cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)as a part of explosive mixtures
2018-10-18SvtoplukZemnAhmedHusseinAhmedEleihMrcelJungov
Svtopluk Zemn,Ahmed K.Hussein,Ahmed Eleih,Mrcel Jungov
aInstitute of Energetic Materials,Faculty of Chemical Technology,University of Pardubice,CZ-532 10,Pardubice,Czech Republic
bMilitary Technical College,Kobry Elkobbah,Cairo,Egypt
Keywords:BCHMX Initiation Sensitivity Explosive strength Penetration
ABSTRACT cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)is a relatively available bicyclic nitramine in the present.Due to the high energetic content of its molecule(deformation of valence angles)it has heat of formation higher than β-1,3,5,7-tetranitro-1,3,5,7-tetrazocane(β-HMX)by nearly three times.As a result,it has heat of explosion and relative explosive strength exceed that ofβ-HMX including their corresponding PBXs.However,penetration abilities of PBXs based on HMX are higher than those based on BCHMX.The relatively high initiation reactivity of BCHMX could be modified in a wide range by its incorporation in a suitable polymeric matrices.Regarding to the performance,coating of BCHMX crystals by 5 wt%Viton A produces the maximum performance while the mixture of BCHMX with polydimethylsiloxane(PDMS)seems to be the optimum composition for the development of PBX with low sensitivity.
1.Introduction
In the eighties of the last century,a number of interesting nitramines specially technical attractive cyclic nitramines were synthesized,one of them is cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)[1-4].Starting from very complicated syntheses[1],and also with intention to find a new route of the 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(HNIW)preparation,a group of Prof.Sysolyatin from Biysk at Siberia has developed the technological method for preparation of BCHMX with a good yield[4-7];however,the corresponding results were reserved as a classified patent for a long time and the first short information in face of international audience was presented in 2005(see quotations in Ref.[4]).
Independently from the Russian colleagues,roughly twenty years later,the same synthesis was developed at University of Pardubice in Czechia[6,7].Then in the Institute of Energetic Materials at this University,activities were started in the research of possible applications of the BCHMX as a plastic bonded explosives(PBX)(see below).This paper deals with some interesting properties and characteristics of BCHMX and its PBXs.
2.Optimum method for BCHMX synthesis
A survey of synthesis of the interesting new nitramines is presented in Ref.[3].Also synthesis of BCHMX is mentioned in Refs.[3,4]but the details about the procedure are protected by the open patents[5,7]and are documented by Schemes 1 and 2.
In the sense of Scheme 1,the mutual reaction of glyoxal,formaldehyde and potassium sulfamate is a nucleophilic addition,catalyzed by selected environment;due to the nature of the reactants it takes place in the polar solvent,preferably in an aqueous environment[4,5,7].TACOS-K separates out as tetrahydrate[8].The fact that this salt contains crystalline water,leads to its certain instability during lengthy storage due to hydrolytic processes[8].During the heating of TACOS-K crystals,evaporation of water in the crystals tends to compete with this hydrolysis;the tetrahydrate of TACOS-K should not be produced for storage,even in normal conditions,and during drying process,there is some possibility of its exothermic decomposition[8].
Nitrolysis of TACOS-K realizes in the fuming nitric acid alone[5,7]or in presence of P2O5[7],or N2O5[5],acetic anhydride[5],sulfuric acid or oleum[7].In addition,the presence of the linear urea-formaldehyde condensate in TACOS-K was tested[7].Due to content of the 11%wt.of water in the TASCOS-K crystals,yields of BCHMX do not exceed of 70%toward theory(mostly about 60%of theory[4]).
3.Important properties of BCHMX and some explosive mixtures on its base
3.1.Properties of BCHMX
cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole
(BCHMX)was predictively considered as a more powerful explosive than β-1,3,5,7-tetranitro-1,3,5,7-tetrazocane(β-HMX)[2].What is a reality?
