CoS2Yolk-Shell Spheres Coated with Carbon Thin Layers as High Active and Stable Electrocatalysts for Hydrogen Evolution Reaction
2018-10-11,,
, ,
MOE Laboratory of Bioinorganic and Synthetic Chemistry, The Key Lab of Low-Carbon Chemistry & Energy Conservation of Guangdong Province, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, P.R. China
(Received 1 June 2018; revised 4 July 2018; accepted 12 July 2018)
Abstract:Though water electrolysis is effective in generating high-quality hydrogen gas, it requires effective electrocatalysts for hydrogen evolution reaction (HER). CoS2have been considered as a promising HER electrocatalyst because of its high ctalytic activity. However, the key limitation for CoS2nanomaterial as HER electrocatalyst is its poor stability, which may be due to the structural breakdown of CoS2nanostructure or the evolution of S during H2evolution in acid media. Coating porous carbon thin layer for protection from structural breakdown and evolution of S is a good way to improve catalytic stability. In addition, coating carbon layer can change electronic structure of CoS2for the moderated hydrogen adsorption energy, leading to enhanced catalytic activity. Here, CoS2yolk-shell spheres coated with carbon thin layers exhibit superior catalytic performance for HER with low overpotential, small Tafel slope, and excellent stability.
Key words:CoS2@C; yolk-shell sphere; electrocatalyst; hydrogen evolution reaction (HER); high stability
0 Introduction
Hydrogen as a sustainable, secure, clean and alternative energy source can ease the energy crisis and environment pollution faced in the present world[1-2]. Electrochemical water splitting is considered as a highly effective method to produce hydrogen[3]. However, it needs electrocatalysts to reduce overpotential due to low kinetics of water splitting. Pt is at present the most active catalyst for the hydrogen evolution reaction (HER)[4-5]. However, its scarcity on the earth and high cost greatly restrict the industrial production of hydrogen. Therefore, it is necessary and urgent to find cost-effective and earth-abundant catalysts with high HER catalytic activity and excellent stability to facilitate translation of H2O to hydrogen.
Transition metal dichalcogenides with generalized formula of MX2(M refers to transition metal; X represents a chalcogen such as S and Se) have received increasing interests due to their low cost and high abundance[6-10]. Among them, MoS2has been considered as a promising candidate because of its high activity and stability[11-14]. However, the experimental and computational studies have concluded that the catalytic activity mainly arises from the active sites located along the edges of 2-D MoS2layers which are under-coordinated and thermodynamically unfavorable, and the basal planes are catalytically inert[15-17]. Although various methods have been adapted to expose more edge sites or enhance the intrinsic activity of the edge sites, the enhancement of HER electrocatalytic performance of MoS2still faces enormous challenges[18-20]. Another transition metal dichalcogenides CoS2, which was often utilized as electrode materials for supercapactiors, Li-ion batteries or electrocatalysts for oxygen reduction reaction (ORR)[21-26], also has shown excellent HER electrocatalytic activity, even higher than MoS2. In addition, CoS2, which is an intriscally conductive metal in contrast to the other MX2such as FeS2[27]and NiS2[28], can improve the electron transportation from catalyst surface to the electrode, thus will reduce the overpotential needed to overcome the energy barriers and decrease the energy consumption. What’s more, CoS2can exhibit high HER activity after conversion from thermodynamically favored semiconducting phase to a metastable metallic polymorph compared wih other transition metal dichalcogenideis, such as MoS2and WS2[29-32]. However, the key limitation for CoS2as HER electrocatalyst is poor stability in acid media, which may be due to the structural breakdown of CoS2nanostructure or the evolution of S during H2evolution[24,33-37 ]. The poor stability of CoS2materials has seriously restricted their practical applications as high-performance HER electrocatalysts. Therefore, it is significant to develop CoS2-based electrocatalysts with excellent stability as well as high catalytic activity.
