Structural evolution,optoelectrical and corrosion properties of electrodeposited WO3integration on Zn-TiO2electrolyte for defence super application
2018-10-18DniynUmoruFyomiPopool
A.A.Dniyn,L.E.Umoru,O.S.I.Fyomi,A.P.I.Popool
a Department of Materials Science and Engineering,Obafemi Awolowo University,Ile-Ife,Nigeria
bDepartment of Chemical,Metallurgical and Materials Engineering,Tshwane University of Technology,P.M.B.X680,Pretoria,South Africa
cDepartment of Mechanical Engineering,Covenant University,P.M.B 1023,Ota,Ogun State,Nigeria
Keywords:Nano-composite Electrocodeposition Corrosion Conductivity and resistance
ABSTRACT Multifunctional nano composite coatings of Zn-TiO2-WO3were deposited electrolytically on mild steel(MS)from Zn bath,having Zn2+ions and uniform dispersion of TiO2and WO3nano particulates.The electrical,optical and corrosion resistance characteristics of the electrocodeposited coatings were assessed by Keithley 2400 Series Source meter with Multimeters,Newport Solar Simulator and a PGSTAT30 Autolab potentiostat respectively.The morphological characteristics of the composite coatings were characterized by scanning electron microscope(SEM)equipped with energy dispersive spectrometer(EDS).The result revealed that the electrocodeposits showed good stability and Zn-TiO2-WO3 nanocomposite deposits displayed enhanced microstructural qualities,good electrical conductivity and exhibited enriched corrosion resistance.
1.Introduction
Metal oxides displayed many important physical and chemical properties in which many of them are semiconductors with large bandgap compounds of conductivity which is n-type.For instance,WO3and TiO2have bandgaps in the range between 3.0-3.4eV[1-3].WO3and TiO2and are two metal oxides with related conducting properties.Recently,great attentions are now drawn to mixed oxides,ever since,it has been established that mixed compounds of TiO2/WO3enhanced electrical charge separation observed under illumination,which has been verified with diverse systems[4,5]and it has been revealed that TiO2-WO3mixed compound found applications in varistor with an electrical behaviour that is not mere linear[6].
Electrocodeposition provides a suitable and multipurpose way to the synthesis of composites of many metal with inorganic oxides[7,8]including Metal with WO3[9]and metal with TiO2[10].Currently,there is wide interest in composite coatings comprising TiO2and WO3in optoelectrical applications.For instance,a greater photo-response was experienced when bilayered composite of TiO2and WO3coatings,compared to the single-component films of each of the constituents[11,12].It has been observed that the photocatalytic activity of TiO2-WO3coatings was found to triple that of pure TiO2coatings in oxidation applications[13].An electron pool observed when WO3was coupled with TiO2coating in the construction of a sensitive PECAS(photoelectrochemical anticorrosion system)with in-built energy storage ability[14].
Apart from the fact that combination of TiO2and WO3is a great asset in the design of photoelectrochromic devices[15],It has also been established that TiO2and WO3possess electrochromic properties that are complementary to each other[16].Nevertheless Nanostructured TiO2-WO3coatings have now be discovered to displayed more excellent electrochromic and photoelectrochemical properties compared to the bulk of their particles,because theycan be brilliantly controlled by adjusting the particle size and some electrocodeposition parameters for coatings,Therefore,this work studied the Structural Evolution,Opto electrical and Corrosion Properties of Electrocodeposited composite of nanostructured TiO2and WO3in Zn matrix for the purpose of developing advance active coating for engineering applications.The focus of this study is the optimization of the WO3nanoparticulates on Zn-TiO2Nanocomposite so achieve enhanced photoelectricity and better energy storage.
2.Material and methods
2.1.Preparation of substrate
The dimension of the mild steel(substrate)used was 45×40×2 mm3sheet and zinc sheets of 85×45×5mm3were prepared as anodes.The cathode was mild steel coupons and anode was commercially pure zinc(99.99%).The mild steel specimens were polished mechanically and chemically pre-treated as described[17].Table 1 shows the chemical analysis of the mild steel substrate used for this study and Fig.1 presents the SEM/EDS spectrum of the mild steel which further revealed its morphology.The EDS analysis established the essential elemental compositions with Fe being the major constituent.
