山梨酸钾/氯化钾复合相变材料制备及热物性分析
2018-10-10章学来王迎辉刘俊名李玉洋韩兴超徐笑锋周孙希
章学来,王迎辉,纪 珺,刘俊名,李玉洋, 韩兴超,徐笑锋,周孙希,刘 璐,刘 升
山梨酸钾/氯化钾复合相变材料制备及热物性分析
章学来1,王迎辉1,纪 珺1,刘俊名2,李玉洋1, 韩兴超1,徐笑锋1,周孙希1,刘 璐1,刘 升3
(1. 上海海事大学蓄冷技术研究所,上海 201306;2. 中建安装工程有限公司,南京 210000 3. 北京市农林科学院蔬菜研究中心,北京 100097)
为开发出高效、性能稳定的微冻保鲜用相变蓄冷剂,保证运输中肉制品等农产品的品质,以山梨酸钾水溶液为主基液,探究添加氯化钾后相变蓄冷材料的热物性及热循环稳定性。对比30 g/L山梨酸钾溶液,当再添加氯化钾浓度为5 g/L时,相变温度为-2.8 ℃,潜热为254.6 J/g,热导率为1.012 W/(m·K),热扩散率为1.01 mm2/s。添加氯化钾对相变温度和潜热几乎没有影响;溶液的热导率提高了23.32 %;热扩散率提高了151.37%。经200次冻融循环后,未添加氯化钾的相变溶液出现了-5.3 ℃的过冷度,含氯化钾的相变溶液没有过冷度,氯化钾在山梨酸钾相变过程中体现出了其良好的成核作用,使得该复合相变蓄冷剂具有优异的热稳定性。试验表明,山梨酸钾/氯化钾复合相变蓄冷剂适合于农产品的微冻保鲜用。
冷藏;相变材料;热力学性质;稳定性
0 引 言
农产品是食品冷链物流中的主要销售对象,由于产品本身的特性,极易出现损害和腐烂,造成严重的损失[1]。随着生活水平的提高,人们对于肉制品、果蔬等微冻保鲜的质量需求增加,为迎合社会发展趋势,在农产品最先一公里和最后一公里中开发出更高效的冷发电和储存技术尤为关键。相变材料在吸放热时的相变潜热可以对能量进行高效地储存,这使得其在食品冷藏保鲜[2-5]、温室应用[6-8]、疫苗低温储运[9-11]等系统中得到广泛的研究与应用。相变材料作为储能技术的重中之重,国内外学者对此展开了大量的研究,集中于有机复合[12-15]、无机复合[16-17]、有机–无机复合相变材料的研究[18-19]等。一些盐溶液由于其较大的潜热以及合适的相变温度,被广泛应用为蓄冷材料[20-21],但由于部分材料过冷度较大且易于相分离、热稳定性差,成为盐溶液作为理想相变蓄冷材料的主要棘手问题[22]。
有机盐溶液[23-25]克服了有机物的低热导率和较小潜热、无机材料过冷度大和相分离的缺点。纪珺等[26]通过试验测试知质量分数29.4 %的甲酸钠水溶液相变温度为-16℃,潜热250.4 J/g,热导率为0.938 W/m·K,远高于有机酸类相变材料,且经过多次循环试验后确定有机盐的熔融和结晶性能良好。应铁进等[27]通过调节甘氨酸浓度,得到相变温度及潜热可调的系列有机物水溶液相变蓄冷剂,且无明显过冷和相分离现象。
有机盐山梨酸钾(C5H7COOK)是一种广泛而有效的防腐剂[28],易溶于水,密封状态下稳定且不易分解,有很强的抑制腐败菌和霉菌作用,且价格低廉易得。但是作为相变蓄冷材料,C5H7COOK却少有涉及。氯化钾(KCl)作为常用的低温蓄冷材料之一,作为相变温度调节剂的使用研究居多[29-31]。本文将以目前食品冷链物流研究领域有限的介于水温冷藏区的-2~-3℃为目标温度,采用有机物和无机物复合,以C5H7COOK、KCl和H2O构成的三元复合物作为复合相变蓄冷剂,通过调整配比,研制一种相变温度(Onset温度)在-2~-3℃的蓄冷剂,并通过一系列试验探究该复合相变材料的热物性及热稳定性,旨在为农产品的水温冷藏储运技术的深入研究和应用提供参考。
