High focused Evaluation of Atherosclerotic risk profile in Retinal Thrombosis: Vascular events Incidence, Sex involvement and Interventional outcomes assessed by Ophthalmologists and internists Network – HEART VISION study protocol
2018-10-18StefaniaBasiliElenaPacellaFernandaPacellaGiulioRomitiGiacomoVisioliLudovicaAntoniniSilviaRobuffoRobertoCangemiMassimoMecellaMarcoProiettiValeriaRaparelli
Stefania Basili, Elena Pacella, Fernanda Pacella, Giulio F.Romiti, Giacomo Visioli, Ludovica M.Antonini,Silvia Robuffo, Roberto Cangemi, Massimo Mecella, Marco Proietti, , Valeria Raparelli
1 Department of Internal Medicine and Medical Specialties and Research Center on Gender and Evaluation and Promotion of Quality in Medicine(CEQUAM), Sapienza-University of Rome, Rome, Italy
2 Department of Sense Organs, Sapienza-University of Rome, Rome, Italy
3 Department of Computer, Control, and Management Engineering Antonio Ruberti, Sapienza-University of Rome, Rome, Italy
4 Centre for Outcomes Research and Evaluation (CORE) McGill University Health Centre Research Institute, Montreal, Canada
5 Department of Experimental Medicine, Sapienza-University of Rome, Rome, Italy
Abstract
Key words: retinal vein occlusion; vision loss; cardiovascular risk factors; ophthalmology; prospective study
INTRODUCTION
Retinal vein occlusion (RVO) is a highly prevalent cause of unilateral vision loss and the second leading cause of retinal vascular disease after diabetic retinopathy.1Nonetheless, the epidemiology, pathophysiology and natural history of this condition have yet to be fully elucidated; besides, due to the lack of definitive data in the literature, the disease management still represents an open issue.2
RVO refers to a group of diseases – with different risk factors, prognosis and treatment – all characterized by impaired venous return from the retinal circulation.3Depending on the site of occlusion of the retinal vein, it is possible to distinguish two main clinical presentations of RVO: central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO),the latter representing most of the cases.4A pooled study,which included 11 different cohorts (with a total of 49,869 patients) showed an age and sex- standardized overall prevalence incindence of 0.52% (0.08% for CRVO and 0.42% for BRVO) – which was slightly higher in females, in Asians and Hispanics but with no significant statistical differences – and demonstrated an increased risk with age,4probably related to the higher burden of risk factors (RFs) and predisposing conditions.5,6
It has been assumed that RVOs shares the same RFs of cardiovascular disease (e.g.arterial hypertension, diabetes,smoking, dyslipidemia,etc.).7-9However, RVOs, with some differences depending on the type, can also be secondary to other processes such as vasospasm, compression,10inflammation11and other conditions including thrombophilia,12sleep apnea13,14or glaucoma15could also be associated.In this context, a straightforward definition of cardiovascular RFs in the disease development has not yet been provided and the impact of genetic differences, sex and sociocultural background should not be underestimated.16As sex (i.e.biological factors) and gender (i.e.psycho-social-cultural factors including personality traits, socioeconomic status and social relationship) are not independent, exclusively assessing one or the other fails to account for identified variations in health.17-20Therefore, the integration of both sex and gender dimensions is a powerful tool to advance our understanding of the management and outcomes of health disease18,19as it has been already proved in patients with acute coronary syndrome.21
A deeper knowledge on the impact of traditional and nontraditional RFs in the pathogenesis of RVO would represent a cornerstone in the improvement of primary and secondary prevention strategies, and it could help in developing a personalized management approach for the treatment of RVO patients.
Objectives of the study
The primary objectives of the study are:
1) Creation of RVO patient’s national registry to estimate the actual prevalence of the condition.
2) To identify the potentially modifiable RFs and to recognize further potential determinants of the disease related to sex, gender, genetics and socio-cultural background of the population.
3) To verify the real-world management of RVO patients.
4) To gather information for the design of IT application for the management of RVO patients.
METHODS/DESIGN
Study design
The “High focused Evaluation of Atherosclerotic risk profile in Retinal Thrombosis: Vascular events Incidence, Sex involvement and Interventional outcomes assessed by Ophthalmologists and internists Network” (HEART VISION) is a longitudinal, prospective, multi-centric study in Italy (Figure 1).According to the study design, the first step will consist in the identification of specialized services (i.e.ophthalmology emergency room and thrombosis centers) which will enroll patients accessing for suspected RVO.Each center will recruit all the eligible patients, collect written inform consent and all the relevant clinical information according to the study protocol, and will be responsible for the data-entry through the web-based platform.A physician (i.e.“local monitor”) will be responsible for the adherence to the protocol, standardized operating procedures, and for the management of critical sensitive data in accordance with the current local legislation.
