Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases
2018-10-18HongyueGuoFrooqWhAlinBerthodDnielArmstrong
Hongyue Guo,M.Frooq Wh,Alin Berthod,Dniel W.Armstrong,*
aDepartment of Chemistry and Biochemistry,University of Texas at Arlington,Planetarium Place,Arlington,TX 76019,USA
bInstitut des Sciences Analytiques,CNRS,Université de Lyon 1,5 rue de la Doua,69100 Villeurbanne,France
Keywords:Mass spectrometry detection Chiral separation Basic drugs Beta-blockers Superficially porous particle Fully porous particle Fast separation Vancomycin
A B S T R A C T Current trends in chiral analysis of pharmaceutical drugs are focused on faster separations and higher separation efficiencies.Core-shell or superficially porous particles(SPP)based chiral stationary phases(CSPs)provide reduced analysis times while maintaining high column efficiencies and sensitivity.In this study,mobile phase conditions suitable for chiral analyses with electrospray ionization LC-MS were systematically investigated using vancomycin as a representative CSP.The performance of a 2.7 μm SPP based vancomycin CSP(SPP-V)10cm × 0.21cm column was compared to that of a corresponding 5 μm fully porous particles based analogue column.The results demonstrated that the SPP-V column provides higher efficiencies,2–5time greater sensitivity and shorter analysis time for a set of 22 basic pharmaceutical drugs.The SPP-V was successfully applied for the analysis of the degradation products of racemic citalopram whose enantiomers could be selectively identified by MS.
1.Introduction
Over the past decades,after the issuance of U.S.Food and Drug Administration(FDA)guidelines relating to the study and pharmaceutical development of individual enantiomers[1],the analysis and quantification of chiral drugs has become a necessity.This is due to the different pharmacological or toxicological effects that the two enantiomers of a chiral active pharmaceutical ingredient may have.Whereas one enantiomer can have the desired beneficial properties,the other can have none or the same or even adverse effects.
High-performance liquid chromatography(HPLC)coupled to mass spectrometry(MS)has become one of the most dominant techniques for analysis in the pharmaceutical field[2].Hyphenation of the high-resolving power of HPLC to MS provides straightforward method development capabilities with excellent analytical linearity,sensitivity and selectivity in the enantioselective analysis of drugs throughout the drug discovery and development process[2,3].Applying tandem mass spectrometry in HPLC-MS adds further selectivity to MS detection of drug molecules and their metabolites in complex biological matrices,which can help avoid the need for more complicated separation or extensive sample clean-up procedures[4].
The recent development of superficially porous particles(SPPs,fused-core or core-shell)based columns is generally considered a breakthrough in column technology that provides reduced analysis time while maintaining high column efficiencies with relatively low operational back pressure[16–18].The increased efficiencies result from lower eddy dispersion,as well as other minimized band broadening effects compared to their fully porous particle(FPP)analogues[19–22].In addition to the above mentioned advantages,SPP materials provide flatter dependence of column performance on the mobile phase flow-rate,primarily due to decreased resistance to mass-transfer compared to FPP(a smaller C-term in the van Deemter equation),hence are better suited for high-speed separations[23].
Although SPP based achiral stationary phases have been developed and have become widely used for drug analysis in recent years,chiral SPP based materials have lagged behind.Recently,several polysaccharide and glycopeptide based chiral selectors have been coated or covalently bonded to SPP silica gel for chiral separations[9,23,24].Higher enantiomeric separation efficiency and resolution were often observed with the use of SPP based CSPs when compared to their corresponding FPP analogues[23–25].Chiral separations done in seconds were achieved with these newly developed materials[9].However,all the enantiomeric separation methods based on these novel SPP columns were developed with UV detection and most of them cannot be directly utilized with HPLC-MS due to various mobile phases and additive incompatibilities.Diminished or lost enantiomeric selectivity/resolution is often observed when simply changing mobile phase solvents and additives of previously developed HPLC-UV methods to achieve MS compatible conditions[25,26].Hence,the LC-MS conditions need to be carefully optimized prior to chiral analysis.
The purposes of this study are:(1)to systematically evaluate mobile phase conditions suitable for chiral LC-MS analysis,and provide a guideline for users in the selection of mobile phase additives in chiral LC-MS,(2)to evaluate the feasibility of using SPP based CSPs for fast and efficient enantioseparation of pharmaceutical basic drugs with MS detection,and(3)to compare the enantioseparation performance between SPP and FPP based CSPs.The macrocyclic glycopeptide vancomycin chiral selector has been used in the enantiomeric separation of a variety of chiral basic drugs.The usefulness of this macrocyclic selectors results from its broad selectivity,making it an ideal candidate for chiral LC-MS analyses[26–29].
2.Experimental
2.1.Chiral drugs and chemicals
22 basic drugs including 11β-blockers,4 antidepressants,4 sedative-analgesics and 3 other drugs were selected as test standards(Table 1)and provided by Millipore Sigma-Aldrich(St.Louis,MO,USA).The mobile phases were prepared from the following compounds(purities>95%)and solvents:formic acid(FA),trifluoroacetic acid(TFA),triethylamine(TEA),ammonium formate(NH4FA),ammonium acetate(NH4Ac),ammonium trifluoroacetate(NH4TFA),and triethylammonium acetate(TEAAc)were all from Sigma-Aldrich;acetic acid(AA)was from J.T.Baker(Center Valley,PA,USA);HPLC-MS grade methanol and water were from Honeywell Burdick and Jackson(Morristown,NJ,USA).Citalopram was obtained from Sigma-Aldrich and standards of its two degradation products were a gift from Lundbeck(Valby,Denmark).
