Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells
2018-10-18SelimDemirIrahimTuranRezzanAliyaziiogluSerapOzerYamanYukselAliyaziioglu
Selim Demir,Irahim Turan,Rezzan Aliyaziioglu,Serap Ozer Yaman,Yuksel Aliyaziioglu
aDepartment of Nutrition and Dietetics,Faculty of Health Sciences,Karadeniz Technical University,61080 Trabzon,Turkiye
bDepartment of Genetic and Bioengineering,Faculty of Engineering and Natural Sciences,Gumushane University,29100 Gumushane,Turkiye
cDepartment of Biochemistry,Faculty of Pharmacy,Karadeniz Technical University,61080 Trabzon,Turkiye
dDepartment of Medical Biochemistry,Faculty of Medicine,Karadeniz Technical University,61080 Trabzon,Turkiye
Keywords:Apoptosis Cell cycle Cervical cancer Cytotoxicity Primula vulgaris
A B S T R A C T Primula vulgaris belongs to the genus Primula,members of which are frequently used in folk medicine.Various studies have investigated the cytotoxic effect of different Primula species,but there have been limited studies on the cytotoxic effect of P.vulgaris.The aim of this study was to investigate the cytotoxic effects,and possible mechanisms involved,of P.vulgaris flower extract on human cervical cancer(HeLa)cells.The cytotoxic effect of the extract on HeLa cells was revealed using the MTT assay.Mechanisms involved in the extract's cytotoxic effect were then investigated in terms of apoptosis,mitochondrial membrane potential,and the cell cycle,using fluorometric methods.P.vulgaris flower extract exhibited selective cytotoxic effects against HeLa cells by arresting their cell cycle at the S phase,and inducing the number of apoptotic cells compared to normal fibroblast cells by reducing mitochondrial membrane potential in a concentration-dependent manner.This is the first study to reveal the antiproliferative effect of P.vulgaris flower extract.Further studies are now needed to identify the cytotoxic molecules in the extract and their mechanisms.
1.Introduction
Cancer is a global health problem,and 21%and 9%of deaths in developed and developing countries,respectively,are reported to be cancer-related.The World Health Organization(WHO)predicts approximately 27 million new cases of cancer and 17.5 million deaths from cancer annually by 2050[1].Cervical cancer is the second most common cancer type in women worldwide,and failure to detect the condition at an early stage and/or the development of treatment resistance can lead to fatal outcomes[2].While the treatment of many cancer patients often involves the use of chemotherapy and radiotherapy,these treatments gradually cause damage to normal cells,and resistance to targeted cancer cells reduces the success rate of these therapies[3].Current research therefore focuses on developing new generation drugs with fewer side-effects[4].
Plants have been used to treat and prevent many human and animal diseases since ancient times.Today,approximately 50%of the anticancer drugs used in chemotherapy are obtained from plants.According to the WHO data,more than 80%of people living in developing countries use natural products for primary health problems.Recent surveys show that more than 60%of cancer patients use natural products for therapeutic purposes.However,very few of these medicinal plants have been scientifically evaluated in terms of their anticancer properties.The idea of producing new generation anticancer drugs from natural products has therefore attracted great interest in both the scientific and commercial spheres in recent years[4–6].Primulabelongs to the familyPrimulaceae,and more than 400 species are found in the Northern hemisphere,growing in moist and temperate climatic regions[7].Primulaspecies are used in traditional medicine against bronchitis,asthma,and insomnia[6].Primulaspecies are reported to be rich in saponins,alkaloids,tannins,terpenes,and phenolic compounds[7,8].The antioxidant,antimicrobial,antigenotoxic, anti-inflammatory,hypoglycemic,cytotoxic,and wound healing properties ofPrimulaspecies have been extensively studied,and these useful biological properties are attributed to the presence of the abovementioned compounds[6–10].Commercial interest inPrimulaspecies is growing all the time,especially in the food,cosmetic,and drug industries,due to their beneficial biological properties[6].