Strengths and limitations
Strengths
- Longitudinal, prospective, multi-center study aimed at determining the epidemiology, potentially modifable risk factors and the determinants of retinal vein occlusion (RVO).
- Real world data on underestimated relevant health problems were obtained to promote high-quality patient-centered care.
- Generating hypothesis on mechanisms underlying RVO and cardiovascular outcomes.
Limitations
- Findings will be country-specific with limited external validity.
- We could not account for all potential confounders or mediating factors in the association between RVO and future cardiovascular outcomes.
In addition, a web-based platform will be developed to enable the management and support to surveys for medical investigation, allowing medical researchers and patients to interact using social features.
Ethical approval
This study protocol (n.1.0, 01.07.2014) was approved by the Sapienza – University of Rome, Ethics Board (Protocol No.1076/14).This study will be performed in accordance with theDeclaration of Helsinki, and it was registered at ClinicalTrials.gov (identifier: NCT02257333).
Study population
All the eligible patients will be consecutively enrolled in the study, according to the inclusion and exclusion criteria defined below.
Inclusion/exclusion criteria
All adults aged > 18 years who access specialized centers with a suspected diagnosis of RVO, and who signed a valid inform consent will be included in the study.Patients would be excluded if a) pregnant, b) affected by a condition which would reduce the life-expectancy to less than the mean time of planned follow-up.
Baseline data collection
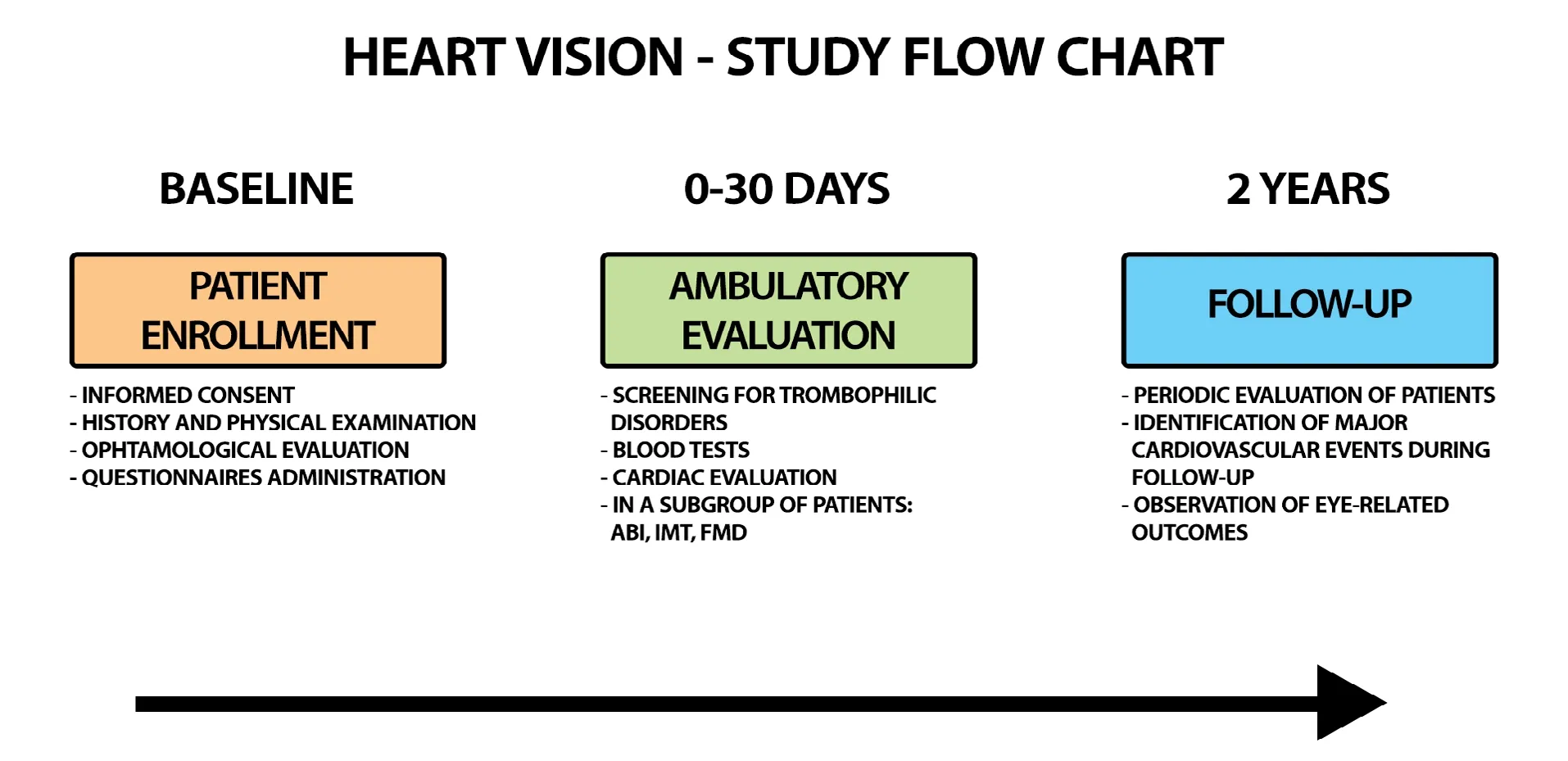
Figure 1: HEART VlSlON study flow chart.