2.2.Chromatography
Table 2 lists the characteristics of the chiral vancomycin stationary phases used in this work in two 10cm×0.21cm columns.The Astec-Chirobiotic V®column was obtained from Supelco(product 11018A-ST,Millipore-Sigma,a division of Merck KGaA,Darmstadt,Germany).The VancoShell®column was obtained from AZYP(product LS2002,Azyp,Arlington,Texas,USA).The columns were mounted in a Prominence LC-20AT HPLC system(Shimadzu,Columbia,Maryland,USA)coupled to an MS-8040 triple quad mass spectrometer(Shimadzu)with an orthogonal electrospray ionization(ESI)source.Signal acquisition and data handling were performed with the LabSolutions v5 software(Shimadzu).Due to the lack of ionization with apolar normal-phase solvents,the two columns were evaluated with pure methanol mobile phases in polar ionic mode(PIM)and hydro-organic mobile phases in reversed-phase(RP)mode.
Unless otherwise indicated,the flow rate was 0.3mL/min producing a dead time of about 40s with the 10cm columns with 0.21cm internal diameter(mobile phase velocity:0.25cm/s).The basic drugs were dissolved in methanol in stock solutions at 1mg/mL,and stored at 5oC.The injection loop had a 2μL volume.All experiments were done at room temperature.
This pattern repeated itself every weekend for almost 10 months. We grew more and more fond of German and we looked forward to his coming. We stopped thinking about where he belonged-he belonged to us. We took comfort in his strong, warm presence, and we felt safe with him near us. When we saw German come to attention and perk9 up his ears, and heard that low growl10 begin deep in his throat, we knew we were protected.
2.3.Forced decomposition study of citalopram
The antidepressant citalopram was subjected to a 2-day hydrolytic degradation.A 10mg solution of citalopram in 100mL 0.2M NaOH(pH 13.3)was heated at 80°C for 48h,and 10mL solution portions were taken at time 12h and 48h and neutralized with a drop of acetic acid.The hydrolytic degradation products were extracted by adding 10mL dichloromethane and vigorously shaking the biphasic mixture.The separated lower organic layer was dried with argon and reconstituted in methanol prior to LC-MS analysis.
3.Results and discussion
3.1.Mobile phase and mass spectrometry detection
3.1.1.Flow rate effect
A UV detector is a non-destructive concentration sensitive detector,while the MS detector is a mass sensitive destructive detector.The difference between the two types of detector can be put simply:if the mobile phase flow rate is stopped when a solute is in the detector,the concentration sensitive UV signal will stay constant;the mass sensitive MS signal will drop to zero as soon as the ion input ceases.The critical consequence is a strong difference in signal intensity that depends on the mobile phase flow rate with the MS detector and not with the UV detector.This signal intensity difference makes the integrated surface area obtained with the UV concentration sensitive detector inversely proportional to the mobile phase flow rate.This surface would not be sensitive to flow rate with the MS mass sensitive detector if the ESI efficiency was not flow rate dependent.Unfortunately,ESI becomes less efficient when there are more or bigger droplets to ionize and this effect also makes the experimental MS surface area decrease when the flow rate increases[30].With the MS instrument in this study,a flow rate of 0.3 mL/min provided the optimal compromise between chromatographic separation duration and ESI signal intensity and it was used for all mobile phase composition testings.
3.1.2.Mobile phase composition effect
Ions must be produced by the ESI source.The mobile phase chemical composition greatly affects ionization.A systematic investigation is difficult since changing the mobile phase composition also affects the chromatographic separation modifying retention time,peak efficiencies and chromatographic resolution.Table 3 gathers the observed effects of mobile phase composition on ESI-MS detection.
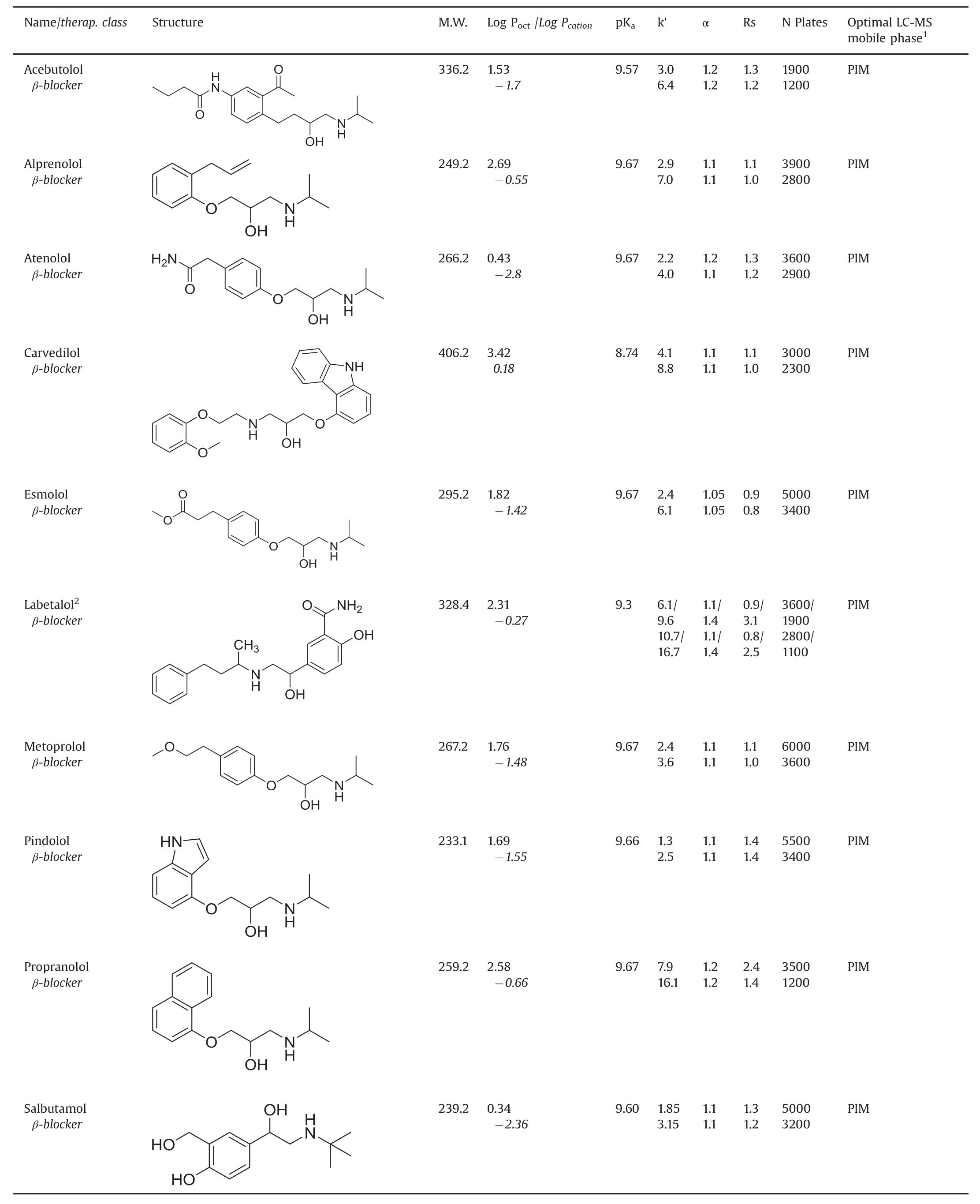
Table 1 Names,structures,properties and best separation conditions of the studied basic drugsa.