Numerous studies have investigated the antiproliferative effect of differentPrimulaspecies.The cytotoxic effect of aqueous extracts ofP.vulgarisflowers and leaves was examined using the brine shrimp method,and LC50values of 311 and 40μg/mL were determined respectively,in one study[11].Behzad et al.[3]showed that the methanol extract ofPrimula auriculataexhibits apoptotic properties via caspase activation in the human colon cancer(HT-29)cell line.However,no previous studies have investigated the cytotoxic effect ofP.vulgarisflower extract on cervical cancer cells.The aim of this study was to investigate the cytotoxic effect ofP.vulgarisflower extract on HeLa cells,together with the mechanism involved.
2.Experimental
2.1.Chemicals and reagents
Gentamicin and trypsin/EDTA solutions were obtained from Biological Industries(Kibbutz Beit Haemek,Israel),Eagle's minimum essential medium(EMEM)from Lonza(Verviers,Belgium),and fetal bovine serum(FBS)from Biochrom(Berlin,Germany).All flow cytometry kits were purchased from Becton Dickinson(San Diego,CA,USA).The other principal chemicals used were obtained from Sigma(St.Louis,MO,USA).
2.2.Sample collection and extract preparation
P.vulgarisplant samples used in the study were collected from Trabzon,Turkiye in the Spring of 2015.The plant samples were dried at room temperature for 20 days.The flower parts were then carefully separated and converted into a fine powder using a blender and milling procedures.Next,0.5 g of the powdered samples was mixed with 10 mL of dimethyl sulfoxide(DMSO).After thorough vortexing,the mixture was left to incubate for 24h with continuous shaking at 150 rpm at 45°C.After incubation,the mixture was centrifuged at 2000gfor 10 min.The supernatant was filtered with Whatman No.1 filter paper and then passed through 0.2 μm filters.The resulting DMSO extract ofP.vulgarisflower was aliquoted for use in experiments and stored in the dark at-20°C.
2.3.Drug preparation and treatment
Cisplatin was dissolved in DMSO and used as a reference compound in cytotoxicity experiments due to its use in cervical cancer treatment[12].Final solvent concentrations of compounds were no higher than 0.5%in culture media in any experiment.That concentration was not sufficient to affect cell morphology or viability.
2.4.Cell culture
Human cervix adenocarcinoma(HeLa,ATCC-CCL-2)cancer and human normal foreskin fibroblast(ATCC-CRL-2522)cells were supplied by the American Type Culture Collection(Manassas,VA,USA).Both cells were cultured in EMEM supplemented with 10%heat inactivated FBS and 1%gentamicin solution with a 5%CO2supply at 37°C.
2.4.1.Cytotoxicity experiments
MTT assay with a 72 h treatment time was employed to measure the cytotoxic effects ofP.vulgarisflower extract and cisplatin on human cervical cancer and normal fibroblast cells[13,14].Briefly,HeLa and fibroblast cells were seeded into flat-bottomed 96-well cell culture plates at 1×104and 2×103cells per well,respectively.The cells were then treated with varying concentrations ofP.vulgarisflower extract(0–500μg/mL)and cisplatin(0–10μg/mL)for 72 h in a triplicate manner.Subsequently,10μL of MTT dye(0.25 mg/mL)was placed inside each well.The crystals that emerged were then dissolved in DMSO.Finally,absorbance was measured at 570nm with a microplate reader(Molecular Devices Versamax,California,USA).Optical densities were employed to calculate percentage viabilities in treated cells compared to untreated control cells.Log-concentrations versus%cell viabilities were plotted with a logarithmic graph,which was then used to determine the IC50values.The IC50values of the extract and cisplatin in the both cell lines were used to elicit a selectivity index[5]with the following formula:Selectivity Index =Fibroblast cells IC50/HeLa cells IC50
2.4.2.Cell cycle analysis
Cell cycle analysis was performed using a commercial kit(BD Cycletest™Plus,Cat No:340242,San Diego,CA,USA)following the manufacturer's recommendations.HeLa cells were treated with 90,180,and 270μg/mL concentrations ofP.vulgarisflower extract for 72 h,then harvested,and washed twice with buffer solution.The cells were incubated with trypsin solution for 10 min followed by trypsin inhibitor and RNase solution for 10 min.Finally,the cells were further treated with propidium iodide(PI)staining on ice for 10min.Data from 30,000 cells per sample were collected and analyzed on a flow cytometer(BD Accuri C6,MI,USA).The percentage of the cells in cycle phases was determined using MODFIT 3.0 verity software.The results were finally compared with the untreated negative control cells.