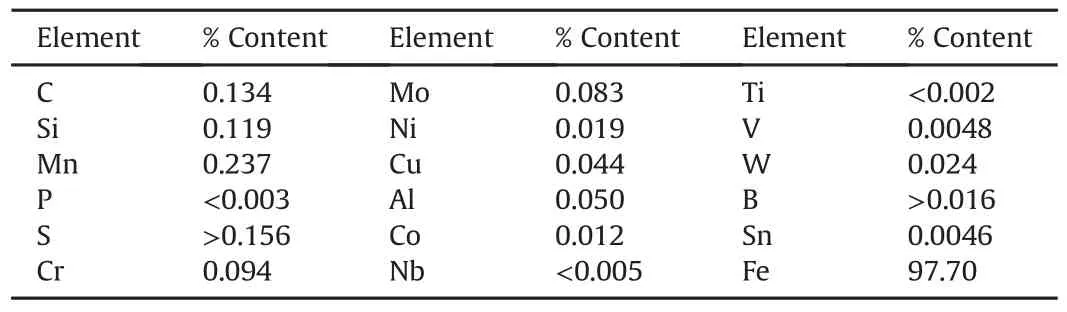
Table 1 Chemical composition of as-received mild steel[18].
The mild steel substrate prepared earlier was activated by dipping into 10%HCl solution for about 5s,thereafter rinsed in deionized water.Chemicals of analar grade and deionized water were used in preparing the coating solution at ordinary(room)temperature before coating.The bath formulation was prepared a day before the coating process and subjected to continuous stirring at 400 rpm and 70oC constant heating throughout the coating process,to obtain suspension stability(so as to prevent particles' agglomeration)and to enhance the mobility electrophoresis of the solution.The compositions of bath used for the diverse coating matrix were as presented inTable 2.KCl was included principally to increase the conductivity of the prepared electrolyte,2-Butyne 1,4 diol and Cetylpridinium Chloride were incorporated to serve as surfactants in order to reduce the surface tension of the suspension which in actual sense lowering the surface energy,so as to aid good adhesion.Thiourea was added to facilitate coating's stability(to promote particles incorporation).Table 3 further presents the concise formulation of the nanocomposites.This is in line with[18-20].
The deposition parameters were chosen in accordance with the electrocodeposition mechanism and based on the previous works[19].The arranged zinc electrodes were connected to the d.c source at different current between 1.0 and 1.5 A(i.e.,current density 560 and 830 A/m2)for constant 20 min as displayed in Fig.2.After each coating exercise,the coated samples were rinsed in water and air dried.Afterwards,parts of the coated steel were sectioned for the microstructures,electrical and optical properties of the nanocrystalline composite coatings.

Table 2 Bath composition of Zn-TiO2/WO3co-depostion[18].
Table 3 Formulation designed bath composition for Zn-TiO2/WO3nano-composites.
2.2.Characterization of the coated samples
The surface adhesion and chemical reaction of the electrocodeposits on the substrate resulted to the development of different phases.These phases formed were studied;the microstructural evaluation of the phases were carried out by optical and scanning electron microscope,and Potentiodynamics assessment were used to characterize the corrosion properties of the coated steel.
2.2.1.Structural test
The coated materials synthesized were characterized with JEOL FIELD EMMISSION JSM-7600F Scanning electron microscope coupled with EDS.
2.2.2.Corrosion test
The electrochemical corrosion measurements were carried with an Autolab potentiostat(PGSTAT30 computer controlled)furnished with the General Purpose Electrochemical Software(GPES)package version 4.9.Potentials were plotted against the logarithmic values of corrosion current.All the measurements were taken at room temperature using 3.5%NaCl solution.The solution for the study was prepared from analytical grade reagents and distilled water.An electrochemical cell consisting of the working electrode(samples)graphite rods as the counter electrodes and a silver/silver chloride reference electrode(SCE).The corrosion potential(Ecorr),corrosion current density(Icorr)and corrosion rate were evaluated afterwards.
2.2.3.Electrical and optical characterization
The electrical studies were achieved using four-point probe system with Keithley 2400 Series Source meter,interfaced by a Lab View Tracer software coupled with multimeters.Keithley 2400 Series Source meter is specifically designed for systems that demand tight connection with measuring source.It is likewise used for measuring sheet resistance and I-V characteristic.It has precision,low noise and read back power source characteristics whereas the multimeter abilities include low noise and high reproducibility.It offers quicker test times.
