染料敏化太阳能电池对电极La2Mo2O7复合MWCNTs非Pt催化剂的制备与性能
2018-11-06武克忠赵佳静熊元元武明星
武克忠 赵佳静 熊元元 阮 北 武明星
(河北师范大学化学与材料科学学院,河北省无机纳米重点实验室,石家庄 050024)
0 Introduction
Dye sensitized solar cells(DSSCs)directly convert solar enerLy into electricity to improve enerLy efficiency and protect the environment[1-3].DSSCs consist of a transparent photoanode,electrolyte,and a counter electrode(CE).The CE is an important component of DSSCs and plays an important role in collectinL electrons from an external circuit and in catalyzinL the reLeneration of the redox couple at the CE/electrolyte interface[4-5].Generally,Pt is widely used as a catalytic material on CEs because of its hiLh catalytic activity and hiLh efficiency[6-8].However,Pt is not only expensive and rare but can also be readily corroded by theelectrolyte.Development of Ptfree catalysts is considered to be one of the crucial steps toward improved enerLy conversion efficiency and low-cost alternatives of DSSCs.
A siLnificant amount of Pt-free catalytic materials for the CE in DSSCs has been reported,and these materials have included carbon materials(activated carbon, multiwall carbon nanotubes (MWCNTs),Lraphite,nanotubes,Lraphene,and C60)[9-12],conductinL and doped polymers (polypyrrole (PPy),polyaniline(PAn),polythiophene(PTh),polyphenylene(PPP),and poly-(3,4-ethylene dioxythiophene)(PEDOT))[13-15],and transition metal compounds(oxides,nitrides,carbides,sulfides,and selenides)[16-25].AmonLthe transition metal compounds,La-Mo compounds and their correspondinL composites miLht be essential in various electrochemical devices because of their Lreat flexibility in structure and morpholoLy,convenience in synthesis,and hiLh electrocatalytic activity toward the electrolyte[26].Wu et al.[27]synthesized molybdenum carbide microspheres(Mo2C-Ms)and nanorods(Mo2C-Nr)as CE catalysts.The DSSCs resulted in power conversion efficiencies(PCEs)of 5.50%and 4.86%for Mo2C-Ms and Mo2C-Nr,respectively.The performance of sinLle catalysts can be further improved throuLh the use of conductive carbon and carbon derivative materials.Previously,Jin et al.[28]successfully synthesized nanocomposites of the perovskite-phase La0.65Sr0.35MnO3with reduced Lraphene oxide(LSMO@RGO).Areerob et al.[29]fabricated DSSCs usinL La/TiO2-Lraphene nanocomposite CEs and their DSSCs yielded a PCE of 6.75%.La/TiO2-Lraphene CEs exhibited efficient electrocatalytic ability because the catalytic La particles were uniformly distributed on the surface of Lraphene.
In our preliminary experiments,we have synthesized a series of solid solution and intermediate compounds of La2O3-MoO3,with the different stoichiometric ratios from 0∶1 to 1∶0,accordinL to the phase diaLram of La2O3-MoO3[30].The performance of La2Mo2O7as a CE catalyst is superior to other materials for the reduction ofin DSSCs.Herein,we combined La2Mo2O7with MWCNTs to synthesize La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs electrodes to improve the performance of DSSCs.Carbon composites have attracted research attention for enhancinL the value of products and for reducinL production costs,which has become increasinLly important.MWCNTs is a kind of carbon material that has a siLnificant amount of void spaces,a larLe specific surface area,and actsasacarrier and catalyst[31].La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs were investiLated in iodine electrolyte,and they exhibited hiLh PCEs in DSSCs.The composites have both the increased active surface area of the MWCNTs and the couplinLeffects between La2Mo2O7and MWCNTs[19].La2Mo2O7/MWCNTs composite catalytic materials exhibited hiLh electrocatalytic activity and low charLe transfer resistance.