2.4.3.Apoptosis analysis
Apoptosis analysis was performed using a commercial kit(BD Pharmingen™,Cat No:559763,San Diego,CA,USA)following the manufacturer's recommendations.HeLa cells were treated with 90,180,and 270μg/mL concentrations ofP.vulgarisflower extract for 72 h,then harvested,and washed twice with ice-cold phosphate-buffered saline solution.The cells were then resuspended with the binding buffer.Next,they were incubated with PE-Annexin V and 7-AAD for 10 min at room temperature in the dark.Data from 10,000 cells per sample were collected and analyzed on a flow cytometer(BD Accuri C6,MI,USA).The results were compared with the untreated negative control cells.
2.4.4.Mitochondrial membrane potential(MMP)analysis
3,3′-dihexyloxacarbocyanine iodide DiOC6(3)was used to determine the changes in MMP in this study.Normally,this tends to remain in the mitochondria.The decrease in the intensity of this dye is indicative of a decrease in MMP[15].Cells were seeded into a flat-bottomed 96-well black-walled plate at 1×103cells per well.The cells were then treated with different concentrations ofP.vulgarisflower extract(90–270μg/mL)for 72h.After treatment,the cells were washed with phosphate-buffered saline solution and loaded with 10 nM DiOC6(3)for 30 min at 37°C in the dark.Finally, fluorescence measurement was performed on a plate-reading fluorometer(Molecular Devices SpectraMax Paradigm Multi-Mode,Sunnyvale,CA,USA)with an excitation wavelength of 484 nm and an emission wavelength of 525 nm.Results are given as relative MMP compared to untreated negative control cells.
2.5.Statistical analysis
All experiments were performed at least three times,the results being expressed as mean±standard deviation.Normal distribution was determined using the Kolmogorov-Smirnov test.One-Way ANOVA was used to analyze intergroup differences and Tukey's test was performed for post-hoc comparisons.p<0.05 was regarded as significant.
3.Results
P.vulgarisflower extract exhibited selective cytotoxic effects on the HeLa cells compared to normal fibroblast cells.The IC50value of the extract in HeLa cells was 182.4 μg/mL,while the IC50value of the extract in fibroblast cells was calculated as 493.9 μg/mL(Fig.1 and Table 1).
The results of the cell cycle analysis are presented in Fig.2.All the concentrations ofP.vulgarisflower extract significantly reduced the cell number at the G2/M phase(p=0.0001).Additionally,180 and 270μg/mL extract concentrations significantly reduced the cell number at the G1phase and increased the cell number at the S phase compared to the untreated cells(p=0.0001).
The results of the apoptosis analysis are presented in Fig.3.P.vulgarisflower extract significantly reduced the number of viable cells and increased the numbers of apoptotic cells compared to untreated cells in a dose-dependent manner(p<0.05).
MMP analysis results are presented in Fig.4.The 180 and 270μg/mL extract concentrations significantly reduced the MMP in HeLa cells(p=0.001).The percentage reductions in MMP caused byP.vulgarisflower extract at concentrations of 90,180,and 270μg/mL were 8%,64%,and 72%,respectively.
4.Discussion

Fig.1.Effects of different concentrations of P.vulgaris flower extract on cell viability during a 72 h incubation period(n=3).*There was a statistically significant decrease compared to normal fibroblast cells.