To improve the electrocatalytic activity and stability of CoS2for HER, carbon thin layer coating will be a promising method because of following advantages: (1) the carbon thin layer can well prevent CoS2from structural breakdown and the evolution of S; (2) the electronic interaction between CoS2and thin carbon layer will change the electronic structure of CoS2, which will be beneficial for the improvement of electrocatalytic activity; (3) The carbon layer will provide “super-highways” for electron transfer to promote HER due to its high electrical conductivity. Based on the above considerations, we devote our attention to designing and synthesizing the novel CoS2yolk-shell (YS) spheres coated with carbon thin layers (CoS2YS@C spheres) as highly efficient HER electrocatalysts by a simple hydrothermal method. The CoS2YS@C spheres own hollow structure and high specific surface area, and they exhibit excellent catalytic activity with low onset potential of only about 20 mV, small Tafel slope of about 55 mV/dec, and small overpotential of about 90 mV at 10 mA/cm2in acidic solution. Especially, CoS2YS@C spheres also exhibit excellent stability at 10 mA/cm2for 10 h. This work provides a new revenue for the development of CoS2-based electrocatalysts with high catalytic activity and excellent stability for HER.
1 Results and Discussion


Fig.1 Schematic of fabrication of CoS2YS@C spheres

Fig.2 SEM image of CoS2YS spheres, SEM image of broken CoS2YS spheres, SEM and TEM images of CoS2YS@C spheres, HRTEM image of Area① in Fig.2(d) of CoS2YS@C sphere, FFT pattern measured in Area① in Fig.2(d), and FFT pattern measured in Area② in Fig.2(d)
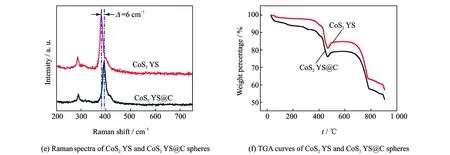
Fig.3 XRD pattern of CoS2YS@C spheres, XPS spectra of Co 2p region of CoS2YS and CoS2YS@C spheres, XPS spectra of S 2p region of CoS2YS and CoS2YS@C spheres, XPS spectra of C 1s region of CoS2YS@C spheres, Raman spectra of CoS2YS and CoS2YS@C spheres, and TGA curves of CoS2YS and CoS2YS@C spheres
The electrocatalytic activities of CoS2YS@C spheres with different carbon layer thickness are studied by linear sweep voltammetry (LSV) in 0.5 M H2SO4solution at 2 mV/s. When the carbon layer thickness is 10 nm, the electrocatalytic activity of CoS2YS@C spheres reaches the highest level (the carbon layer thickness of CoS2YS@C spheres was kept to be 10 nm in all the following experiments). The electrocatalytic activities of CoS2YS@C spheres, CoS2YS spheres and carbon with the same loadings (1.02 mg/cm2) were compared and their polarization curves are shown in Fig.4(a). Obviously, the HER catalytic activity of CoS2YS@C is much better than that of CoS2YS spheres and the carbon almost has no catalytic activity. The onset potential of CoS2YS@C is about 20 mV, which is much lower than those of CoS2YS spheres (85 mV), as shown in Fig.4(b). The overpotential of CoS2YS@C at 10 mA/cm2is 89.3 mV, which is much smaller than 184.2 mV of CoS2YS spheres. In addition, the current density of CoS2YS@C at a given potential is much higher than that of CoS2YS spheres, as shown in Fig.4(b). For instance, when the overpotential is 200 mV, the current density of CoS2YS@C spheres is about 81.23 mA/cm2, which is about 6.5 times higher than that of CoS2YS speheres, as shown in Fig.4(c), suggesting the important role of carbon thin layer for the enhancement of electrocatalytic activity of CoS2YS@C speheres.
The linear portions of Tafel plots were fit to Tafel equation (η=a+blogj, wherejis current density,bis Tafel slope), yielding Tafel slope of about 55 mV/dec for CoS2YS@C spheres (Fig.4(d)), which is much lower than that of CoS2(77 mV/dec). The Tafel slope of 55 mV/dec for CoS2YS@C indicates Volmer reaction has been taken place[41-42], and the process to convert the protons into absorbed hydrogen atoms on CoS2YS@C surfaces becomes rate-determining step during HER. The exchange current density (j0) of the catalyst can be calculated by extrapolating the Tafel plot. As expected,j0of CoS2YS@C spheres is 0.265 mA/cm2(Fig.5(a)), which is much larger than that of CoS2(0.062 mA/cm2). Therefore, the CoS2YS@C spheres exhibit outstanding HER activity with low onset potential, high current density, low Tafel slope and high exchange current density, which are superior to most of the CoS2-based electrocatalysts that have been ever reported in the acidic electrolyte.
