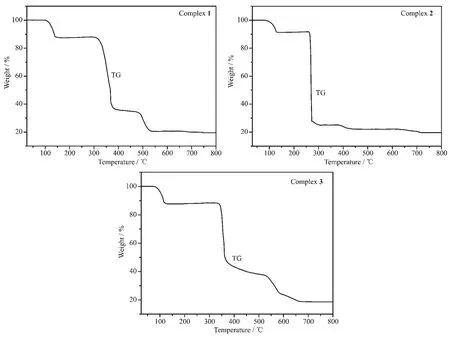
Fig.11 TG curves of complexes 1,2 and 3
2.4 EB-DNA binding study by fluorescence spectrum
The interactions of the ligand (HMTZA)and complexes with calf thymus DNA (ct-DNA)were studied by ethidium bromide fluorescent probe.The experiment was carried out by adding different volumes of compound solution to 10 mL colorimetric tube,which contained 1.0 mL 200 μg·mL-1ct-DNA,1.0 mL 200 μg·mL-1EB and 2.0 mL Tris-HCl buffer solution (pH=7.40).And then,different amounts of ligand and complexes solution (0.10 mmol·L-1)were diluted with distilled water to the scale.The reactions were carried out for 12 h at room temperature.The fluorescence spectra of the composite system were obtained at the wavelength of 530~690 nm with 255 nm as the excitation wavelength.
The effects of the ligand and complexes on the fluorescence spectra of EB-DNA system are presented in Fig.12.With the increasing concentration of the complexes,the fluorescence intensities of EB bound to ct-DNA at 592 nm showed remarkable decreasing trend,indicating that some EB molecules are released into solution after the exchange with the compounds that result in the fluorescence quenching of EB.According to the classical Stern-Volmer equation[36]:I0/I=1+Ksqr,where I0and I represent the fluorescence intensities in the absence and presence of quencher,respectively.Ksqis the linear Stern-Volmer quenching constant,r is the ratio of the concentration of quencher and DNA.And Ksqwas obtained as the slope of I0/I versus r plot.
From the inset of Fig.12,the Ksqvalues are 0.849,1.684,1.546 and 0.942 for the HMTZA ligand and complexes 1,2 and 3.The results indicate that the interactions of the complexes with DNA are stronger than the ligand,because the complexes have higher rigidity to bind the base pairs along DNA.
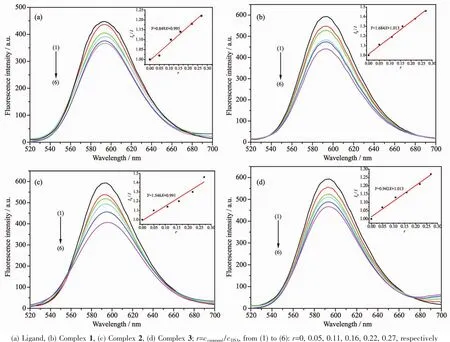
Fig.12 Influence of the compounds on the fluorescence spectra of EB-DNA system
3 Conclusions
In summary,one kind of thiazole derivative HMTZA was purposely synthesized based on ethyl bromopyruvate.Its three novel complexes,[Co(MTZA)2(H2O)2]·3H2O (1),[Cu (MTZA)2(H2O)]·2H2O (2)and[Zn (MTZA)2(H2O)2]·3H2O (3),were synthesized.The complexes 1 and 3 exhibit extended 3D framework by rich intermolecular hydrogen bonding interactions.Complex 2 displays a 2D framework by intermolecular hydrogen bonding interactions.Complexes 1 and 2 have more stronger interactions with DNA than complex 3,which can release more free EB molecules from EB-DNA.