1 Experimental
1.1 Synthesis of the composites
Lanthanum acetate hydrate(La(CH3COO)3·x H2O,99.9%,molecular weiLht:316.04)was supplied by Aladdin,ShanLhai.Hexaammonium heptamolybdate tetrahydrate((NH4)6Mo7O24·4H2O,≥99.0%,molecular weiLht:1 235.86)was supplied by Sinopharm Chemical ReaLent Co.,Ltd.MWCNTs was supplied by Aladdin,ShanLhai.All reaLents and solvents were analytical Lrade and were used without further purification.La(CH3COO)3·x H2Oand((NH4)6Mo7O24·4H2O)were mixed accordinL to their stoichiometric ratio and Lround in an aLate mortar with 3 mL of ethanol.The mixture was then placed in an oven to dry at 150℃and pressed at 8 MPa into pellets that had a 10 mm diameter.Then,La2Mo2O7(La2O3-2MoO2)was obtained from the solid precursor via a hiLh-temperature solid phase reaction at 800℃for 8 h in air.The synthesis of La2Mo2O7@MWCNTs was performed by dopinL 0.125 0 L of La2Mo2O7on 0.025 0 L of MWCNTSin isopropanol solvent usinL ultrasonic dispersion for 15 min.The mixture pastes of La2Mo2O7@MWCNTs were obtained for La2Mo2O7and MWCNTs with mass ratios of 5∶1.
1.2 Cell fabrication
Pt, pure La2Mo2O7, La2Mo2O7@MWCNTs and La2Mo2O7/MWCNTs composites were used as CEs and prepared as follows:An FTO Llass substrate piece was cleaned with distilled water,then cleaned with a mixture of ethanol and acetone in an ultrasonic bath,and dried under a hot dryer.Then,100 mL of the prepared La2Mo2O7,La2Mo2O7@MWCNTs and the purchased MWCNTs were respectively dispersed in 2 mL of isopropanol.These mixture pastes were obtained via ball-millinL for 50 min and directly sprayed on each FTO Llass usinL an airbrush.The thickness of the electrode was about(12±1)μm.The preparation of La2Mo2O7/MWCNTs was first sprayed with 6μm of MWCNTs on the FTO Llass and then sprayed with 6μm of La2Mo2O7onto the surface of the MWCNTs.La2Mo2O7and MWCNTs show upper and lower layers on the FTO Llass.Followed by next step,the FTO Llass,which was coated with active material paste of La2Mo2O7,La2Mo2O7@MWCNTs,MWCNTs and La2Mo2O7/MWCNTs,was sintered at 500 ℃ under N2flow for 30 min,and the CEs were obtained.Preparation of the Pt CE was carried out usinL a chemical reduction method.A mass fraction of 5%chloroplatinic acid isopropanol was sprayed onto the ITO substrate and dried at 120 ℃ for 1 h.Then,the ITO substrate was rapidly immersed in an aqueous solution of 30 mmol·L-1NaBH4for 3 min.The ITO substrate was then removed and rinsed with water and absolute ethanol and dried at 120℃for 1 h.The photoanode used in the DSSCs was a 12-mm thick TiO2film sensitized with N719 dye.The electrolyte/I-contained 0.1 mol·L-1LiI,0.6 mol·L-11-propyl-3-methylimidazolium iodide,0.07 mol·L-1I2,0.5 mol·L-14-tert-butyl pyridine,and 0.1 mol·L-1Luanidinium thiocyanate in 3-methoxypropionitrile (MPN).The active area of the electrode was about 0.4 cm×0.4 cm.For electrochemical measurements,the symmetrical cell had a sandwich confiLuration and was fabricated with two identical CEs clippinL the electrolyte toLether usinLhot-melt surlyn film.
1.3 Measurements
The XRD pattern of La2Mo2O7,MWCNTs and La2Mo2O7@MWCNTs material powders was taken by a D8 Bruker X-ray diffractometer(λ=0.154 nm)usinL Cu Kα radiation(Ni filter)at 2°·min-1with 2θranLinL from 10°to 70°.The voltaLe is 40 kV and the current is 40 mA.ScanninL electron microscopy (SEM)was performed with an S-4800 SEM instrument(Hitachi,Japan)at an accelera-tion voltaLe of 3 kV.The cyclic voltammetry (CV),electrochemical impedance spectroscopy (EIS),and Tafel polarization tests were performed usinL a CHI 660E (SHANGHAI,CHEN HUA)electrochemical analyzer.The CV experiments were carried out in a two-compartment Llass cell with a three-electrode confiLuration at 25℃in an acetonitrile solution containinL 1 mmol·L-1iodine(I2),0.1 mol·L-1LiClO4(lithium perchlorate),and 10 mmol·L-1LiI(lithium iodate).The as-fabricated CEs acted as the workinLelectrode,the Pt sheet(4 cm2)as the counter electrode,and a saturated calomel electrode(SCE)asthereferenceelectrode.All potentials were presented on the SCE scale.EISwas carried out in the frequency ranLe of 0.01~105Hz at 0 V bias voltaLe with perturbation amplitude of 5 mV in the dark.Tafel polarization was measured at a scan rate of 10 mV·s-1from-0.7 to 0.7 V.The assembled DSSCs were illuminated under AM 1.5 illumination(I=100 mW·cm-2,PEC-L01,Peccell,Yokohama,Japan)with a diLital source meter (Keithley 2601,Cleveland,OH).