In order to further provide the insight to CoS2YS@C sphere electrocatalysts, the electrochemical active surface area (ECSA) and electrochemical impedance spectroscopy (EIS) measurements were performed. Though it is difficult to obtain the accurate value of ECSA owing to the unclear capacitive behavior, it can be visualized by double layer capacitance (Cdl), which is proportional to the electrochemical surface area. The calculation ofCdlby cycle voltammograms (CVs) in 0.5 M H2SO4is used to make comparison of ECSA. TheCdlof CoS2YS@C spheres is caculated to be 13.13 mF/cm2(Figs.5(b) and (c)), which is much larger than that of CoS2YS spheres (6.56 mF/cm2), indicating that CoS2YS@C spheres own much larger ECSA than CoS2YS spheres. Nyquist plots of CoS2and CoS2YS@C spheres are shown in Fig.5(d). The semicircle in the high frequency region is attributed to the charge transfer resistance (Rct), which is related to the electrocatalytic kinetics, and a low value of semicircle is consistent with a fast reaction rate[43]. From Fig.5(d), it is clearly seen that CoS2YS@C spheres have much lowerRctthan CoS2YS spheres, indicating much faster reaction rate for CoS2YS@C spheres.
HER electrocatalytic activity of CoS2YS@C spheres is significantly better than those of CoS2and other CoS2-based electrocatalysts reported in the litertatures. The enhancement of the catalytic activity of CoS2YS@C spheres can be ascribed to the rapid charge transfer based on analyses of EIS results and the large ECSA with more exposed active sites. Actually the better catalytic activity of CoS2YS@C spheres as electrocatalysts for HER is also due to the natural properties of CoS2YS@C spheres. As we all know, HER activity is strongly correlated with the chemisorption energy of atomic hydrogen to the electrocatalyst surface, and the hydrogen binding energy for an excellent HER electrocatalyst should be neither too high nor too low[44-45]. The positive hydrogen binding energy on CoS2indicates a weak adsorption of H on CoS2surface[45], which will be unfavourable to the reduction of H+(i.e., Volmer step). Thus, an optimization of the electronic features is desired. It is notable that the surrounding elements have an important effect on the electron density around Co active sites. As we all know, carbon has a lower electronegativity compared with S, so the electron density around Co will increase by embedding carbon onto the surface of CoS2. This phenomenon has been well demonstrated by XPS and Raman results. XPS binding energy of Co 2p of CoS2YS@C spheres obviously decreases compared with that of CoS2, as shown in Fig.3(b), indicating that the valence of Co in CoS2YS@C shperes is below +2 and the electronic density of Co will increase. In addition,EgandAgpeaks of CoS2YS@C spheres shifting to low Raman shift compared with that of CoS2YS spheres also confirms the strong electronic interactions between CoS2and carbon thin layer, as shown in Fig.3(e). Therefore, the increase of electronic density of Co will consequently enhance the strength of hydrogen binding energy to promoteHadsadsorption and thus will improve HER catalytic activity of CoS2YS@C spheres.