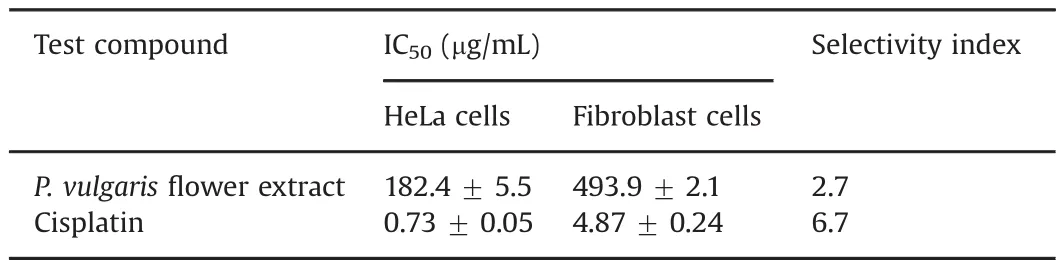
Table 1 Cytotoxic effects(IC50,μg/mL)and selectivity index of the test compounds.
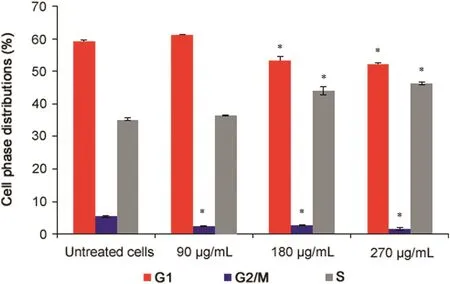
Fig.2.Cell cycle analysis of P.vulgaris flower extract-treated HeLa cells after 72 h(n=6).*Represents significant result(p<0.05)compared with untreated cells.
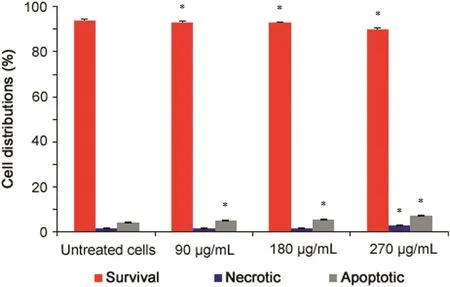
Fig.3.Apoptosis analysis of P.vulgaris flower extract-treated HeLa cells after 72h(n=4).*Represents significant result(p<0.05)compared with untreated cells.
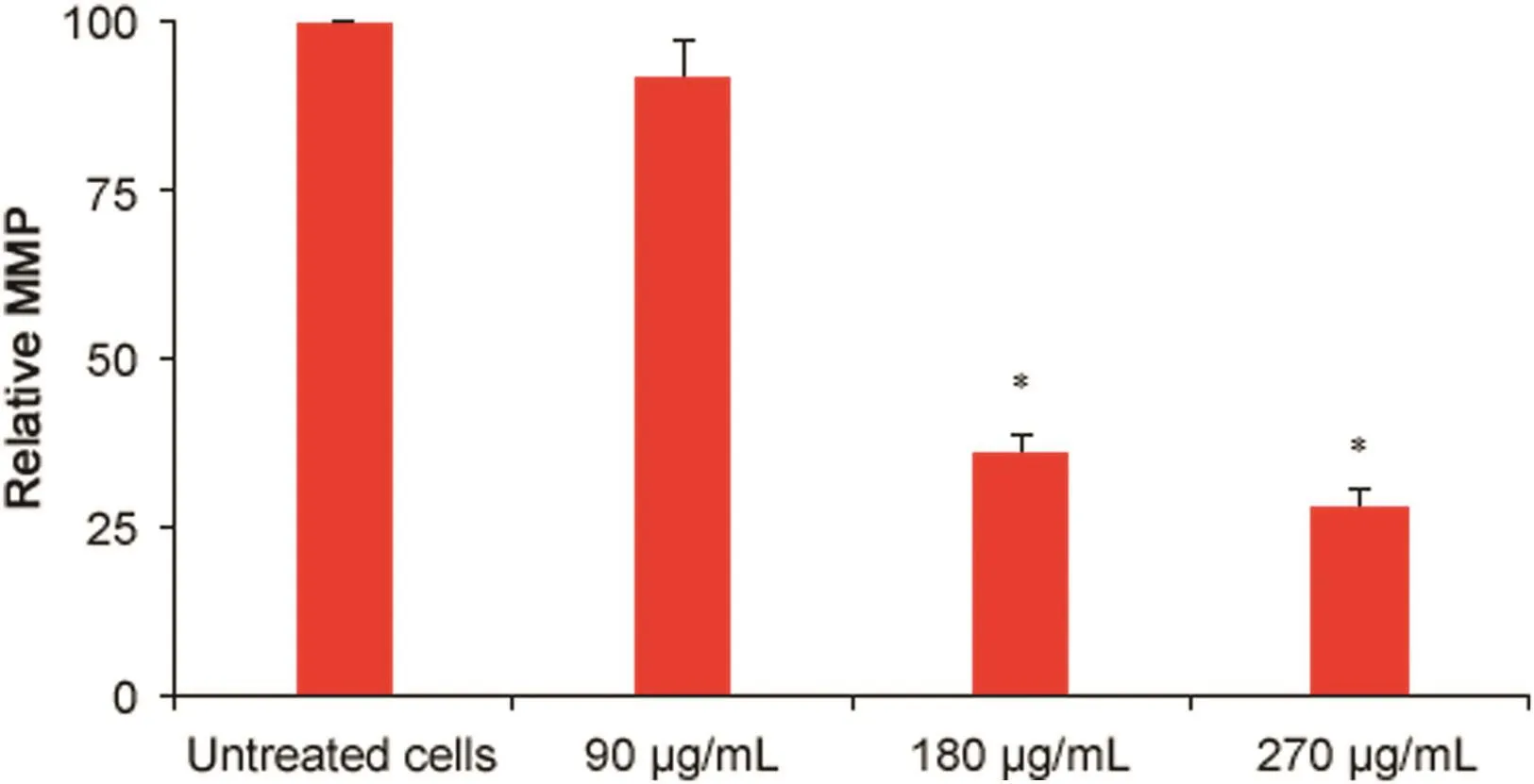
Fig.4.Effects of P.vulgaris flower extract on the integrity of the mitochondrial membrane of HeLa cells after 72 h(n=3).*Represents significant result(p<0.05)compared with untreated cells.
Cancer is one of the most important causes of disease-related mortality in the world.The WHO reports that between 2005 and 2015 approximately 84 million people lost their lives due to cancer[3].Chemotherapy is frequently used in cancer treatment,but the toxic effects that occur over time limit the use of these drugs.Both the high mortality rate in cancer cases and the serious side-effects of chemotherapy and radiotherapy have encouraged the search for alternative and/or complementary treatments[4,5].Natural products are today regarded as potential raw materials for new drug discoveries,and phenolic compounds found in natural products are particularly significant in this context due to their structures and activities.Investigation of the anticancer effects of both natural product extracts and compounds isolated from such products has become one of the popular research fields in recent years.Various criteria need to be met for an effective and acceptable anticancer agent,including its effects on normal cells being harmless[5,15].We therefore planned cytotoxicity experiments in HeLa cells coupled with human normal foreskin fibroblast cells.Numerous methods,such as trypan blue,MTT assay,neutral red,and lactate dehydrogenase,are frequently used for the determination of cell viability,proliferation rates,and cytotoxicity.In order to determine the cytotoxic effect of the extract,we employ the MTT,a reliable,sensitive,quantitative,and colorimetric method,previously used in similar studies[5,14,15].P.vulgarisflower extract exhibited selective toxicity(2.7-fold)against HeLa cells compared to normal fibroblast cells.Deterioration of cell cycle control and decreased apoptosis are two of the characteristic features of cancer cells.Anticancer activity studies investigating