1 材料和方法
1.1 试剂与仪器
C5H7COOK、KCl,均为AR分析纯;山梨酸钾是国际粮农组织和卫生组织推荐的高效安全的防腐保鲜剂,广泛应用于食品、饮料、农药等行业,有效地解决了蓄冷剂在长期使用下存在的细菌滋生问题。
试验仪器主要有:电子分析天平(精度0.1 mg);安捷伦温度时间采集仪;T型热电偶(精度± 0.01 ℃);数显磁力恒温搅拌器;差示扫描量热仪DSC200 F3(温度精度0.1 ℃,热焓精度0.1 %,量热灵敏度0.1W);热常数分析仪Hot Disk TPS 2500S型(误差2%);热成像仪FLIR(热灵敏度< 0.05℃,测温准确度1.0 ℃);低温恒温槽;高低温交变试验箱YSGJW-100C型。
1.2 试验方法
1.2.1 C5H7COOK的浓度优选
本文选择C5H7COOK水溶液作为主储能剂,利用高精度电子天平称取不同质量的山梨酸钾,配制50 mL C5H7COOK浓度分别为10、20、30、40、50、60 g/L的水溶液,并将其分别放在磁力搅拌器上加热至30℃,并用磁性粒子搅拌30 min使其混合均匀。通过C5H7COOK溶液的步冷曲线确定试验蓄冷工况,通过DSC曲线图确定满足水温冷藏区温度要求的浓度。
1)步冷试验:根据试验要求,搭建的试验平台如图1所示,量取50 mL充分混合的复合相变材料置于100 mL烧杯中放入-30℃的低温恒温槽中,将T型热电偶插入烧杯后做好密封处理。按3 s的时间间隔记录样品温度变化,并根据温度与时间关系,绘制步冷曲线。

图1 步冷试验装置图
2)DSC测试:称量5~10 mg样品放置在铝制坩埚中心部分,压机密封后放入仪器。为了消除试样的热历史,对样品在-30℃(温度下限)与20℃(温度上限)之间以20 ℃/min的速率降升温2次即可。在温度降至-30℃时恒温3 min确保材料完全凝固后,以5 ℃/min的速率升温至20℃。在得到的DSC融解曲线中,吸热峰的最大斜率与基线相交得到的温度即为相变温度(Onset温度),虽然Onset温度不是起始融解温度,但是Onset温度之后才开始大量吸热,所以考察Onset温度更符合实际意义[32]。
1.2.2 C5H7COOK/KCl制备与性能测试
向30 g/L C5H7COOK溶液中分别添加不同质量的KCl,制备的KCl浓度分别为3、5、7、10和20 g/L的复合相变蓄冷剂,使用磁力恒温搅拌器将其混合均匀。通过DSC试验结果得到满足条件的C5H7COOK/KCl最佳配比,通过测量其热导率、蓄冷特性以及热循环稳定性分析C5H7COOK/KCl复合相变材料热性能。
热物性测试:采用热常数分析仪测量材料的热导率、热扩散率、比热。仪器预热30 min,为防止空气对流影响试验结果,测量过程中将热导率测量探头C5465插入复合相变材料中并放置在密闭箱中,环境温度维持在20 ℃。
热循环稳定性测试:为了准确模拟夏季蓄冷材料的使用工况,用烧杯量取30 mL复合相变材料,密封处理后放入温度段设置为-20~40 ℃的高低温交变试验箱内,循环周期为90 min。完成200次冻融循环后,对比溶液循环前后的热性能变化。
2 结果与分析