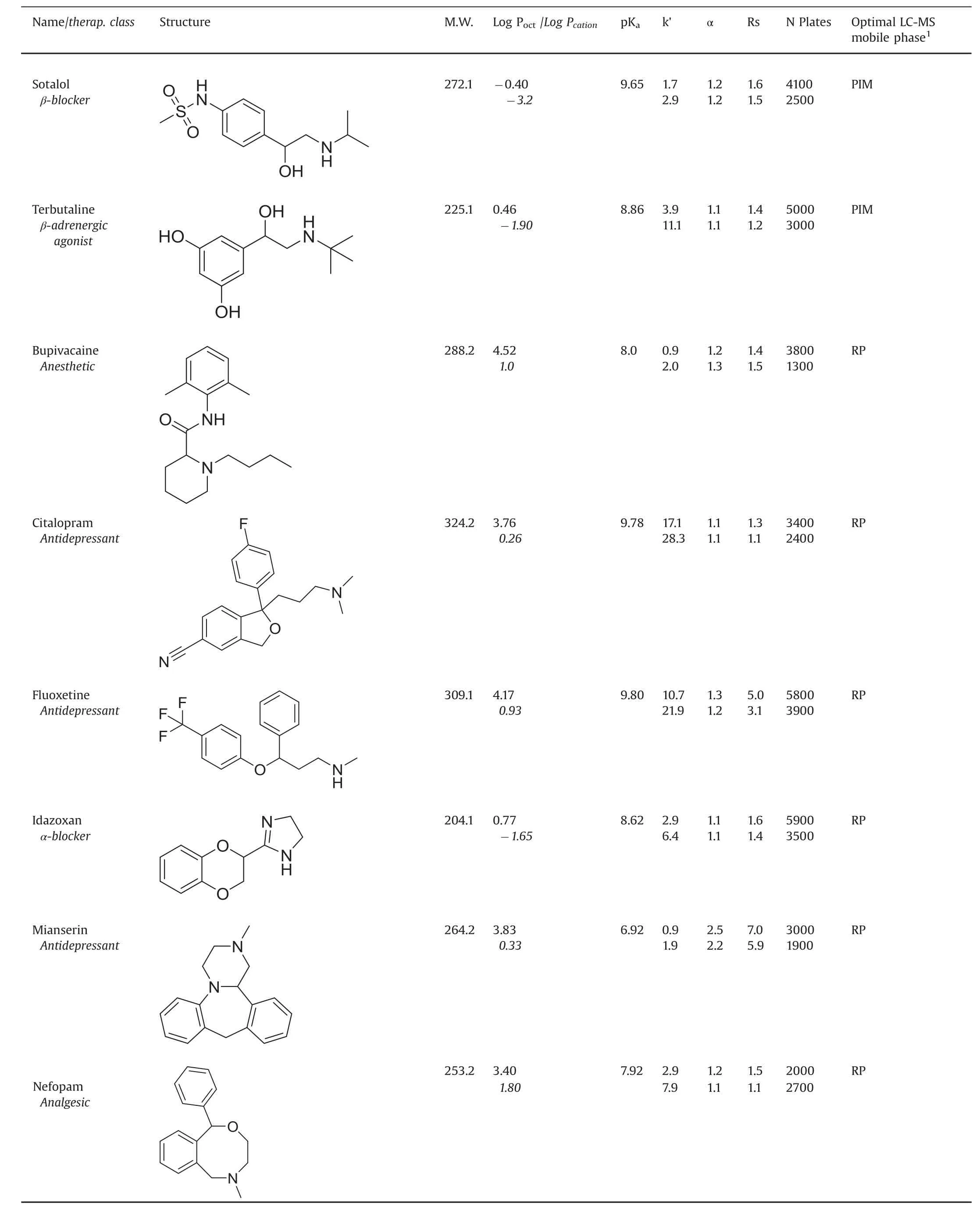
Table 1(continued)
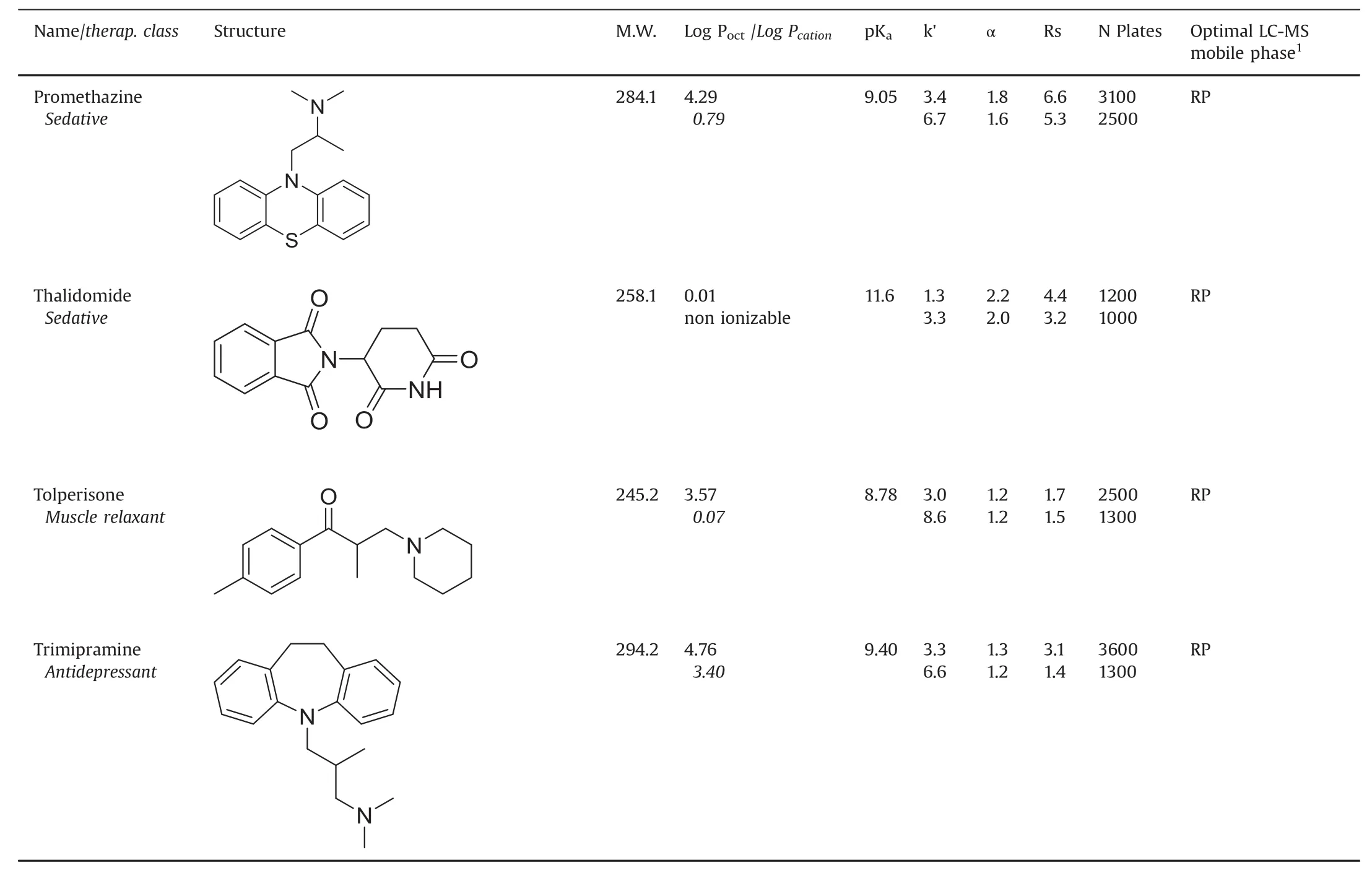
Table 1(continued)
The apolar mobile phases used in the normal phase mode do not solubilize ions.Also they preclude efficient molecule ionization;hence this mode is inappropriate with MS detection.The PIM uses non-aqueous polar mobile phases in which small amounts(0.05%–0.5%)of amine and organic acid are added to modify the ionization state of both the solutes and the stationary phase.Pure methanol was used as the PIM solvent with different added acids,bases or salts.Table 3 shows that good ionization was obtained with ammonium salts,either formate,acetate or trifluoroacetate.The best signal was obtained with 0.5–1mM TEA acetate.However,this salt has a trade-off since higher than 1mM concentration produced faster separation(lower retention times),but it also degraded MS sensitivity due to ionization suppression.The in-situ formation of TEA acetate by adding proportion of TEA and AA to methanol gave similar results with ionization suppression observed when more than 0.02%(v/v)was added.0.02%TEA corresponds to 1.44mM and 0.02%AA is 3.5 mM,the combination of which makes 1.44 mM TEAAc with an excess of about 2mM AA.Pure acids induce solute MS ionization,but they are not recommended for chromatographic reasons:the protonated basic drugs of our set were eluted at the dead volume not giving any separation,since they were electrostatically repelled from the positively charged stationary phase.
The polar aqueous reversed phase mobile phases were appropriate for MS detection.The best salt additive was ammonium acetate in our working conditions with methanol:water(90:10,v/v).TEAAc,NH4FA and NH4TFA also could be used giving poorer peak shapes but with an acceptable signal.Concentrations of TEAAc higher than 0.5mM and addition of TEA and AA higher than 0.05%(v/v)produced significant ionization suppression.Since higher salt concentrations provided faster separations,the optimum reversed phase composition was methanol/water(90:10,v/v)with 5mM NH4Ac(Table 3).