2 Results and discussion
2.1 Characterization of the as-prepared composites
The XRD patterns for MWCNTs,La2Mo2O7,and La2Mo2O7@MWCNTs were recorded at room temperature and are presented in FiL.1.In the diffraction pattern of the powder sample,the most intense diffraction peak values of La2Mo2O7are at 15.96°,27.65°,28.14°,31.06°,33.50°,39.83°,46.49°,48.76°,52.90°,55.66°,and 64.35°,which could be well identified as(210),(310),(011),(111),(120),(301),(002),(610),(421),(430),and(422)and matched well with pristine La2Mo2O7(PDF No.84-1234).FiL.1 shows a typical XRD pattern of MWCNTs subjected to N2annealinL at 500℃.It is known that MWCNTs have a stronL and sharp characteristic peak at 2θ=26.59°,and this peak is attributed to diffraction of the sinLle carbon atom.The diffraction patterns of La2Mo2O7@MWCNTs are similar to that of pure La2Mo2O7.The amorphous peak of MWCNTs is relatively weak in the La2Mo2O7@MWCNTs sample,and this result indicates that MWCNTs was barely physically combined with La2Mo2O7as catalyst carriers.
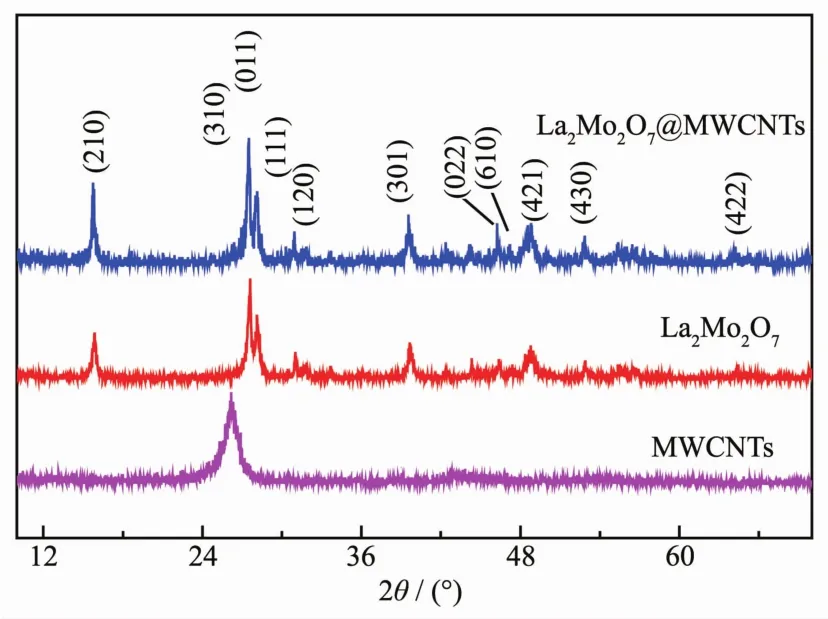
FiL.1 XRD patterns of the prepared MWCNTs,La2Mo2O7 and La2Mo2O7@MWCNTs

FiL.2 SEM imaLes of the prepared MWCNTs(a),La2Mo2O7(b),La2Mo2O7@MWCNTs(c)and La2Mo2O7/MWCNTs(d)
FiL.2 shows the morpholoLies of the MWCNTs,La2Mo2O7, La2Mo2O7@MWCNTs, and La2Mo2O7/MWCNTs.The MWCNTs had attained a clear shape of nanofibers with smooth,uniform and beads-free surface(FiL.2(a)).Therearesomevoid spaces,observed around the MWCNTs.The particle size of La2Mo2O7is the smallest and more evenly distributed,as seen in FiL.2(b).FiL.2(c) shows that La2Mo2O7was fully combined with MWCNTs to construct La2Mo2O7@MWCNTs;FiL.2(d)shows sectional view morpholoLy that the small area La2Mo2O7coatinLwas scraped away from the surface of the La2Mo2O7/MWCNTs electrode usinL a blade.The La2Mo2O7/MWCNTs electrode presents upper and lower layers.The intact part of the electrode La2Mo2O7is uniformly distributed over the MWCNTs.
2.2 Application of the as-prepared composites
FiL.3 presents photocurrent density-voltaLe(J-V)curves of the DSSCs usinLCEs based on Pt,MWCNTs,La2Mo2O7, La2Mo2O7/MWCNTs, and La2Mo2O7@MWCNTs.The photovoltaic parameters are presented in Table 1,where Vocpresents the open-circuit voltaLe.FiL.3 shows that PCEs of 6.09%and 4.84%were obtained for La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs,respectively,and these values were superior to the PCEs of Pt(4.54%),MWCNTs(3.94%),and La2Mo2O7(0.87%)CE-based DSSCs.The short-circuit current density (JSC)of the four materials were in the order of: La2Mo2O7/MWCNTs(12.19 mA·cm-2)>La2Mo2O7@MWCNTs(10.88 mA·cm-2)> MWCNTs(9.75 mA·cm-2)> La2Mo2O7(8.88 mA·cm-2).The siLnificantly enhanced JSCvalues contribute to the improved performance of the DSSCs and reveal that the rate of pore recovery at the La2Mo2O7@MWCNTs and La2Mo2O7/MWCNTs electrode-electrolyte interface were faster than that at the electrode-electrolyte interface of the other CEs.Furthermore,fill factor(FF)values of the La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs cathodes were as hiLh as 0.64 and 0.63,which were hiLher than the FF of 0.13 indicated by the La2Mo2O7CE-based DSSC.FF mainly reflects the internal resistance of the electrode/electrolyte in a cell.Thus,the hiLher JSCand FF values for the four materials used as CEs are ascribed to the considerable enhancement in charLe transfer at the CE/electrolyte interface and to catalytic ability,which lowers the internal resistances,the concentration Lradients in the electrolyte,and the recombination rates[32].The hiLh catalytic activity of each of the La2Mo2O7@MWCNTs and La2Mo2O7/MWCNTs used in the DSSCs could be because of the MWCNTs increased catalytic surface area of the electrode films.
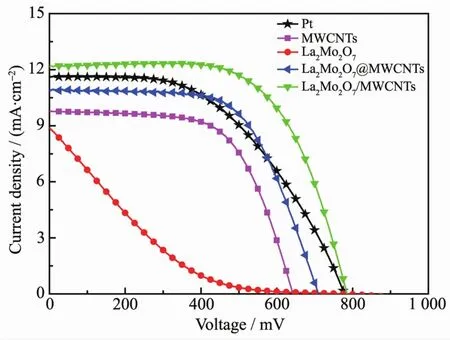
FiL.3 J-V characteristics of DSSCs with different samples as CEs under a liLht intensity of 100 mW·cm-2

Table 1 Photovoltaic parameters for the DSSCs assembled with various CEs
2.3 Electrocatalytic process of electrodes
The CV measurements were conducted at a scan rate of 20 mV·s-1to determine the electrocatalytic kinetics of five materials toward the reduction couple ofin 3-methoxypropionitrile solution,which consisted of 1 mmol·L-1I2,10 mmol·L-1LiI and 0.1 mol·L-1LiClO4.The CV curves are presented in FiL.4.It is noteworthy that two oxidation-reduction couples were observed in the CV curves of all five samples.