2.1 C5H7COOK的浓度优选
2.1.1 不同蓄冷工况下过冷度研究
过冷度作为相变材料的重要特性,蓄冷工况对相变材料的过冷度以及蓄冷时间有很大的影响。C5H7COOK水溶液步冷试验结果如图2所示,在-10℃的蓄冷环境下,浓度分别为10、20、30、40、50、60 g/L的C5H7COOK水溶液均存在着较大的过冷度,分别为7.754、7.333、6.23、4.411、6.276、6.067 ℃。在-20℃的蓄冷环境下,只有浓度为10 g/L的C5H7COOK溶液存在1.427 ℃的过冷,其余浓度的过冷度已经消除,且相变蓄冷时间明显缩短。在-30℃的蓄冷环境下,不同浓度的C5H7COOK溶液均无过冷现象。
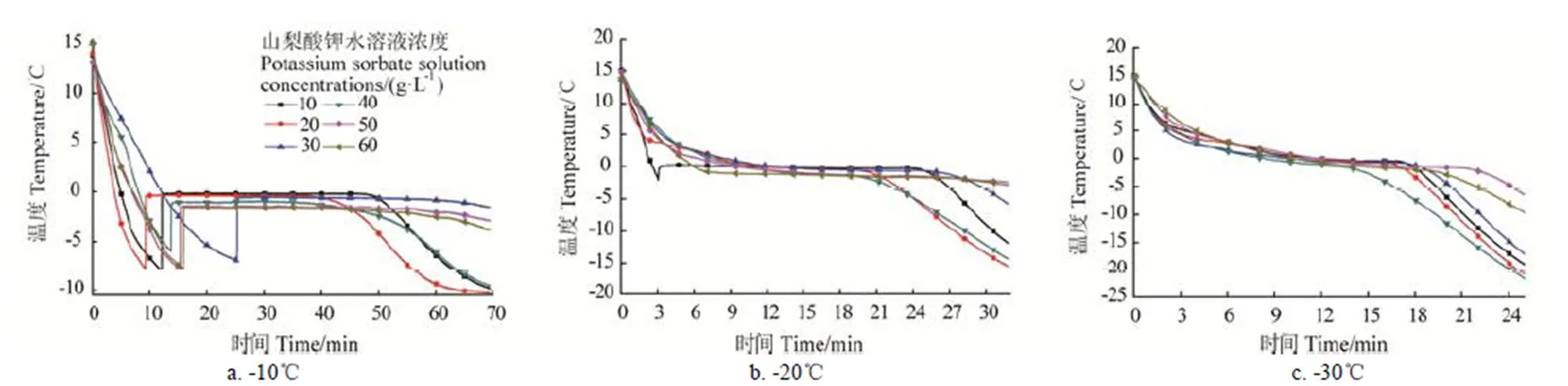
图2 不同蓄冷工况对山梨酸钾水溶液过冷度的影响
出现该情况的原因可能是随着蓄冷环境温度的降低,促成C5H7COOK溶液在相变阶段产生二次成核现象,该过程中成核基体的存在,可以大大降低成核位垒,使得成核在较小的过冷度下即可进行。考虑到耗能以及排除过冷度的影响因素,本文选择-20℃为蓄冷工况进行以下试验。
2.1.2 DSC测试
相变温度和潜热对蓄冷材料的应用起到了关键性作用。山梨酸钾水溶液的DSC(differencial scanning calorimentry)试验结果如图3、图4所示。

图3 不同浓度山梨酸钾水溶液的DSC曲线
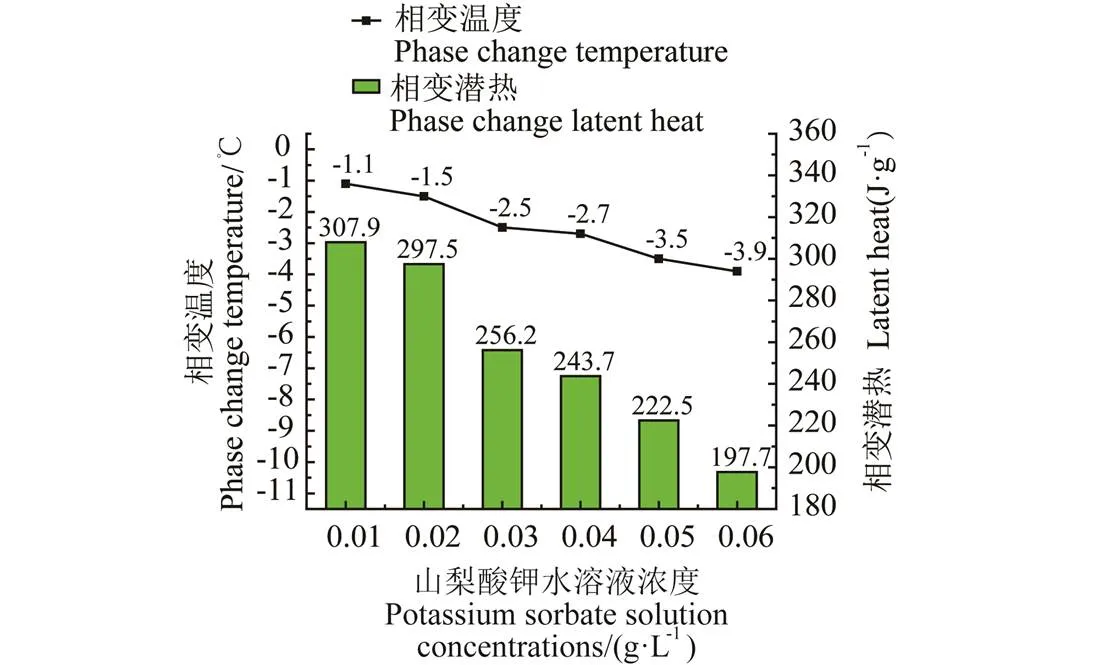
图4 不同浓度的山梨酸钾水溶液相变温度及潜热
由图3的DSC曲线可知,该复合溶液相溶性较好,且随着其浓度的增加,相变起始点逐渐向左偏移,曲线所围成的面积也在逐渐减小;由图4更加直观地表达了随着山梨酸钾水溶液浓度的增加相变温度及潜热逐渐减小。这是由于山梨酸钾水溶液中,水的相变潜热较大,随着山梨酸钾浓度的增加,水的占比减小,其潜热值也逐渐减小。
在浓度为10 ~60 g/L范围内,山梨酸钾水溶液的相变潜热由307.9减小至197.7 J/g。为了筛选出符合水温冷藏区所要求的温度-2~-3 ℃,浓度为30、40 g/L的山梨酸钾水溶液相变温度都在该范围内,选择潜热稍高的30 g/L山梨酸钾水溶液作为复合相变材料主基液,其Onset温度为-2.5 ℃,潜热为256.2 J/g;且在-20 ℃蓄冷工况下,由步冷曲线知山梨酸钾溶液在该浓度下无过冷。
2.2 C5H7COOK/KCl储能剂复配
2.2.1 C5H7COOK/KCl最佳配比
C5H7COOK/KCl复合相变材料的DSC测试结果如图5所示。当KCl浓度为3和5 g/L时,复合相变材料DSC曲线只有1个峰,C5H7COOK与KCl的相溶性较好;当KCl浓度为7 g/L时,DSC曲线开始出现2个峰,山梨酸钾水溶液不能再溶解更多的KCl,在相变过程中开始发生相分离,随着KCl浓度的增加,2个峰值越加明显。当KCl浓度为5 g/L时,Onset温度为-2.8 ℃,潜热为254.6 J/g;对比未添加KCl前,相变温度降低了-0.3 ℃,潜热降低了1.6 J/g,该变化符合浓度配比的线性关系,说明山梨酸钾和KCl没有发生化学反应。综上,较少量的KCl对山梨酸钾水溶液的相变温度及潜热影响不大。因此,选择山梨酸钾、氯化钾水溶液浓度分别为30、5 g/L作为复配材料的最佳浓度配比。