3.Theory/calculation
3.1.The electrocodeposition of metal oxide nanoceramics
The chosen deposition admixes nano powders used for this work are:Titanium oxide(TiO2),tungsten oxide(WO3),with average particle sizes of 40 nm and 70 nm,respectively(from Sigma-Aldrich)were used to form an electrocodeposition formulation.TiO2was chosen because of its distinctive anticorrosion properties.When a metal coated with TiO2is excited with ultra violetlight(sunlight),photogenerated electrons are released into the metal.WO3was selected for the energy storage ability.It has the ability to store photogenerated electrons during the day and gradually release it when it is dark.It can also control corrosion via barrier.All the powders are in nanoparticles because of the exceptionality of nanostructured particles.When reducing materials to nanoparticulates,they exhibited distinctive properties that are quite different from what they show in their bulk form[22,23].
Where t is the thickness of the thin film,ρin Ω·cm Then the sheet resistance is
RSinΩ.
The electrical resistivity can then be obtained by the equation
Therefore,the electrical conductivity is given as
The optical monitoring was done with the aid of a solar simulator.A solar simulator(artificial sun)is a device that gives illumination which is approximate to the natural sunlight.The purpose of using solar simulator is to provide an adjustable indoor test capability applying laboratory environments.
The solar simulator machine used for this study has optimum intensity of 1000 Watt per square metre nevertheless,the experiment was done at 750 Watt per square metre working intensity with 1.5 A.M air mass(equivalent to that of actual atmosphere).
4.Results and discussion
4.1.SEM/EDS of deposited nanocomposite
Figs.3 and 4 display the SEM/EDS of Zn-TiO2and Zn-TiO2/WO3structures nano-composites matrices coated at 830 A/m2respectively on mild steel.Considering the two Figs,it is clear that the crystallites of the electrocodeposits are uniformly distributed on the substrates.Figs.5 and 6 further revealed the spot analysis of SEM/EDS Spectrum showing TiO2-riched and WO3-riched portions with Tables 4 and 5 showing the percentage of different elements present in the EDS spot analysis on the coating respectively.It is quite obvious that with the integration of WO3nanoparticulates,a visible crystallite of the nanocomposite structure along the boundary was noticed.Moreover,there are two typical phases,one is unvaryingly alike and the second consisting of network of aggregate nodules.Evidently,the integration of the WO3in the zinc interface could be clear to display networks of structures with better nodular structures.
The plating surface and the interface of Zn-TiO2/WO3are reasonably attractive with good adhesion due to synergistic and complementary of the WO3and TiO2nano ceramics particles incorporated to strengthen the coating system.This result was as anticipated since the route of nucleation commenced from the zinc metal as load bearer,the dissemination of the particulates involves the nucleation domains and improved the formed nanocomposites[24-26].Furthermore,it is imperative to indicate that the microstructure change might be traceable to the presence of WO3nanoparticulates integrated in the nanocomposite coatings resulting to enriched precipitation and improved reinforcement.Also,with reference to[27],the induced current density together with other coating constraints in conjunction with the amount of surfactant can also play a very important contribution in modification of the coating and surface quality of an electrocodeposited material.
Table 6 and Fig.7 present the hardness trend for the coatings before and after the annealing heat treatment at 250oC.The graph is plotted in sequential trend from sample 1 to 6.The highest value of hardness achieved was Zn-TiO2/WO3coated at 1.0 A.This can be correlated with the fact that an increase in the bath loading provides more number of particles for adsorption at the cathode leading to a large number of particles getting codeposited and this has resulted in increased hardness of the composites,as stressed in adsorption theory by Achi[19].The grain filling and dispersive strengthening effects become stronger with increase in WO3particles thus the hardness of the composite coatings increased with the incorporation of WO3particles in the coating and is in accordance with the results reported by other authors[21,25].It is clear that,virtually all the coatings' hardness increased after the annealing heat treatment,which could be attributed to the homogenizing effect in the structure of the coatings system,though the little discrepancy experienced by samples 6 could be as a result of insufficient homogenizing of some localized spots in the coatings structure.