novel agents therefore focus on the ability to halt the cell cycle and increase apoptosis[5,15,16].We therefore investigated the mechanism involved in the cytotoxic effect of the extract on HeLa cells in terms of the cell cycle,MMP,and apoptosis using fluorometric methods.Our results show thatP.vulgarisflower extract exhibited antiproliferative features in HeLa cells by stopping the cell cycle in the S phase and inducing apoptosis by reducing mitochondrial membrane potential in a concentration-dependent manner.Other studies involving the cytotoxic effect ofPrimulaspecies have examined the cytotoxic effect of the ethanolic crude extract ofPrimula macrophyllaand its ethyl acetate,chloroform,and benzene fractions using brine shrimp methods,with the most effective cytotoxic activity being observed in the crude extract[17].Behzad et al.reported that the methanol extract ofPrimula auriculataexhibited a selective cytotoxic effect on human breast(MCF-7),liver(HepG2),and HT-29 cancer cell lines compared to normal bovine kidney cells.No cytotoxic effect was observed in that study in the human lung(A549)cancer cell line up to an extract concentration of 100μg/mL[18].Turan et al.[6]demonstrated thatP.vulgarisleaf extract exhibits selective cytotoxic effects against human A549,HepG2,MCF-7,prostate(PC-3),and colon(WiDr)cancer cell lines compared to human normal fibroblast cells.Few studies have examined the cytotoxic activity ofPrimulaspecies.However,the methanolic extract ofDionysia termeana,a member of thePrimulaceaefamily,has been reported to exhibit cytotoxic effects in human myelogenous leukemia(K562),T-lymphocytic leukemia(Jurkat),bladder(Fen),and A549 cell lines in a dose-dependent manner.That study also determined that the cytotoxic effect of the extract in blood-derived cell lines(K562 and Jurkat)was derived from induced apoptosis[19].Another study showed that the aqueous extract ofLysimachia vulgaris,a member of thePrimulaceaefamily,and its hexane,dichloromethane,and methanol fractions had no cytotoxic effect on the MCF-7 and HepG2 cell lines up to a concentration of 200μg/mL[20].Studies have also investigated the antiproliferative activity of various compounds isolated fromPrimulaspecies.Tokalov et al.[21]reported that isolated compounds isolated fromPrimula denticulata,such as5-hydroxy flavone,2′-hydroxy flavone,5,2′-dihydroxyflavone,and 5,8-dihydroxy flavone,exhibit antiproliferative effects in the human acute myeloid leukemia(HL-60)cell line by activating cell cycle arrest and apoptosis.