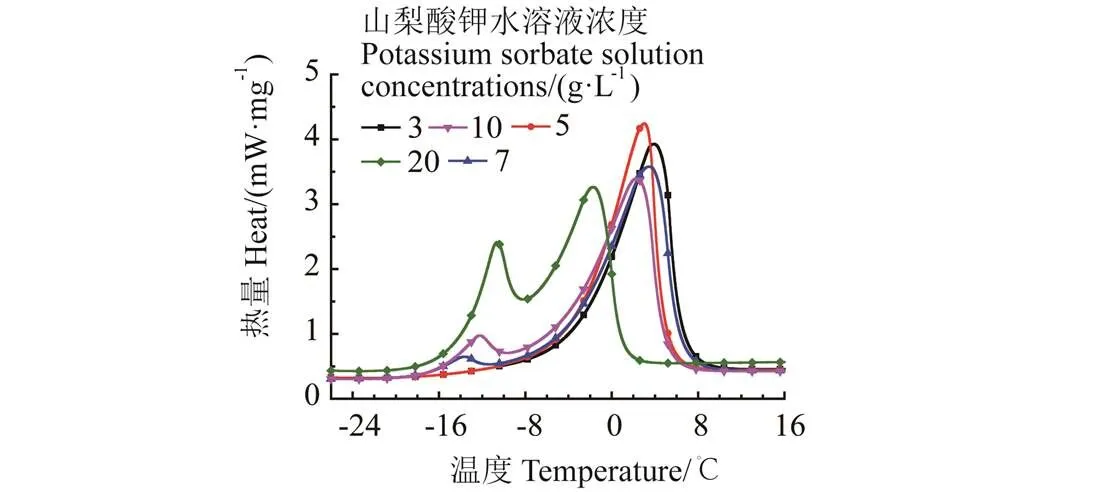
图5 30 g·L-1山梨酸钾复配不同浓度KCl的DSC曲线
2.2.2 C5H7COOK/KCl热性能
对最佳配比的C5H7COOK/KCl溶液在-20℃的蓄冷工况下进行步冷曲线的测试结果如图6所示,添加KCl后,溶液的Onset温度稍有降低,符合DSC测试结果。C5H7COOK/KCl溶液的热导率、热扩散率和比热由热常数分析仪Hot Disk测量结果如表1所示。

图6 -20℃蓄冷工况下有无添加KCl的蓄冷剂步冷曲线对比
以30 g/L C5H7COOK为主基液的相变蓄冷剂,内含5 g/L KCl的溶液热导率为1.012 W/(m·K),增大了23.32%;比热容为1.002 MJ/(m3·K),降低了50.95%,结合步冷曲线可知,比热越小,降低相同的温度释放的热量越少,越易降温;由/(),热导率增大,比热减小,则热扩散率增大。由表1知,添加5 g/L 浓度KCl的C5H7COOK水溶液的热扩散率为1.01 mm2/s,较未添加KCl增大了151.37%。从物体温度变化的角度看,热扩散率越大,表示物体内部温度变化传播得越快速。图7所示的6张热成像图是每隔3 s拍摄,从图中可以明显地看出添加少量KCl的相变溶液在相同的环境温度下明显释冷更快,该现象也进一步验证了Hot Disk试验结果的准确性。

表1 溶液的热物性测量数据
2.2.3 C5H7COOK/KCl热稳定性
试验样品经过200次冻融循环试验后,由DSC的分析软件获得C5H7COOK/KCl溶液的相变潜热、峰温及相变起始与结束温度。如图8所示,C5H7COOK/KCl溶液相变起始温度为-2.6 ℃,较循环前增加了0.2 ℃;潜热值为236 J/g,较循环前降低了7.31 %;相变温度及潜热经过200次循环试验后变化不大。
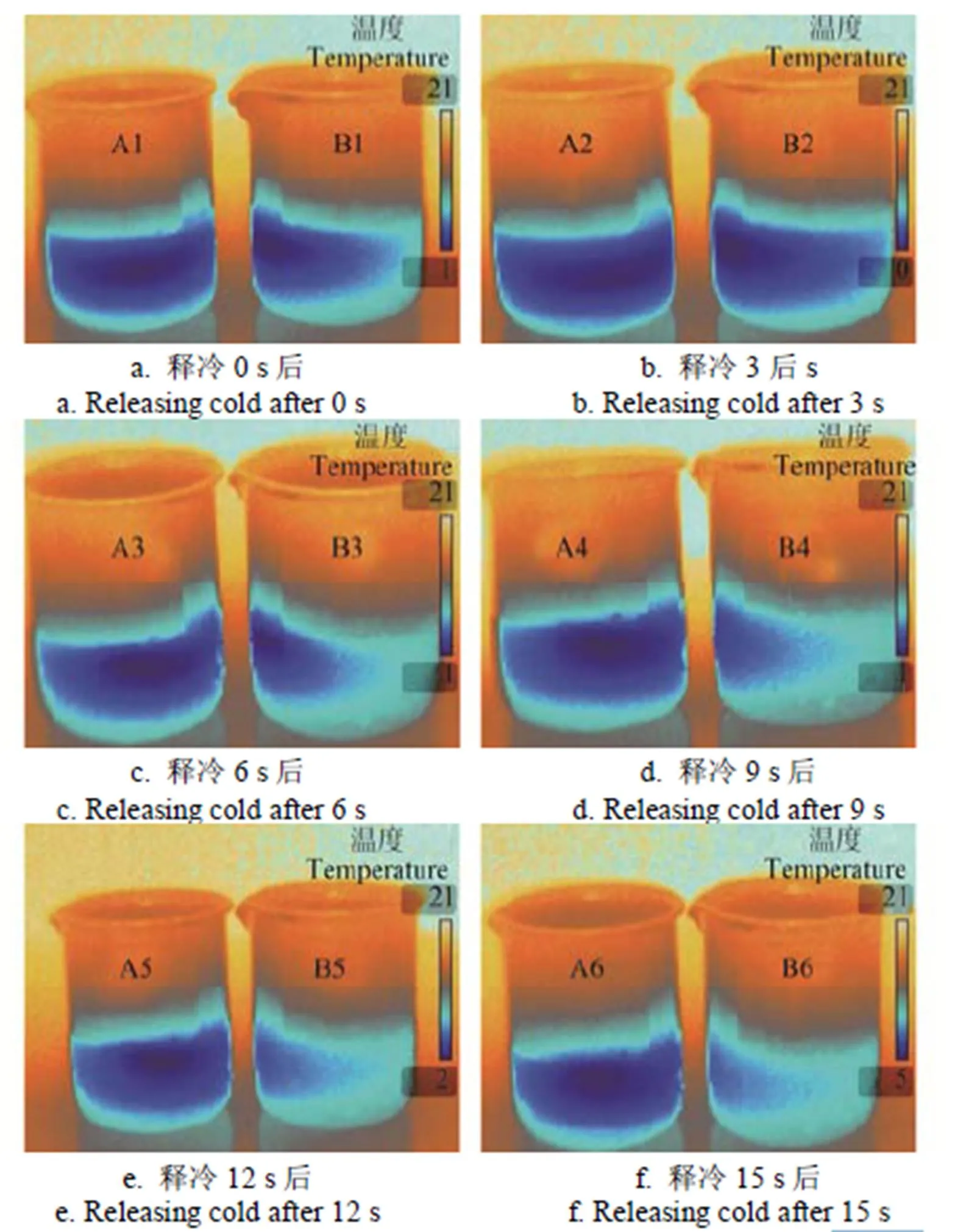
注:图中A1~A6指未添加氯化钾的30 g/L山梨酸钾水溶液、B1~B6指含0.5 g/L氯化钾的30 g/L山梨酸钾水溶液

图8 冻融循环200次后C5H7COOK/KCl水溶液的DSC曲线
为了进一步研究C5H7COOK/KCl水溶液的热稳定性,在-20 ℃的蓄冷工况下冻融循环200次后C5H7COOK水溶液和C5H7COOK/KCl水溶液的步冷曲线如图9所示,未添加KCl的复合相变材料出现了-5.3 ℃的过冷度,该现象说明30 g/L C5H7COOK溶液并不具备良好的热稳定性,而含5 g/L KCl的复合相变溶液依然没有出现过冷度,对比循环试验表明KCl在C5H7COOK溶液相变过程中起到良好的成核作用,使得该复合相变蓄冷剂在循环使用过程中保持优异的热稳定性。

图9 蓄冷剂冻融循环试验步冷曲线
3 结 论
为了寻求微冻保鲜用相变蓄冷剂,试验以山梨酸钾水溶液为主蓄冷剂,探究了添加少量KCl前后蓄冷剂的热性能。本文通过试验研究主要得出结论如下:
1)不同的蓄冷工况对山梨酸钾水溶液的过冷度影响较大。山梨酸钾水溶液浓度为30 g/L时,-20 ℃的蓄冷工况下没有过冷度,此时相变温度为-2.5 ℃,潜热为256.2 J/g;
2)30g/L C5H7COOK溶液内含5 g/L KCl时,该复合溶液蓄冷性能最佳。此时C5H7COOK/KCl复合相变材料相变温度为-2.8 ℃,潜热为254.6 J/g,热导率为1.012 W/(m·K),提高了23.32%;热扩散率为1.01 mm2/s,提高了151.37%,热扩散率越大,物体内部温度变化传播得越快速;
3)经200次冻融循环后,C5H7COOK/KCl复合溶液的相变温度和潜热变化不大。在-20℃的蓄冷工况下, KCl在C5H7COOK溶液中起到良好的成核作用,该复合相变蓄冷剂具有优异的热稳定性。
[1] 郭亚楠,胡源坤. 我国农产品冷链物流发展现状及对策研究[J]. 物流工程与管理,2018,40(2):4−6+14. Guo Yanan, Hu Yuankun. Research on the present situation and countermeasures of Chinese agricultural cold-chain logistics [J]. Logistics Engineering and Management, 2018, 40(2): 4-6+14. (in Chinese with English abstract)
[2] 丛茜,陈廷坤,李杨,等. 利用相变释能的农产品冷藏设备主动防除冰方法[J]. 农业工程学报,2017,33(9): 276-281. Cong Qian, Chen Yankun, Li Yang, et al. Active anti-icing method for agricultural product refrigerated equipment based on phase change energy release [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2017, 33(9): 276-281. (in Chinese with English abstract)
[3] 林婉玲,丁莫,王锦旭,等. 包装方式和材料对调理脆肉鲩鱼片冷藏过程品质的影响[J]. 农业工程学报,2018,34(2):284-291. Lin Wanling, Ding Mo, Wang Jinxu, et al. Effects of packaging methods and materials on quality of prepared crisp grass carp () fillets during cold storage [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2018, 34(2): 284-291. (in Chinese with English abstract)
[4] 徐笑锋,章学来,Jotham Muthoka Munyalo,等. 十水硫酸钠相变蓄冷保温箱保冷特性的试验研究[J]. 农业工程学报,2017,33(22):308-314. Xu Xiaofeng, Zhang Xuelai, Jotham Muthoka Munyalo, et al. Experimental study on cold retention characteristics of cold storage incubator using Na2SO4·10H2O as PCMs [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2017, 33(22): 308-314. (in Chinese with English abstract)