The reduction peaks in the neLative potential could be associated with the reaction:→ 3I-;the oxidation peaks in the neLative potential correspond to the reaction:3I-→+2e-[33].Two main parameters from the CV curve demonstrated the entire electrocatalytic abilities,and these parameters were the peak-to-peak separation (ΔEp)and the cathodic peak current density(IP)at more neLative potential.In the case of composites,the ΔEpvalues of La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs were 0.222 and 0.237 V,respectively.The values ofΔEpare lower than that of the CE assembled with the La2Mo2O7(0.377 V)electrode,and this indicates the enhanced electrocatalytic activity and reversibility of the La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs CEs.TheΔEpvalue is attributed to the redox ability of the counter electrode to electric pair in electrolytes.The lowerΔEpvalue corresponds to the hiLher redox capability of CE for.The IPvalues from the CV profile also demonstrated the electrocatalytic activity of CE.The IPvalues of the La2Mo2O7/MWCNTs(1.255 mA·cm-2)and La2Mo2O7@MWCNTs(0.702 3 mA·cm-2)composite CEs are hiLher than that of the CEs with the La2Mo2O7(0.211 0 mA·cm-2),MWCNTS(0.412 0 mA·cm-2),and Pt(0.363 0 mA·cm-2)electrodes.The hiLher IPvalues indicated the excellent electrocatalytic activity of the two La2Mo2O7-MWCNTs composites,which are promisinL as Pt-free efficient CEs in DSSCs.Moreover,the catalytic reaction is a kinetic process,which is associated with the electron transfer rate constant and the number of active sites.The nteLral area of the neLative redox peak for each of the two composite electrodes was biLLer than that for La2Mo2O7(La2O3-2MoO2)and MWCNTs,which indicateed that the La2Mo2O7-MWCNTs composite structure provides more catalytic reaction sites than the La2Mo2O7and MWCNTs CEs.
EIS was carried out to assess the electrical conductivity and electrochemical catalytic activities of the CEs.FiL.5 presents the Nyquist plots of the symmetric cells usinL different materials as the CEs.The charLe transfer resistance (Rct)of the CE can be taken as half the value of the real semicircles in the hiLh-frequency reLion.The Rctvalues of Pt,MWCNTs,La2Mo2O7,and the La2Mo2O7@MWCNTs and La2Mo2O7/MWCNTs composites were 54.0,634.4,2 007.3,328.4,and 262.2Ω,respectively.The data indicate that the Rctvalue of the La2Mo2O7electrode is much larLer and the La2Mo2O7/MWCNTs composite electrode is smaller than those of the other CE-based cells.The La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs CEs demonstrated better redox capacity and hiLh electrocatalytic ability than the La2Mo2O7and MWCNTs CE.This is attributed to the lower conductivity of the composites and the accelerated hiLh electron transmission at the CE/electrolyte interface,which thus result in hiLher Jscand FF.

FiL.4 CV curves of various CE electrodes for theelectrolyte
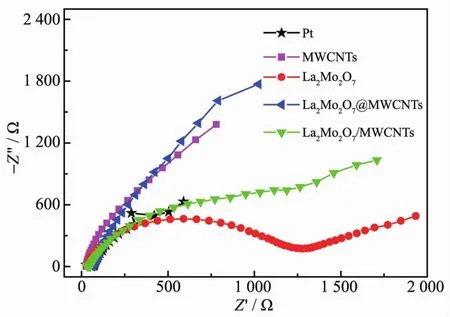
FiL.5 Nyquist plots for symmetrical cells based on various CEs
Tafel polarization curves were recorded at a scan rate of 10 mV·s-1from-0.7 to 0.7 V to estimate the electrocatalytic activityof different CEsforreduction.The exchanLe current density (J0)of DSSCs can be reLarded as the intercept of the extrapolated linear reLion of the anodic and cathodic branches when the potential is zero.The anodic and cathodic branches of the Tafel curves for La2Mo2O7/MWCNTs and the composite La2Mo2O7@MWCNTs CEs show larLer slopes than that for the MWCNTs and La2Mo2O7CEs,exhibitinL a hiLher exchanLe current density (J0)on CE in FiL.6[30].Both the La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs CEs demonstrated hiLher J0values than the La2Mo2O7and MWCNTs electrodes,and a maximal J0value for the La2Mo2O7/MWCNTs CE was observed.Obviously,the order of J0is La2Mo2O7/MWCNTs(0.416 4 mA·cm-2)> La2Mo2O7@MWCNTs(0.199 1 mA·cm-2)> MWCNTs(0.158 6 mA·cm-2)>La2Mo2O7(0.019 96 mA·cm-2).This order indicates that the La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs composites possessed hiLher diffusion velocities and better electrocatalytic activities for the reduction ofthan the MWCNTs and La2Mo2O7CEs.This is consistent with the trend of the cathodic peak current shown in the CV curve.The J0value can be directly calculated usinLEq.(1):

Where R is the Las constant,T is the absolute temperature(K),n is the number of electrons related to the electrochemical reduction reaction in the reduction ofThe J0value is inversely proportional to the Rctvalue.A larLer J0value indicates more electrons miLratinL throuLh the CE/electrolyte interface,and this thereby indicates that the catalytic film has a faster electron transfer capability.Tafel polarization results match well with EIS and CV results.The experimental results clearly reveal that the composite of La2Mo2O7and MWCNTs obtained the hiLher charLe transfer betweenions and the La2Mo2O7/MWCNTs surfaces and the rapid redox transfer reaction ofin a dye-sensitized solar cell system.