As a result of the C2-C4 bond creation in case of BCHMX(see Fig.1)from the skeleton of HMX(see Fig.2),the deformation of the valence angles of the original HMX molecule is changed to form BCHMX molecules in a certain internal tension[6].This tension is proved in higher energetic content of the BCHMX molecule in comparison with the HMX one(roughly three time higher heat of formation of BCHMX-see in Table 1).The mentioned skeleton deformation is visible on the angular conformation of the BCHMX molecule and relatively very long length of the N3-N7 bond while the other N-N bond lengths are comparable with those in the HMX molecule[6].The longest N-N bond is a bearer of initiation reactivity of this nitramine[6](see higher sensitivity data of BCHMX compared with HMX inTable 1).In the impact sensitivity,BCHMX is roughly in the same level of pentaerythritol tetranitrate(PETN)[9].
Due to angular conformation and lower molecular symmetry,the BCHMX molecule cannot be as perfectly placed into crystal lattice,as in the case of HMX analogue,and its maximum theoretical density is thus lower in comparison with HMX.On the other hand,the maximum heat of explosion is higher(by 4.7%)compared to HMX,and that is due to the higher heat of formation of BCHMX.This difference is transferring into the explosion heats of their corresponding PBXs(see Fig.3).
3.2.Properties of some explosives based on BCHMX
Already Qui and Xiao published a molecular dynamic study of the plastic bonded explosives(PBXs)containing BCHMX and poly fluorinated binders[12].They claim that BCHMX is suitable for special plastic bonded explosives(PBXs)or applications in propellants[12].Several of our publications presented the effect of different polymeric matrices on the performance of the BCHMX,such as polystyrene-butadiene rubber(SBR)[13],Viton A 200[8,14,16],polydimethylsiloxane(PDMS)[10,17-19],acrylonitrilebutadiene rubber(NBR)[8,15,16],polyisobutylene(PIB)[8,17],poly-(methyl methacrylate)(PMMA)[8],TNT[19],GAP[21]and HTPB[22].For comparison also PBXs with these binders were studied which were filled by 1,3,5-trinitro-1,3,5-triazinane(RDX),β-1,3,5,7-tetranitro-1,3,5,7-tetrazocane (β-HMX) and ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(ε-HNIW).Regarding to the recent studies[9,10,18,19],it was concluded that the PDMS matrix considers one from the optimumbinders to be used in the explosives production:it has a neutral effect on thermal stability of the nitramines studied and has roughly the same influence on the thermochemistry of detonation(according to the volume heat of detonation)as those obtained with fluorinated hydrocarbon elastomers,although it does not provide the same performance values(D2ρ)[10](see also Fig.5).Moreover,this matrix increases the safety of explosives against mechanical impulses.For the purpose of research in the sense of Fig.1,the PDMS oligomers were used as viscous oils(trimethylsilyl terminated products of Wacker®AK).For completeness'sake is needed to mention that also the Sylgard binder in amount of 15%wt.in PBX was used for preparation and subsequent testing of extruded charges filled by the same nitramines[23];results from the corresponding PBXs sensitivity testing are close to those presented in Ref.[10].
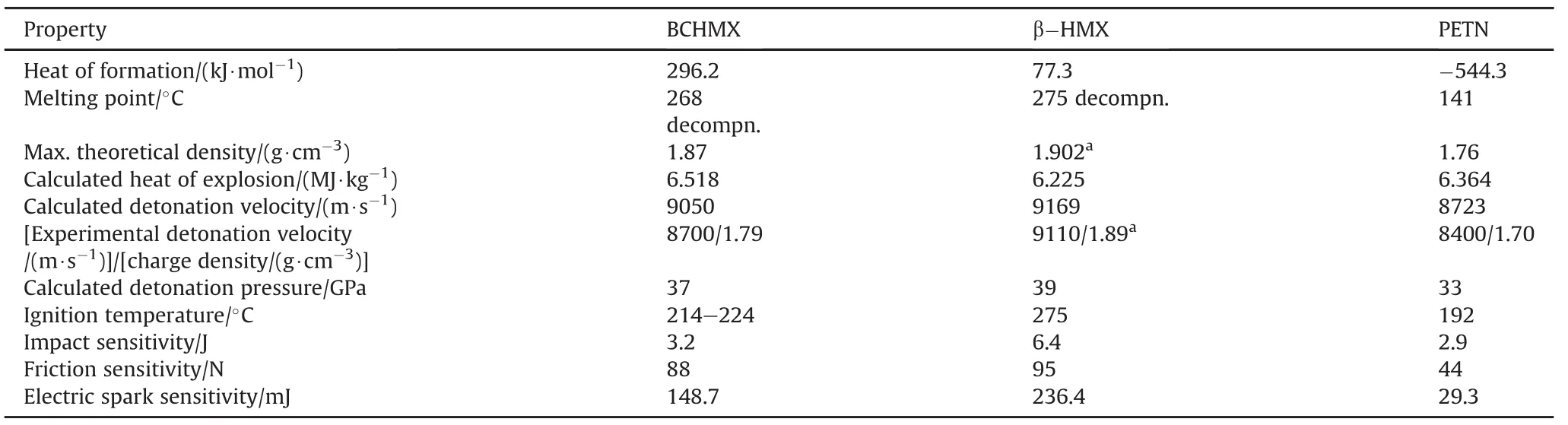
Table 1 Main characteristics of BCHMX,taken from papers[6,10];the calculated heat of explosion and detonation velocity were obtained by means of Explo5 code.