[5] Kowata H, Sase S, Ishii M, et al. Cold water thermal storage with phase change materials using nocturnal radiative cooling for vegetable cooling [J]. Proceedings of the World Renewable Energy Congress WII, Cologne (Germany), 2002.
[6] 蒋自鹏,铁生年. 芒硝基相变材料性能及其在简易温室中升温效果试验[J]. 农业工程学报,2016,32(20):209-216. Jiang Zipeng, Tie Shengnian. Property and heat storage performances of Glauber’s salt-based phase change materials for solar greenhouse in Qinghai-Tibet plateau [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2016, 32(20): 209-216. (in Chinese with English abstract)
[7] 周莹,王双喜.复合相变储能保温砂浆在日光温室中的应用效果[J].农业工程学报,2017,33(20):190-196. Zhou Ying, Wang Shuangxi. Application effect of composite phase change energy storage thermal insulation mortar in solar greenhouse [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2017, 33(20): 190-196. (in Chinese with English abstract)
[8] 鲍恩财,邹志荣,张勇. 日光温室墙体用相变固化土性能测试及固化机理[J]. 农业工程学报,2017,33(16):203-210. Bao Encai, Zou Zhirong, Zhang Yong. Performance test and curing mechanism of phase change cured soil for solar greenhouse walls [J]. Transactions of the Chinese Society of Agricultural Engineering(Transaction of the CSAE), 2017, 33(16): 203-210. (in Chinese with English abstract)
[9] BelénZalba, José M a Marı́n, Luisa F, et al. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications [J]. Applied Thermal Engineering, 2003, 23(3): 251-283.