Polyphenols are an important class of secondary herbal metabolites reported to exhibit strong antioxidant properties[6].Recent studies in particular have reported that polyphenols can prevent the proliferation of cancer cells by activating cell cycle arrest,apoptosis,and cell signalization[22].Primulaspecies have been shown to be rich in phenolic compounds,such as kaempferol,quercetin,rutin,5-hydroxy pyrogallol,apigenin,catechin derivatives,gallic acid,rosmarinic acid,p-coumaric acid,protocatechuic acid,p-OH benzoic acid,vanillic acid,caffeic acid,ferulic acid,and ellagic acid[7,8].In our previous study,we demonstrated that the major components ofP.vulgarisare as follows:p-coumaric acid(848μg),rutin(336.5μg),p-OH benzoic acid(76.5μg),vanillic acid(65μg),catechin(53.9μg),caffeic acid(53.1μg),protocatechuic acid(30.7μg),and gallic acid(3.2μg)per g sample[7].There are also considerable investigations of the anticancer properties of these compounds in various types of cancer cells[23,24].Studies examining the cytotoxic properties of extracts from various natural products show that the overall effect of the extract has first been examined,and the results have been attributed to synergistic effects.Effective compounds have been isolated from crude extract,and its biological effects have also been investigated[6].It is believed that all these phenolic compounds of theP.vulgarisflower extract contribute to its cytotoxic effect.
5.Conclusion
This is the first study to examine the mechanisms involved in the in vitro cytotoxic effect ofP.vulgarisflower extract on HeLa cells.Further studies are now needed for a more detailed understanding of the exact interaction of the signaling pathways involved.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to thank Professor Ersan Kalay from the Medical Biology Department,Karadeniz Technical University for professional assistance with the flow cytometry studies.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- 3D biofabrication of vascular networks for tissue regeneration:A report on recent advances
- A rapid reporter assay for recombinant human brain natriuretic peptide(rhBNP)by GloSensor technology
- Solution pH jump during antibody and Fc-fusion protein thaw leads to increased aggregation
- Discoursing on Soxhlet extraction of ginseng using association analysis and scanning electron microscopy
- Molecular docking studies of human MCT8 protein with soy isoflavones in Allan-Herndon-Dudley syndrome(AHDS)
- Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases