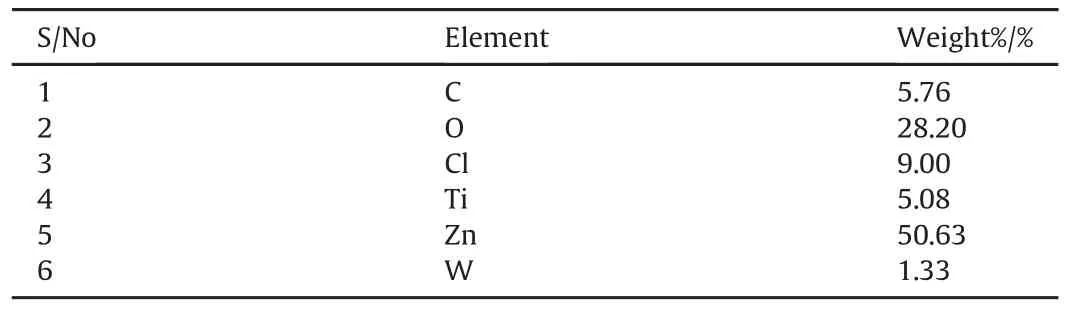
Table 4 Percentage of different elements present in the EDS spot analysis of TiO2-riched portion.

Table 5 Percentage of different elements present in the EDS spot analysis of WO3-riched portion.
4.2.Electrical characterization
Figs.8 and 9 displayed the characteristic I-R(current-resistance)plots of nano-composites coatings of Zn-TiO2and Zn-TiO2/WO3on mild steel during light and dark environments.Table 7 showed of the electrical conductivity of nano-composites coatings in line with deposition parameters.The value of conductivity was higher for Zn-TiO2/WO3sample which was 24.5 Ω-1⋅cm-1compared to the value of 4.47 Ω-1⋅cm-1for Zn-TiO2sample.The reason for this improvement might be due to better stability of photon current by the active nature of ZnTiO2/WO3coating system.The plots show the pattern in which the electric current flows through the nanocomposites coating at different resistance during light and dark environments.It was observed that the presence of light enhances the flow of current in the coatings.Both the nano-composites coating showed better performance under the light than the dark position.This could be traceable to the improved electrons that were photo generated during illumination[26-30].Likewise,it can also be observed from the Figs that the current flow under light decreases more linearly with resistance when compared to the dark situation.This could be attributed to the photo electron stability of light energy from the Solar Simulator[28].More so,a kind of sinusoidal plot was observed when it was dark condition(Fig.9)which could be attributed to the presence of a material that stores energy(WO3)in the coating matrix.The result is also in good agreement with that of Okada et al.[31]and Chong and his group[32]who revealed that composite of TiO2as an electrode for high electron injection as well as multi-layered structures in devices that are photovoltaic,offers direct electrical conduit for photogenerated electrons which increases the electron transport rate and leads to a greater efficiency.
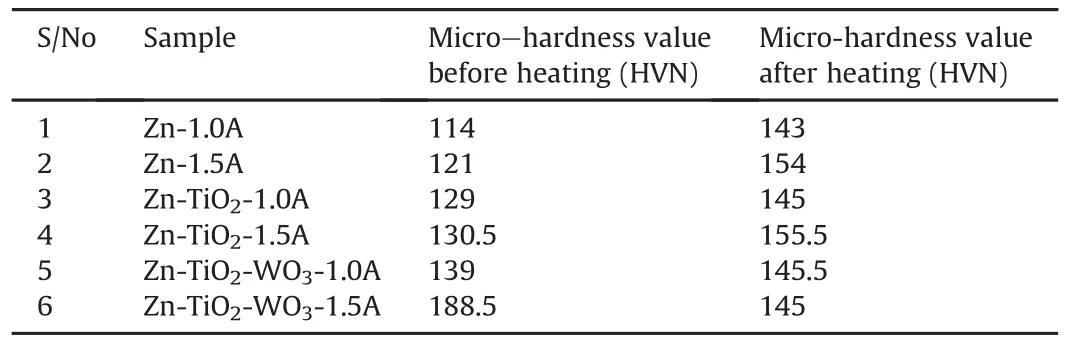
Table 6 The values of the coatings' hardness before and after annealing thermal treatment at 250oC for 5 h.
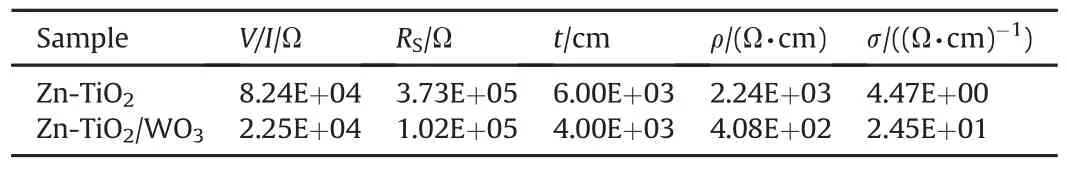
Table 7Electrical properties of the coated nano-composite.