[10] Jose Pereira da Cunha, Philip Eames. Thermal energy storage for low and medium temperature applications using phase change materials – A review [J]. Applied Energy, 2016, 177(177): 227-238.
[11] Manish K. Rathod, Jyotirmay Banerjee. Thermal stability of phase change materials used in latent heat energy storage systems: A review [J]. Renewable and Sustainable Energy Reviews, 2013, 18(18): 246-258.
[12] Liu C, Yuan Y, Zhang N, et al. A novel PCM of lauric–myristic–stearic acid/expanded graphite composite for thermal energy storage [J]. Materials Letters, 2014, 120(4): 43-46.
[13] Zhang N, Yuan Y, Yuan Y, et al. Effect of carbon nanotubes on the thermal behavior of palmitic–stearic acid eutectic mixtures as phase change materials for energy storage [J]. Solar Energy, 2014, 110: 64-70.
[14] 李玉洋,章学来,徐笑锋,等. 正辛酸-肉豆蔻酸低温相变材料的制备和循环性能[J].化工进展,2018,37(2):689-693. Li Yuyang, Zhang Xuelai, Xu Xiaofeng, et al. Preparation and cyclic properties of low temperature phase change materials of n-caprylic acid and myristic acid [J]. Chemical industry and engineering progress, 2018, 37(2): 689-693. (in Chinese with English abstract)
[15] 应铁进,苏党,白家玮. 用于非冷冻低温区运输的复合有机物相变蓄冷剂[J]. 农业机械学报,2017,48(8):309-314. Ying Tiejin, Su Dang, Bai Jiawei. Organic phase change compound materials for non-freezing cold chain [J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(8): 309−314. (in Chinese with English abstract)
[16] 展佳,秦善,高静. 无机水合盐中水的状态与相变潜热的关系[J]. 北京大学学报:自然科学版,2018,54(1):80-86. Zhan Jia, Qin Shan, Gao Jing. Storage of water in inorganic salt hydrates and the implications to latent heat in phase changes [J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2018, 54(1): 80-86. (in Chinese with English abstract)
[17] 纪珺,陈跃,章学来,等. 甘露醇水溶液低温储能相变材料的制备及热物性[J]. 化工进展,2018,37(3):1111-1117. Ji Jun, Chen Yue, Zhang Xuelai, et al. Preparation and thermophysical properties of mannitol aqueous solution PCMs for thermal energy storage [J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1111-1117. (in Chinese with English abstract)
[18] Yu Q, Tchuenbou-Magaia F, Al-Duri B, et al. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage [J]. Applied Energy, 2018, 211: 1190-1202.
[19] 熊梦雅,冯妍卉,冯黛丽,等. 金属有机骨架复合体的相变热特性测试[J]. 工程热物理学报,2018,39(1):165-171. Xiong Mengya, Feng Yanhui, Feng Daili, et al. Thermal properties of material organic frameworks composite with phase change [J]. Journal of Engineering Thermophysics, 2018, 39(1): 165-171. (in Chinese with English abstract)
[20] 徐笑锋,章学来,陈跃,等. 不同球径的多孔球层内无机盐溶液过冷度的试验研究[J]. 化工学报,2018:69(15)-7. Xu Xiaofeng, Zhang Xuelai, Chen Yue, et.al. Experimental investigation on inorganic salt solution’s supercooling degree under porous spherical layer with different spherical diameters [J]. CIESC Journal, 2018: 69(15) 1-7. (in Chinese with English abstract)
[21] 陈文朴,章学来,黄艳,等. 甲酸钠低温相变材料的研制及其在蓄冷箱中的应用[J]. 制冷学报,2017,38(1):68-72. Chen Wenpu, Zhang Xuelai, Huang Yan, et al. Sodium formate as low temperature phase change material in cold storage insulation box [J]. Journal of Refrigeration, 2017, 38(1): 68-72. (in Chinese with English abstract)
[22] 张仁元. 相变材料与相变储能技术[M]. 北京:科学出版社,2009.
[23] 曾现炜. -19 ℃至-26 ℃低温冰袋:CN103743184A [P]. 北京:标准出版社,2014-04-23.