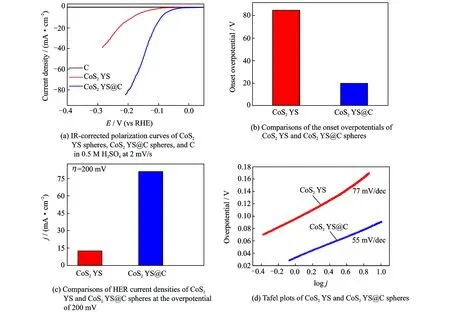
Fig.4 IR-corrected polarization curves of CoS2YS spheres, CoS2YS@C spheres, and C in 0.5 M H2SO4at 2 mV/s; comparisons of the onset overpotentials of CoS2YS and CoS2YS@C spheres; comparisons of HER current densities of CoS2YS and CoS2YS@C spheres at the overpotential of 200 mV; and Tafel plots of CoS2YS and CoS2YS@C spheres
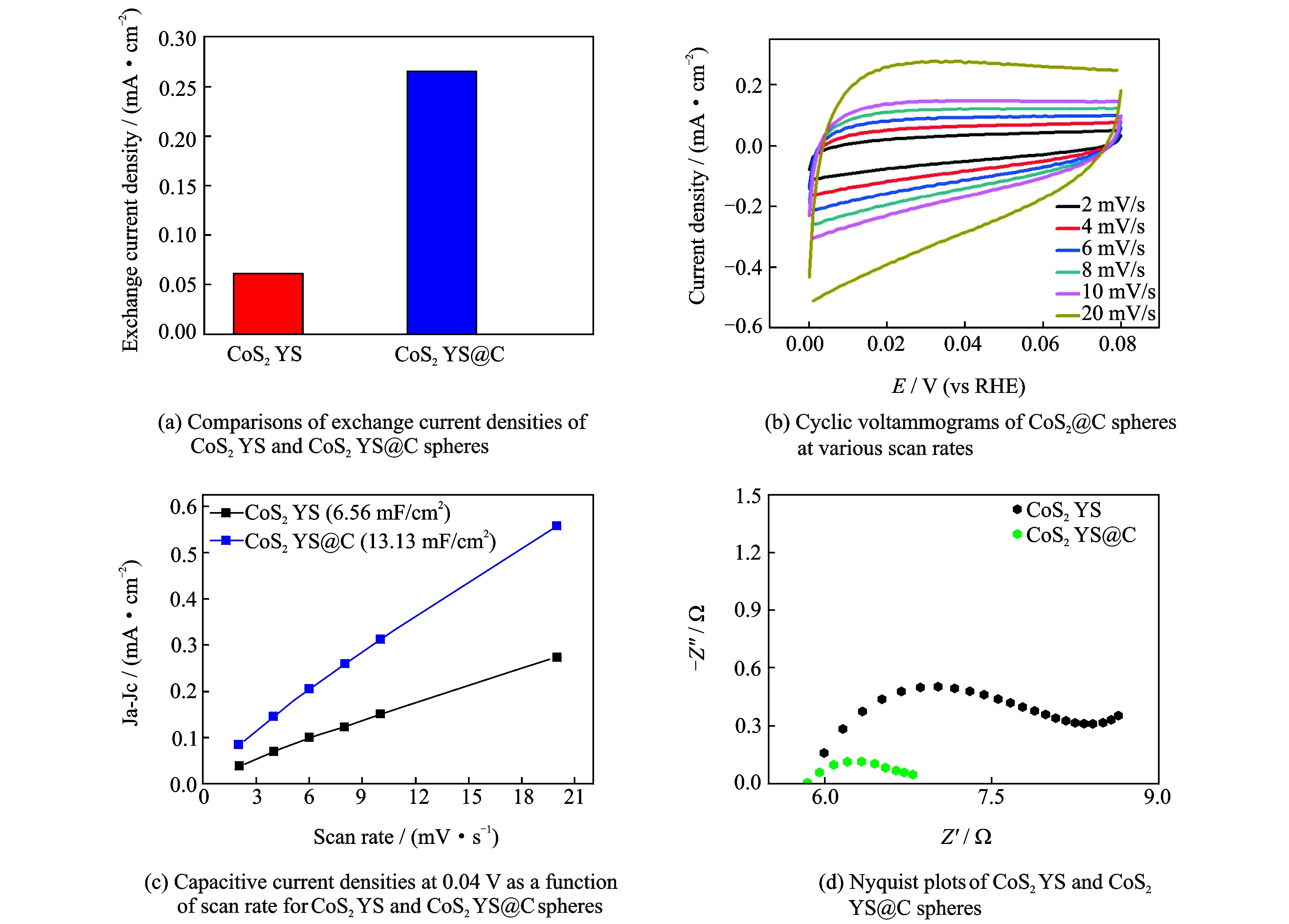
Fig.5 Comparisons of exchange current densities of CoS2YS and CoS2YS@C spheres, cyclic voltammograms of CoS2@C spheres at various scan rates, capacitive current densities at 0.04 V as a function of scan rate for CoS2YS and CoS2YS@C spheres, and nyquist plots of CoS2YS and CoS2YS@C spheres
Besides the HER electrocatalytic activity, the stability is also one important criterion in evaluating the performance of electrocatalyst. The long-term HER electrocatalytic stabilities of CoS2YS spheres and CoS2YS@C spheres were tested through continuous electrolysis at 10 mA/cm2in 0.5 M H2SO4for 10 h. As shown in Fig.6(a), it is clearly seen that the CoS2YS@C spheres exhibit high durability with slight overpotential increase of about 32 mV at 10 mA/cm2after 10 h, whereas CoS2YS spheres exhibit very poor durability with obvious overpotential increase of about 517 mV after 8 h. To further gain insight into the stability of electrocatalysts, CoS2YS spheres and CoS2YS@C spheres after stability tests were further studied by SEM and XPS. It is observed that the surface morphology of CoS2YS@C still remains very well after stability tests, as shown in Fig.6(b). However, for CoS2YS spheres, their surface morphology is seriously damaged after stability tests. So here the carbon thin layer plays a very important role for the protection from structural breakdown of CoS2YS@C spheres, as illustrated in Fig.6(e). XPS characterization was also performed on CoS2and CoS2YS@C spheres after stability tests. It is clearly seen that the XPS peaks of Co 2p of CoS2YS@C spheres almost remain unchangeable compared with those of CoS2YS@C spheres before stablity tests, suggesting high chemical stability of CoS2YS@C spheres. However, for CoS2YS spheres, besides the peaks at 778.8 and 794.0 eV of Co 2p, two large new peaks appear at 783.1 and 799.9 eV, as shown in Fig.6(c), which can be attributed to other forms of Co because of the oxidation of CoS2. In addition, for CoS2YS spheres, a large peak appears at 169.2 eV in S 2p region, as shown in Fig.6(d), indicating the evolution of S from CoS2. However, for CoS2YS@C spheres, the oxidation of CoS2or the evolution of S are not observed as shown in Figs.6(c) and (d). There fore, after coating carbon thin layers, CoS2YS@C spheres can efficiently prevent from the structural breakdown, CoS2oxidation or S evolution, which all are beneficial for the improvements of catalytic activity and stability.