4.3.Polarization studies
Fig.10 presents the polarization curves of the mild steel(control),Zn-TiO2and Zn-TiO2/WO3nano-composites coatings on mild steel.The accompanied summary of the results for the polarization measurements are shown in Table 8 which were obtained from Tafel plots.The values of Ecorr,Icorr,corrosion rate(CR)and polarization resistance(RP)of the samples in 1M HCl are extrapolated from Tafel slope.The results revealed the corrosion resistance behaviour of the coatings in the test solutions.It was found that the addition of the nanoparticles in the coating altered the shape of the polarization curve but causes a considerable increase in the value of the Ecorrinthetestmedia.The characteristic shapes of the polarization curve differ in the solution and there is noticeable passivity of the anodic polarization of all the samples in the acidic solution.These characteristics indicate the behaviour of each material in the medium and that,an increase in additive concentration enhances this process.Also the additions of these nanoparticulates provide better effect of corrosion resistant property.The trend of corrosion resistance of the specimens in the acid medium for the samples was Zn-TiO2/WO3-1.5A>Zn-TiO2/WO3-1.0A>Zn-TiO2-1.5A>Zn-TiO2-1.0A>Control.
Therefore,the optimal performance for the coating with least corrosion rate of 0.23677 mm/yr in the acidic solution.This implies that addition of WO3,nanoceramics loaded at 1.5 A(870 A/m2current density)during electrocodeposition provides best effect of corrosion resistant property in the acidic environment.It is worth noting that increase in current density during the electrocodeposition of the coating enhances the coating performance in the service environment,thus increase in the current density reduces the corrosion rate and increases polarization resistance.According to[33]this could be attributed to the type and effectiveness of the passive film formed by the nano composites film on the surface of the coated steel.Generally,appreciable improvement in potential was observed for all the coatings,which may primarily be due to creation of barrier by the film,between the steel surface and the corrosive environment[34].These results were in good agreement with the results obtained[34-36].
5.Conclusions
(1)TiO2and WO3nanoparticulates were used to produce Zn-TiO2/WO3nanocomposite coating from chloride bath.
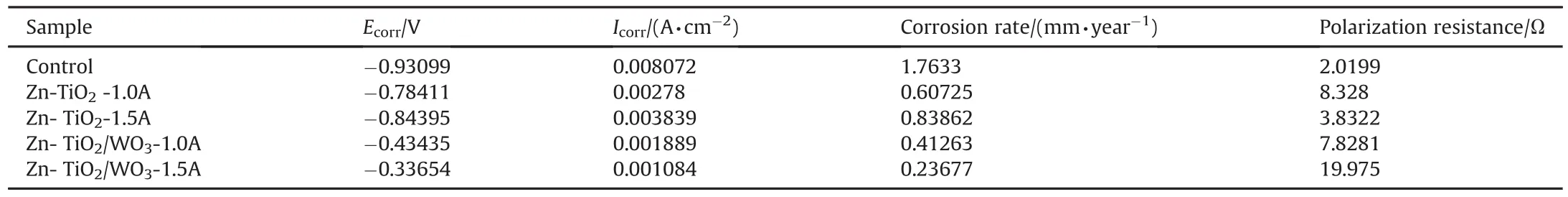
Table 8 Polarization data extrapolated from Tafel slope for matrix Zn-TiO2/WO3composite coating.
(2)The incorporation of TiO2and WO3in Zn matrix of the coating was confirmed by EDX
(3)The integration of the TiO2/WO3nanoceramics composite particles in the zinc matrix as reinforcement improved the structural morphology of nanocomposite.
Acknowledgement
The work is based on the financial support by National Research Foundation and the equipment support by Surface Engineering Research Centre,Tshwane University of Technology,Pretoria,South Africa.
杂志排行
Defence Technology的其它文章
- Overview of Al-based nanoenergetic ingredients for solid rocket propulsion
- Implications of fine water mist environment on the post-detonation processes of a PE4 explosive charge in a semi-confined blast chamber
- Systematic research on the performance of self-designed microwave plasma reactor for CVD high quality diamond
- cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)as a part of explosive mixtures
- Joining and machining of(ZrB2-SiC)and(Cf-SiC)based composites
- Influence of spark plasma sintering on microstructure and corrosion behaviour of Ti-6Al-4V alloy reinforced with micron-sized Si3N4 powder