At baseline, and after the signature of a valid inform consent,for each patient with suspected RVO the following data will be collected: a) complete personal data and medical history(familiar, past and present) and physical assessment (anthropometric data and vital signs), b) comprehensive ophthalmologic evaluation including eye history and examination, visual acuity, eye fundus examination, optical coherent tomography(OCT) and ocular tonometry, c) data on gender-related variables (including psychosocial factors and socio-economic status), d) data on ongoing treatment and questionnaires on medications adherence,22demographic and social aspects(including marital status, educational level, income, working status risk taking behaviors)23and Mediterranean diet.24These data will be recorded on the online registry at baseline.Medical management and prescriptions before the confirmation of the final diagnosis will be also recorded.After 1 month from the index event, the patients will be evaluated through an outpatient examination.During the first month, the patient will undergo a specialist evaluation for the confirmation of the diagnosis (which could also include an evaluation through OCT, microperimetry and retinal fluorescein angiography).Moreover, etiologic factors and a complete evaluation of existing RFs and co-morbidities will be reassessed.At the thrombosis center, the patient will undergo the following instrumental and laboratory investigations:
1) Assessment of the presence/absence of congenital and/or acquired thrombophilic disorders (including antiphospholipid syndrome, hyper-homocysteinemia, Factor V Leiden mutation, prothrombin mutation, protein C and S deficiencies,anti-thrombin III deficiency);
2) Routine blood tests (including whole count cell, creatinine,prothrombin time, liver enzymes);
3) Cardiac evaluation with electrocardiography, transthoracic echocardiography with US-Doppler evaluation (i.e.left ventricular hypertrophy screening and cardiac morphology and motility).
A subgroup of patients with an established RVO diagnosis,according to the availability and the consent of the subjects,will be involved in an ancillary evaluation of several markers of subclinical atherosclerosis and endothelial dysfunction,i.e.:
1) Ankle-brachial index: at baseline, a measurement of upper and lower limb systolic blood pressure for ankle-brachial index calculation will be performed as previously described and a value equal or inferior to 0.90 will be considered as pathological;
2) Carotid intimal-medial thickness (cIMT): according to the American Society of Echocardiography consensus statement on the use of carotid ultrasound to identify subclinical vascular disease, tracing far wall blood-intima and media-adventitia interfaces using leading edge–to–leading edge method at 1 cm from the carotid bulb will assess cIMT.A value of cIMT above 0.90 mm or the presence of a carotid surrounding thickening more than 1.50 mm will be defined as “pathological cIMT”;
3) Brachial artery flow-mediated dilation (FMD): ultrasound assessment of endothelial dependent and independent FMD of brachial artery will be evaluated.Briefly, the study will be performed in a temperature-controlled room (22°C) with the subjects in a resting, supine state between the hours of 8 a.m.and 10 a.m.; brachial artery diameter will be imaged using a 7.5-MHz linear array transducer ultrasound system equipped with electronic callipers, vascular software for twodimensional imaging, color and spectral Doppler, and internal electrocardiogram; the brachial artery will be imaged at a location 3–7 cm above the ante-cubital crease; to create a flow stimulus in the brachial artery, a sphygmomanometric cuff will be placed on the forearm; the cuff will be inflated at least 50 mmHg above systolic pressure to occlude artery inflow for 5 min; all vasodilatation measurements will be made at the end of diastole; FMD will be expressed as a change in post-stimulus diameter evaluated as a percentage of the baseline diameter;
4) Blood and urine samples collection for the assessment of the hemostatic milieu and redox status: blood samples will be properly maintained until batch analysis in freezers at –80°C.All assays will be performed in a blinded fashion.The samples analyzed by immunoassay methods will be tested in duplicate,and those with concentrations exceeding the standard curve will be assayed again after appropriate dilution.We plan to evaluate the following parameters to study interaction between platelet function and endothelial dysfunction in RVO patients,including soluble CD40 ligand (sCD40L) and soluble P-selectin, as members of the inflammatory molecules released from platelets, plasma thromboxane B2 and von Willebrand factor as marker of endothelial activation.