Fig.6 Stability tests of CoS2YS and CoS2YS@C spheres at 10 mA/cm2, SEM image of CoS2YS@C spheres after HER test for 10 h, XPS spectra of CoS2YS and CoS2YS@C spheres in Co 2p region after HER tests for 10 h, XPS spectra of CoS2YS and CoS2YS@C spheres in S 2p region after HER test for 10 h, and schematic of the advantage of CoS2YS@C spheres for long-term HER
2 Conclusions
CoS2YS spheres coated with carbon thin layers (CoS2YS@C spheres) were designed and fabricated as high-performance electrocatalysts for HER in acid media. The unique YS structure provides large space for the reactant and resultant, which is beneficial for the improvement of utilization of active sites. The carbon thin layer coating can efficently change the electronic structure of CoS2for the appropriate hydrogen adsorption energy to promote the Volmer step of HER, and it also can protect CoS2from the structural breakdown and can prevent the evolution of S from CoS2for the improvement of elelctrocatalytic activity and stability. Because of the above advantages, CoS2YS@C spheres exhibit superior catalytic activity with low onset potential of about 20 mV, small Tafel slope of about 55 mV/dec, and small overpotential of about 90 mV at 10 mA/cm2. Especially, CoS2YS@C spheres also exhibit high durability with overpotential increase of only about 8% at 10 mA/cm2for 10 h. This work provides a new avenue for the design of high-performance electrocatalysts that are unstable during the catalysis process.
Acknowledgements
This work was supported by the National Basic Research Program of China (Nos.2015CB932304, 2016YFA-0202603), the Natural Science Foundation of China (No.91645104), the Natural Science Foundation of Guangdong Province (Nos.S2013020012833, 2016A010104004), and the Fundamental Research Fund for the Central Universities (No.16lgjc67).
杂志排行
Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- Adaptive Energy Efficient Power Allocation Scheme for DAS with Multiple Receive Antennas
- Parametric Modeling of Circuit Model for AC Glow Discharge in Air
- Solid-State Electrolytes for Lithium-Sulfur Batteries
- Facile Fabrication of Hierarchical Porous N/O Functionalized Carbon Derived from Blighted Grains Towards Electrochemical Capacitors
- Solar Cells Based on All-Inorganic Halide Perovskites: Progress and Prospects
- Modeling and Optimal Design of Planar Linkage Mechanism of Coupled Joint Clearances for Manufacturing