Fig.3 represents a generally well-known relationship between performance and sensitivity of energetic materials[25].It is obvious that the relative explosive strength of PBXs with content of 12%wt.of PDMS(suffix-Si)in the most cases are surpassing those mixtures based on 9%wt.of PIB(-C4),9%wt.of Viton A(-V9)and 15%wt.of acrylonitrile-butadiene caoutchouc NBR(-sem).Certain exceptions are PBXs filled by HNIW;in the case of the polyfluorinated bindersacertain pressure and temperature in detonation of nitramine PBXs should exist which induces a shift in fluorine chemistry of the poly fluorinated binders during detonation process[26].This change of detonation chemistry is apparent in case of HNIW-V9 mixture(see position of its data in Fig.3 and finding of paper[27]).This statement about the shifting mechanism might be valid also for the HNIW-C4 and other PBXs with HNIW[27].The HNIW mixtures distinguish can be connected with its brisance,which is higher than the other nitramine fillers studied in this research.
The PDMS binder,however,is not able to decrease the sensitivity of BCHMX to the level that data of BCHMX-Si explosive correlate with straight line C in Fig.3.Nevertheless,this PBX has higher explosive strength and resistance to impact in comparison with the well-known Czech explosive Semtex 10(84-86%wt.of PETN bonded by softened NBR)or classical RDX-C4.In case of 50 wt%of BCHMX in the mentioned PBX is substituted by 3-nitro-1,2,4-triazol-5-one(NTO),a low sensitive explosive for Low Vulnerable Ammunition(LOVA)might be obtained[18].It was marked as BCHMX/NTO/Si which has obvious decrease in the explosive strengths(see in Fig.3);it is logic result due to the existence of a reciprocal proportion of the explosive strength(i.e.performance)and sensitivity[25](which Fig.3 clearly documents).Decreasing of the BCHMX/NTO/Si performance is due to the relatively low heat of explosion of NTO[18]where NTO acts as a “cooler”in this case.Analogical substitution of BCHMX by 1,1-diamino-2,2-dinitroethene(FOX-7)does not have as thus expressive “cooling”effect[19]which is illustrated by position of the BCHMX/FOX-7/Si data in Fig.3(these data do not achieve straight line C);admixture of FOX-7 also somewhat decreases density of corresponding PBX in comparison with density of BCHMX-Si[19].
Another different study including the penetration ability of the BCHMX PBX is presented in Fig.4.It shows that the penetration ability represented bythe holes produced in the aluminum cylinder by the shaped charges with Cu liner[12].The explosive fillers of the charges were bonded by 13%wt.of the softened SBR(PBXs of the Formex type)[13].It was proved that the order in the penetration ability corresponds to detonation velocity of the PBXs.Similar experiment was recently realized with the same nitramines PBXs,but bonded by 5%wt.of Viton A[28]and in terms of the penetration depth into laminated rolled homogenous armor target.Autodyn numerical hydrocode was implemented to determine the shaped charge jet's characteristics and its penetration depth[28].Results from this research correspond to those PBXs,presented on Fig.4,are more detailed explained[28](the BCHMX-V5 explosive exhibited 12.5%increase in the penetration-ability compared with RDX-V5 analogue while HMX-V5 produced 22.4%increase[28]).