Patients in which diagnosis will not be confirmed will be used as controls.
Follow-up and outcomes
Patients with an establisheddiagnosis of RVO will be prospectively followed for 2 years.
After 3 months from the index events, a short-term followup will be executed to evaluate the recovery of visual acuity in relation to the type of received treatment according to the ophthalmologist clinical judgement.
Patients will be periodically evaluated with follow-up visits, and a dedicated website will be developed for their management.
The following 2-year follow up events will be collected:
1) Major adverse cardiovascular events including ischemic heart disease (i.e.acute coronary syndrome or stable chronic angina), cerebrovascular events (including stroke or transient ischemic attack), cardiovascular death, venous thrombotic events (i.e.deep vein thrombosis and/or pulmonary embolism);
2) Eye-related outcomes (i.e.outcomes of pharmacological and non-pharmacological management, recurrences).
Data management and statistical analysis
Dataset management and statistical analysis will be performed by both the “UOC Prima Clinica Medica – Atherothrombosis Centre at Policlinico Umberto I – Rome” and the “BioMedical Statistics and Clinical Epidemiology Centre”at Sapienza-University of Rome.
The Wilson method will be used for calculating confidence intervals (CI) for proportions.The Kaplan-Meier estimator will be used to calculate cumulative incidence, with the 95% CI.Multivariate analysis will be used in an attempt to identify determinants of outcomes and to control the effect of confounders(i.e.Cox competing risk model and logistic regression).
Likewise, the presence of the center effect will be assessed and eventually, removed.The secondary endpoints will be evaluated with the univariate log-rank test and the Cox model(with time-dependent effects) multivariate analysis.A logistic regression analysis will be performed to establish all clinical sex- and gender- related factors significantly associated with RVO.
Sample size calculation
It is planned to enroll about 1,000 patients for the study (about 50 patients per research center) during a 2-year period.An expected prevalence of 13%, which would produce a CI of 95%with amplitude of 0.043, was used for calculating the dimension of the study population.SPSS and STAT-SOFT Statistical Software will be used for performing statistical analysis.
DISCUSSION
RVO represents an important open issue of concerns for the need of a multidisciplinary approach to patient care.The disease management necessitates the involvement of physicians from a number of different clinical specialties, from ophthalmologists to vascular medicine specialists.At present, lack of definitive data on epidemiology, RFs and their specific contribution to the disease pathogenesis along with the absence of an established treatment algorithm for RVO generates some controversies.HEART VISION study will help to clarify these gray spots and overcome this potential source of pitfalls.It will pursue, as a mandatory objective, to fill in the lack of evidence on the pathogenesis of RVO and, concurrently, to bridge the gap between research and clinical practice with its prognostic and therapeutic implications.Furthermore, it will also represent the starting point for the medical community towards the development of further studies and research.
TRIAL sTATUS
We are currently recruiting participants.
Additional files
Additional file 1: Ethical Approval Documentation.
Additional file 2: Model consent form.
Author contributions
Study concept and design and manuscript writing: SB, EP, FP, RC,MP, VR; creation of the electronic registry of data and manuscript writing: MM; literature integration and manuscript writing: GFR, GV,LMA, SR.All authors approved the final version of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Financial support
This study was funded by Sapienza-University of Rome in 2014 -C26A147HC8.
Institutional review board statement
This study protocol (n.1.0, 01.07.2014) was approved by the Sapienza-University of Rome Ethics Board (Protocol No.1076/14).This study will be performed in accordance with theDeclaration of Helsinki.Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the form the patients will give their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity will be guaranteed.
Reporting statement
This study followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by Prof.Basili S,Department of Internal Medicine and Medical Specialties, Sapienza-University of Rome, Rome, Italy.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
For data sharing, individual participant data will not be available.However, the study protocol and informed consent form will be made available beginning 3 months and ending 5 years following article publication to investigators whose proposed use of the data has been approved by an independent review committee identified to achieve aims in the approved proposal.In order to gain access, data requestors will need to sign a data access agreement.Proposals should be directed to stefania.basili@uniroma1.it.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Systematic review on effectiveness of theory-based intervention on self-care behaviors among patients with type 2 diabetes
- What the future holds for the challenging hereditary spastic paraplegia?
- Accurate identification of potential critical coronary lesions for the reduction of risk of cardiovascular events: study protocol for a randomized, open-label,active-controlled multi-center trial
- Multi-component botanical drugs for degenerative diseases
- Using robotic-assisted technology to improve lower-limb function in people with stroke