3.2.Enantiomer separation with fully porous and superficially porous particles
Table 1 lists the retention,selectivity and resolution factors obtained with the basic drugs and the two chiral columns containing the same macrocyclic glycopeptide vancomycin selector.For each compound,the first line corresponds to the data obtained with the VancoShell®SPP column and the second line gives the Chirobiotic®V FPP column data,all obtained with the same column geometry,mobile phase composition and flow rate.The detection was done by MS for both columns.The PIM mobile phase composition:pure methanol with 2mM TEAAc,provided optimal separations in term of solute retention,resolution and signal for all β-blocker solutes.The other basic drugs were best separated by the reversed mobile phase made of methanol:water(90:10,v/v)with 5 mM NH4Ac,both flown at 0.3 mL/min.
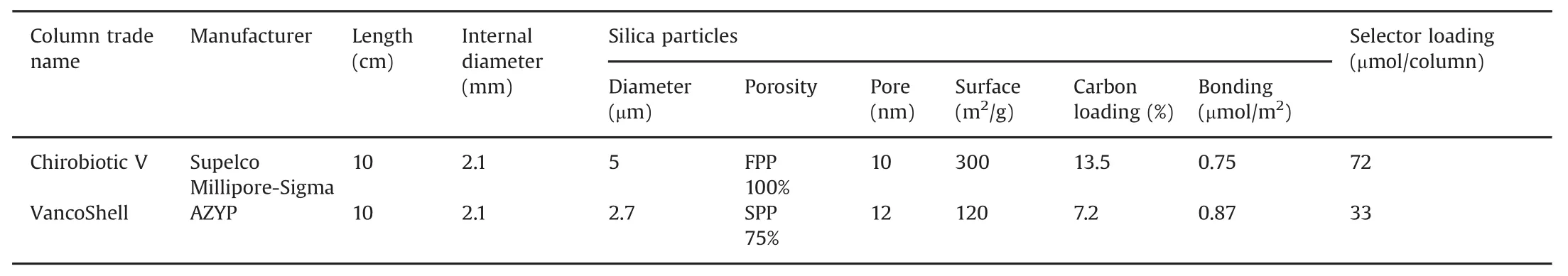
Table 2 Characteristics of the chiral vancomycin columns.
The first general observation is that the Chirobiotic®V FPP column systematically produced longer retention times,seen in almostal ways 50% higher retention factors,k’,than the VancoShell®SPP column for the same solute,mobile phase and flow rate.The second observation is that the enantioselectivity factors obtained with the two columns are very similar.The last general observation is that the peak efficiency obtained with the SPP column is always higher than that observed with the FPP column of equal length.
Pointing that the chiral selector is the same for the two columns,the results can be explained as follows:(i)the difference in retention is due to the fact that the FPP column contains twice more vancomycin selector than the SPP column(72 μmoles versus 33μmoles,Table 2);(ii)this larger amount of selector increases equally the retention of both enantiomers,so the ratio of the two increased retention factors,which