Actually it is interesting to compare the different PBXs based on BCHMX and bonded by different binders as presented in Fig.5.It is clear from this figure that the positions of the individual data are not based only on the thermochemical aspects(kind and amount of binder and kind of explosive)but also the physical properties of nitramine filler.It has influence on both the performance and sensitivity of the final PBX.Regarding to the different methods used for samples preparation(extrusion and pressing methods),the mutual comparison of their explosive properties might be possible.
Positions of data for PBXs with poly fluorinated elastomers in Fig.5 are interesting:both the PBXs,BCHNMX-V5 and BCHMX-V9 have the same loading densities(1.81g/cm3[12])but different detonation velocity values[10],BCHMX with 5 wt%binder has slightly higher value of the ρD2product toward the pure BCHMX(134.24 against 133.97GPa);this fact could be similar to the known effect of adding nonexplosive liquids to solid explosives[30].BCHMX-Fluorel is outside of the correlation due to lower loading density in comparison with the previous PBXs(1.79 g/cm3[10]);this highly poly fluorinated fluorel polymerdoes not match with the PBX binding(PBX with bad pressing process).In the case of BCHMX/NTO/Si explosive,it is possible to see the “cooling”effect of NTO(this PBX has a higher resistance against impact).
From the mutual comparison of Figs.3 and 5,it is possible to see certain mutual difference in the PBXs explosive strengths(performances).This is given by the used conception of this performance and/or its experimental specification in each of these individual cases.As well as in the case of Fig.5 and partially also of Fig.3,it is possible to see decreasing in performance of the derived PBXs from original BCHMX-Si due to substitution of 50%wt.of BCHMX by other explosive;however,this modification by insensitive explosives can lead to the LOVA PBXs(here BCHMX/NTO/Si and BCHMX/FOX-7/Si)with higher performance towards modification by RDXor HMX[18,24].In term of the kind of the polydimethylsiloxane in relation with the performance,it seems that the trimethylsilyl terminated polymer has slightly more advantages than Sylgard(see Fig.5)but the second one can give elastic or solid PBXs.From the point of view of performance,there is no practical difference between the BCHMX-GAP,BCHMX-TNT and BCHMX-C4 explosives while more difference was observed in their impact sensitivity(see Fig.5).On the basis of relationships in Figs.3 and 5,it is possible to stated that the most effective flegamtization of BCHMX is 5%wt.of Viton A 200 and that mixture BCHMX-Si is a good PBX not only for independent application but also for development of other low sensitive PBXs.
4.Conclusion
From the eighties of the last century, cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole (BCHMX) has become available as bicyclic nitramine.Due to the deformation of valence angles in its molecule,a certain tension exists and BCHMX has thus roughly three times higher heat of formation in comparison with β-1,3,5,7-tetranitro-1,3,5,7-tetrazocane(β-HMX).From this fact,higher relative explosive strength of BCHMX thanβ-HMX was concluded while the penetration ability of HMX was higher than BCHMX.To the mentioned higher energetic content,and the molecular skeleton deformation,a higher initiation reactivity of BCHMX was observed and it could be modified in a wide range by its incorporation into suitable polymeric matrix.This incorporation affect totally the performance and impact sensitivity of the final plastic bonded explosives(PBXs).In order to maximize the performance,the best BCHMX modification is its phlegmatization by 5% wt.of Viton A 200.Mixture of BCHMX with polydimethylsiloxane(PDMS)is the optimum due to the high efficiency of PDMS in decreasing the sensitivity of BCHMX in comparison with the other binders.
Acknowledgement
The work described in this paper received financial support from the Students Grant Projects No.SGSFCHT_2016002 of the Faculty of Chemical Technology at the University of Pretoria.
杂志排行
Defence Technology的其它文章
- Overview of Al-based nanoenergetic ingredients for solid rocket propulsion
- Implications of fine water mist environment on the post-detonation processes of a PE4 explosive charge in a semi-confined blast chamber
- Systematic research on the performance of self-designed microwave plasma reactor for CVD high quality diamond
- Joining and machining of(ZrB2-SiC)and(Cf-SiC)based composites
- Structural evolution,optoelectrical and corrosion properties of electrodeposited WO3integration on Zn-TiO2electrolyte for defence super application
- Influence of spark plasma sintering on microstructure and corrosion behaviour of Ti-6Al-4V alloy reinforced with micron-sized Si3N4 powder