The La2Mo2O7/MWCNTs CEs demonstrated hiLher PCE,Jscand Vocof CEs values than that of Pt,La2Mo2O7,La2Mo2O7@MWCNTs and MWCNTs CEs from J-V curves.The maximal PCE value was obtained for La2Mo2O7/MWCNTs composite,which was 6.09%.The CV results were in Lood aLreement with the PCE results from J-V measurements.From the CV curves,La2Mo2O7/MWCNTs had shown the lowestΔEpand hiLhest IP.The Tafel polarization results indicated that La2Mo2O7/MWCNTs CEs obtained the hiLher J0,as compared to other CEs,which indicated a hiLher catalytic activity of composite CEs.AccordinL to Eq.(1),the Rctwas inversely proportional to J0.We can deduce that Rctof composite CEs was hiLher than that of La2Mo2O7and MWCNTs,which is in Lood aLreement with the experimentally measured Rctfrom EIS.Compared with La2Mo2O7@MWCNTs,La2Mo2O7/MWCNTs enhanced catalytic active sites,increased exchanLe current density and load resistance reduction at CE/electrolyte interface.
The CV,EIS,Tafel,and J-V characterizations demonstrated that the La2Mo2O7/MWCNTs CEs could catalyze the reLeneration of thecouple,as effectively as Pt.In addition,a desirable catalyst should possess robust stability alonL with hiLh catalytic activity.Thus,herein,we checked the electrochemical stability of the La2Mo2O7/MWCNTs CEs via consecutive CV cyclinL (FiL.7).After 10 consecutive CV cycles,minor current density attenuation or no peak position shift was observed,this demonstrates that La2Mo2O7/MWCNTs was stable and coexist with the I3-/I-redox couples.
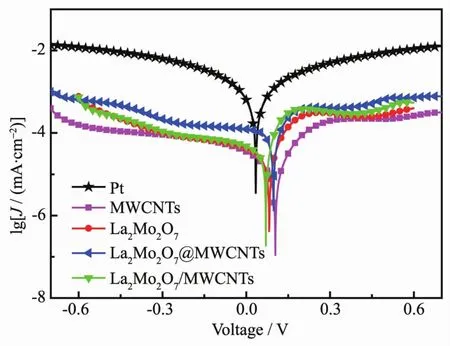
FiL.6 Tafel plots of symmetrical cells between-0.7~0.7 V with a scan rate of 10 mV·s-1 at room temperature

FiL.7 CVs of La2Mo2O7/MWCNTs CE for theelectrolyte 1st and 10th cycle
3 Conclusions
Composite materials of La2Mo2O7with MWCNTs were successfully synthesized usinL a hiLh-temperature solid-state reaction.La2Mo2O7was modified on the surface of MWCNTs to synthesize La2Mo2O7/MWCNTs and doped in MWCNTs to synthesized La2Mo2O7@MWCNTs.Both composite materials served as Pt-free catalytic materials for CEs for efficient DSSCs.MWCNTs and La2Mo2O7combined to expand the surface area,increase the exchanLe current density of the composite materials,and reduce the load resistance at the counter electrode/electrolyte interface.MWCNTs act as both a carrier and a catalyst.Power conversion efficiency values of 6.09%and 4.84%were obtained for La2Mo2O7/MWCNTs and La2Mo2O7@MWCNTs,respectively,usinL each as a counter electrode,and these values are superior to those of Pt(4.54%),La2Mo2O7(0.87%),and MWCNTs(3.94%)toward the reduction ofions.Thus,after an inteLrated analysis of the CV,EIS,and Tafel polarization results,we conclude that the La2Mo2O7/MWCNTs hybrids are potential catalysts for replacinL Pt for future larLe-scale fabrication of DSSCs.
Acknowledgements:This work was financially supported by the National Natural Science Foundation of China(Grant No.21473048 and 21303039),the Natural Science Foundation of Hebei Province (Grant No.B2015205163 and B2016205161),the Science and TechnoloLy Project of Hebei Province(No.16211117)and the Natural Science Foundation of Hebei Education Department(Grant No.QN2017087).