is the enantioselectivity factor,stays constant;and(iii)the peak efficiency in column of equal length is linked to the silica particle diameter which is almost twice smaller(2.7 μm)in the SPP column compared to the FPP one(5 μm,Table 2).It must be noted that the listed peak efficiencies are not obtained at the same retention time so the difference between columns may be even higher.Further,the greater extracolumn volume,associated with MS detection,limits the efficiency gains of the SPP column somewhat[9,19,24,25].
The resolution factor combines retention,selectivity and efficiency in a single quality parameter.The Rs factors obtained with the VancoShell®SPP column are clearly significantly better than their corresponding values obtained with the Chirobiotic®FPP column(Table 1).The advantage of the SPP particles is obvious that faster separations are obtained with better resolution factors.Not surprisingly,these results are fully coherent with previously published works done with the vancomycin chiral selector[6,9,31].
3.3.Sensitivity comparison
The most used chromatographic detector is the UV–vis detector for its versatility,ease of use and cost.It is sensitive down to the nanogram on five orders of magnitude linear range,up to almost a milligram injected.With optimized ionization,the MS detector is known to be almost three orders of magnitude more sensitive thanthe UV detector[32].So the MS detector is not compared to the UV detector in term of sensitivity.The limit of detection(LOD)that can be reached with both SPP and FPP columns in similar conditions were compared.
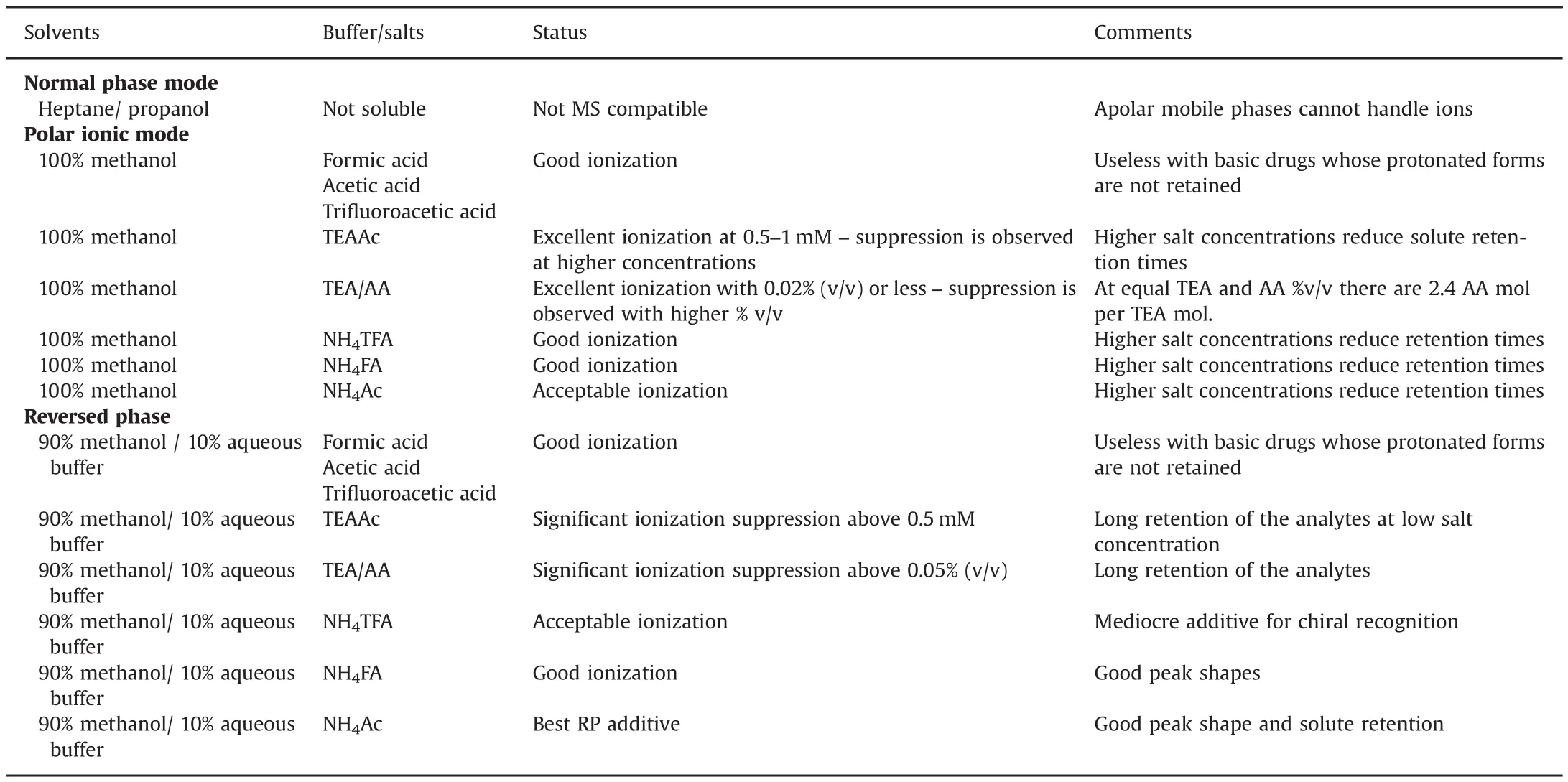
Table 3 Mobile phase compositions and MS detection with electrospray ionization.
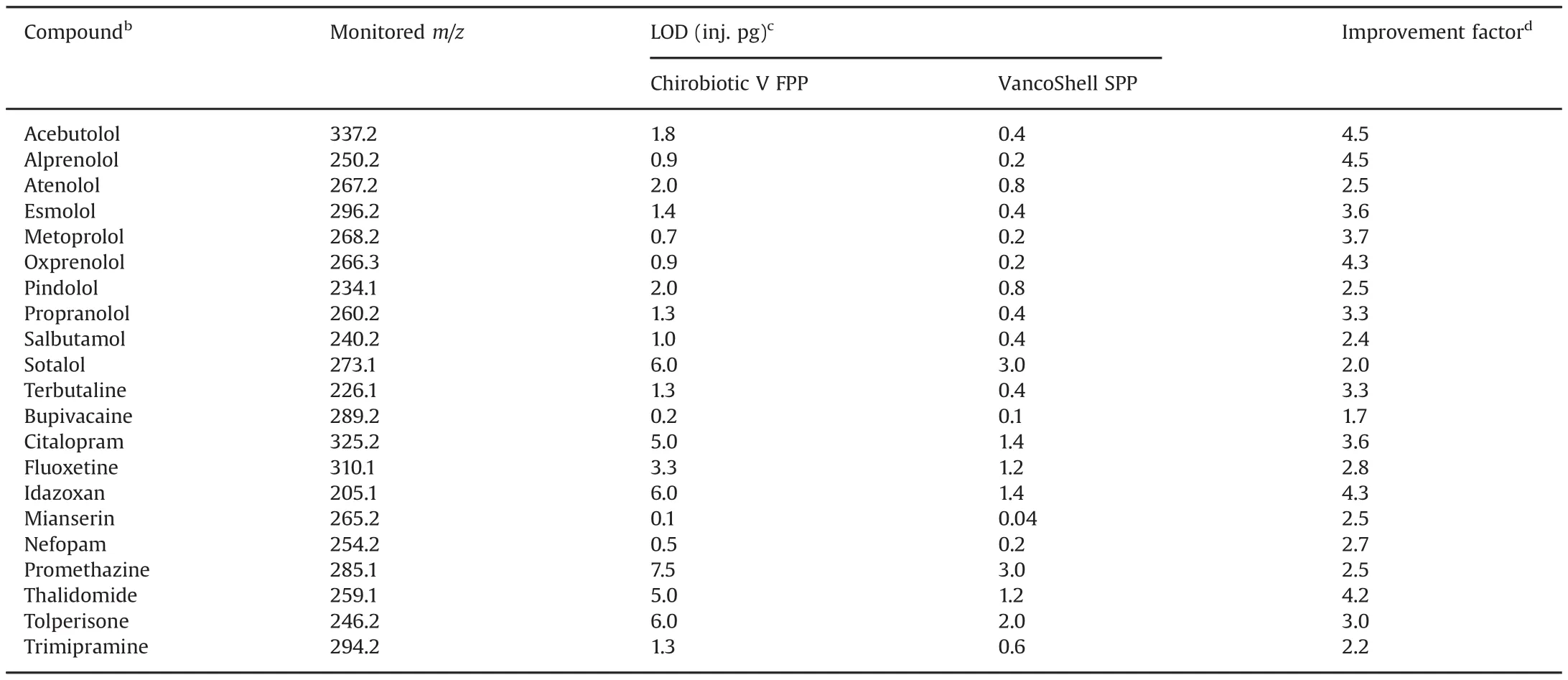
Table 4 Comparison of detection limits(LOD)between the VancoShell SPP and Chirobiotic V FPP columns for the selected basic drugsa.
The LODs in LC-MS were obtained by serial dilution of the standard solution of each compound until a signal-to-noise ratio(S/N)of three was noted in five replicate injections of the diluted sample.The LOD determinations were performed with the column in the positive SIM mode monitoring them/zof the protonated analyte.Table 4 summarizes the detection limits for all these tested drugs after column separation using both the VancoShell SPP and the Chirobiotic V FPP columns under identical mobile phase andm/zSIM MS conditions.As can be seen in Table 4,a LOD as low as 40 fg(0.04 pg in absolute values i.e.2μL of a 20 ng/L dilution injected)was obtained for the mianserin drug with the VancoShell SPP column.This lowest LOD was 2.5 lower than that obtained with the Chirobiotic V FPP column for the same compound.The LODs with the VancoShell SPP column were all down to the picogram injected and 2–5 times lower than the corresponding LODs obtained with the Chirobiotic V FPP column in the same MS conditions.
3.4.Fast SPP separations
The SPP core-shell particles were developed for reducing separation duration and especially solvent consumption[6,9].As seen in Table 1,in optimal enantioselective conditions at 0.3mL/min,the VancoShell SPP column separates the listed enantiomers two to four times faster than the Chirobiotic V FPP column with a better resolution.Since fast chiral separations are needed in two-dimensional chromatography,the MS response was tested in separations done in seconds.Fig.1 shows the separation of three drugs achieved in less than a minute.To obtain such fast separations some compromises had to be made.The faster flow rate produced a lower ESI response compensated by injecting 20pg of solute.Also the PIM mobile phase was selected because it gave lower drug retentions;however,the resolution factors also were significantly lower.The promethazine resolution factor dropped from 6.6 with optimized RP mobile phase(Table 1)to 1.4 with the fast PIM mobile phase(Fig.1)mainly due to the great drop in efficiency(from 3100 plates to 400 plates)with broadening peaks at this fast flow rate.However,even in these conditions,the ESI signal is perfectly usable and ESI-MS detection could be used in a fast second dimension in 2D-LC with baseline separation of the enantiomers[33].
3.5.Application:following citalopram basic degradation
The characterization of all possible impurities and degradation products in a drug substance and product is required by regulatory agencies[30].Since many drugs are chiral,the advantage of using CSPs for the degradation studies is that not only degradation products but also each enantiomer of the degraded drug products can be identified and quantified.The MS detection adds the information on the nature of the degradation products providing their mass to charge ratio.
A forced degradation study was done using the racemic form of the antidepressant drug citalopram as a representative.Two major peaks(m/z343 and 344)were found in the positive triple-quadripole-scan mode with direct injection of 2μL of the processed sample.The degraded sample was further separated on the SPP-V column and was detected in positive Q3-SIM mode.The peaks were compared to the citalopram standard.As shown in Fig.2,the citalopram standard peaks that appear in the retention window of 12–15min progressively decrease and eventually disappear,indicating the complete degradation of citalopram in less than two days at 80°C,pH 13.The extracted ion chromatograms(EIC)in SIM mode atm/z343 and 344 showed two chiral degradation products.One enantiomeric pair appears at the retention window:3.0–4.5min and the other pair eluting between 18 and 21min.After checking for possible structures corresponding to them/zratios and determining the retention times of the provided degradation product standards(Fig.2),the structure of Product 1 was confirmed to be the amide obtained by hydrolysis of the citalopram nitrile group:
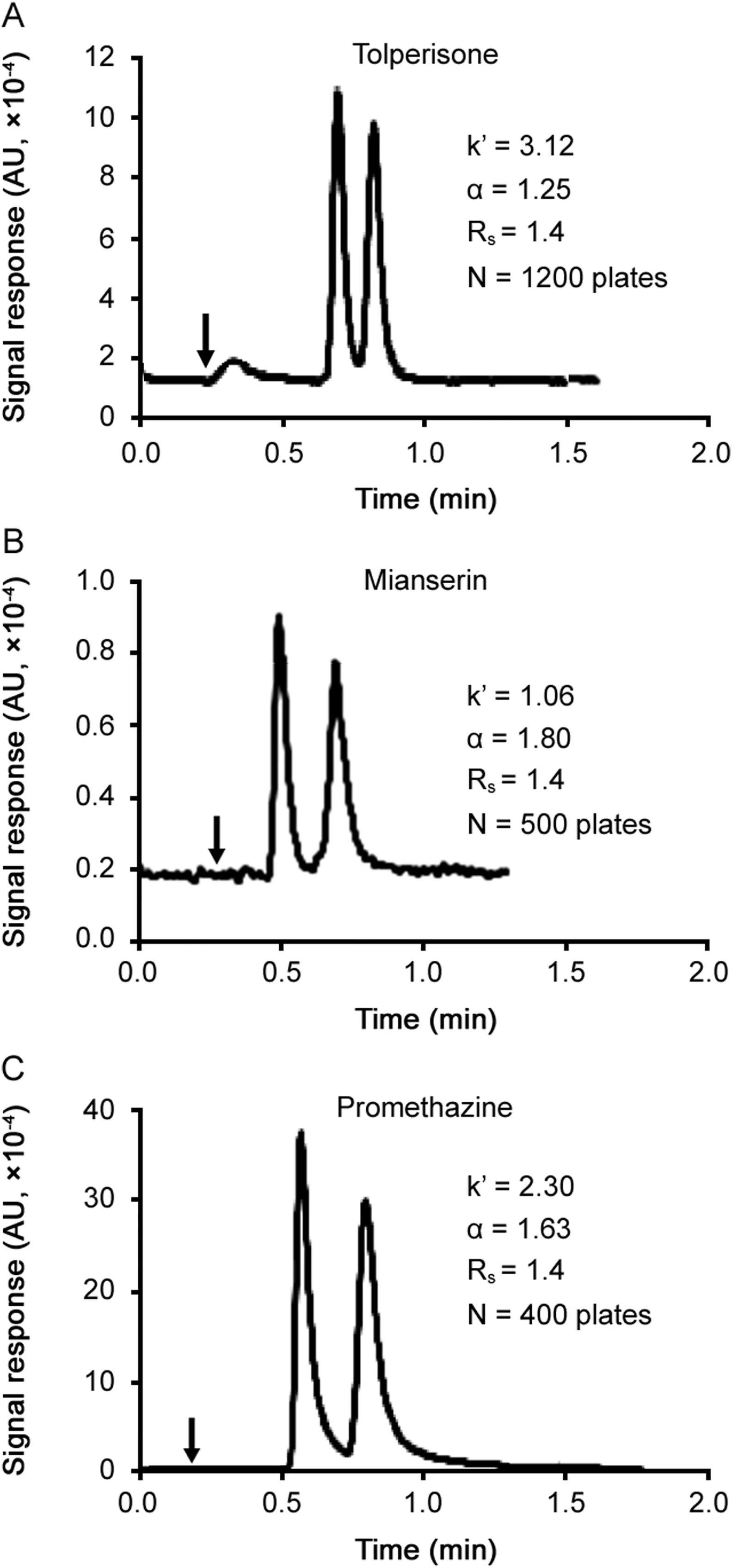
Fig.1.Fast separations of basic drugs using the VancoShell SPP column.Chromatographic conditions:PIM mobile phase:methanol with 2 mM TEAAc; flow rate:(A)1.0 mL/min for tolperisone,(B)0.8 mL/min for mianserin,(C)1.2 mL/min for promethazine;injection volume:2 μL of 10 ng/mL.MS detection in positive SIM mode;monitored m/z:tolperisone 246.2,mianserin 265.2 and promethazine 285.1.The vertical arrows point at the dead time.
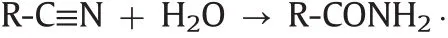
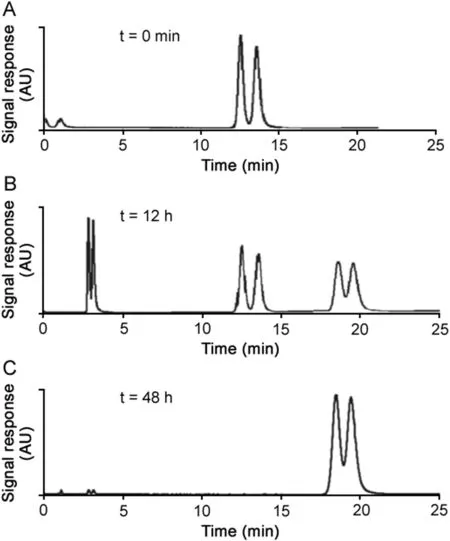
Fig.2.Following the achiral basic degradation of citalopram in aqueous pH 13 solution at 80°C.Chromatographic conditions:column VancoShell 10cm×0.21 cm,2.7μm core shell particles;RP mobile phase,methanol/buffer 5mM NH4Ac(10:90,v/v); flow rate,0.3mL/min;injection volume,2μL.MS conditions:(A)TIC in positive Q3-scan mode,(B)and(C)positive Q3 SIM mode at m/z 343.2 from 0 to 5min(amide),325.2 from 5 to 15min(nitrile),and 344.2 from 15 to 25min(acid derivative).
This amide was itself hydrolyzed in the corresponding acid Product II following R-CONH2→R-COOH.The confirmed structures of degradation Products I and II were consistent with previously reported results in the literature[34,35].
4.Conclusions
An MS detector is suitable to detect separated enantiomers in the polar ionic mode with methanol and appropriate salt mobile phases,and in the reversed phase mode with an aqueous buffer mobile phase rich in organic modifier.Volatile salts must be used to buffer the mobile phase or to adjust the stationary phase ionization for best enantiorecognition.Amounts of triethylamine and acetic acid smaller than 0.05%(v/v)were optimal,allowing to adjust the acidity/basicity by adapting the TEA and AA proportions.Larger amounts of salts produced ESI ionization suppression.Ammonium acetate was the best salt additive in the RP mode when higher than 1 mM concentration was needed for peak shape or lower retention of the basic drugs tested.
The advantages of using a column containing superficially porous particles are faster separations using significantly less mobile phase associated with better efficiencies giving similar or better resolution.These advantages were confirmed by comparing an SPP column to a fully porous column of identical geometry and both containing the same vancomycin chiral selector.In optimized conditions the LODs obtained with the SPP column were between two and five times lower than those obtained with the FPP column.However,higher mobile phase flow rates are associated with a lower ionization yield due to an increased number of larger droplets in the ESI source,suggesting that separations of enantiomers done in seconds will be detected by the MS with lower performance(LOD)than those obtained in optimal(slower)conditions.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank AZYP,LLC,for their technical support for HPLC chiral column technology,and Shimadzu Institute Center for Nanostructured Materials for providing support for all mass spectrometry experiments.This work was supported by the Robert A.Welch Foundation(Y0026)and the French National Center for Scientific Research(ISA-CNRS-UMR5280).
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- 3D biofabrication of vascular networks for tissue regeneration:A report on recent advances
- A rapid reporter assay for recombinant human brain natriuretic peptide(rhBNP)by GloSensor technology
- Solution pH jump during antibody and Fc-fusion protein thaw leads to increased aggregation
- Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells
- Discoursing on Soxhlet extraction of ginseng using association analysis and scanning electron microscopy
- Molecular docking studies of human MCT8 protein with soy isoflavones in Allan-Herndon-Dudley syndrome(AHDS)