[24] 杨颖,沈海英. 复合低温相变蓄冷材料的试验研究[J]. 低温物理学报,2009,31(2):143-147. Yang Ying,Shen Haiying. Investigation on cryogenics cool thermal energy storage phase change composite material [J]. Low Temperature Physics, 2009, 31(2): 143-147. (in Chinese with English abstract)
[25] 唐娟. 低温相变材料的热物性及应用研究[C]// 第九届国家空调、制冷及压缩会议报告文集,2008:233-238. Tang Juan. Thermal properties and application research of low temperature phase change materials[C]// The 9th National AirConditioner, Refrigerator, and Compressor Symposion, 2008: 233-238. (in Chinese with English abstract)
[26] 纪珺,任迎蕾,章学来,等. 甲酸钠/氧化铜复合相变材料的制备与热力学性能测试[J]. 传感技术学报,2016,29(9):1351-1355. Ji Jun, Ren Yinglei, Zhang Xuelai, et al. Preparation and thermal performances of nano-copper oxide/sodium formate composite phase change material [J]. Chinese Journal of Sensors and Actuators, 2016, 29(9): 1351-1355. (in Chinese with English abstract)
[27] 应铁进,朱冰清,戚晓丽,等. 用于农产品保鲜的有机物水溶液相变蓄冷剂[J]. 农业机械学报,2015,46(2):208-212. Ying Tiejin, Zhu Bingqing, Qi Xiaoli, et al. Development of organics solution phase change materials for preservation of agricultural products [J]. Transactions of the Chinese Society for Agricultural Machinery, 2015, 46(2): 208-212. (in Chinese with English abstract)
[28] Engel F, Pinto L H, Del Ciampo L F, et al. Comparative toxicity of physiological and biochemical parameters in Euglena gracilis to short-term exposure to potassium sorbate[J]. Ecotoxicology, 2015, 24(1): 153-162.
[29] 王华,左承基.几种相变蓄冷材料热物性参数的对比分析[J]. 合肥工业大学学报(自然科学版),2012,35(6):748-749+761. Wang Hua, Zuo Chengji. Comparative analysis of thermal properties of several phase change materials for cool storage[J]. Journal of Hefei University of Technology (Natural Science), 2012, 35(6): 748-749+761. (in Chinese with English abstract)
[30] 韩丽蓉. 相变蓄热材料十水硫酸钠的改性及应用研究[D]. 杨凌:西北农林科技大学,2014. Han Lirong. Modification and Application Research of Phase Change Heat Storage Material Glauber Salt [D]. Yangling: Northwest A&F University, 2014. (in Chinese with English abstract)
[31] 戚晓丽,朱冰清,牟望舒,等. 用于冷链运输的复合相变蓄冷剂主储能剂研制[J]. 中国食品学报, 2015,15(10):86-90. Qi Xiaoli, Zhu Bingqing, Mou Wangshu, et al. Development of main storage agent of composite phase change coolant for cold chain transportation [J]. Journal of Chinese Institute of Food Science and Technology, 2015, 15(10): 86-90. (in Chinese with English abstract)
[32] 戚晓丽. 复合相变蓄冷剂开发及在果蔬保鲜上的应用研究[D]. 杭州:浙江大学,2015. Qi Xiaoli. Development of Composite Phase-Changing Coolant and Its Application on Preservation of Fruit and Vegetables[D]. Hangzhou: Zhejiang University, 2015. (in Chinese with English abstract)
Preparation and thermal performance analysis of C5H7COOK/KCl composite phase change material
Zhang Xuelai1, Wang Yinghui1, Ji Jun1, Liu Junming2, Li Yuyang1, Han Xingchao1, Xu Xiaofeng1, Zhou Sunxi1, Liu Lu1, Liu Sheng3
(1.,201306;2..,,210000;3,100097,)
In the process of handover, strict logistics quality standards and inspection methods are lacking in food logistics enterprises. It makes the “broken chain” and “being not cold” of fresh and perishable low-temperature products become common phenomena during fresh and perishable low-temperature products’ transportation. At present, distribution of fresh food is not effective with the application of modern technology and the implementation of the “first mile” sorting and pre-cooling scheme, and the “last mile” is still dominated by the traditional ways of circulation model represented by “ice pack & plastic foam box”. Meanwhile, the cold storage, of which the main function is cargo-storing, often does not have the ability to sort, flow and process, so it restricts the effective improvement of the cold chain logistics’ service level seriously. It is particularly critical to develop more efficient storage technologies with respect to the “first mile” and the “last mile” of agricultural products. Water temperature refrigerated preservation(range from -2 to -3℃) belongs to the temperature interval of micro-frozen and fresh-keeping, and it is also a concept proposed for meat products, fruits and vegetables, etc. It can not only inhibit the growth of microorganisms effectively, but also keep food nutrients from being lost compared to traditional refrigeration technology. In order to develop a stable and efficient phase change coolants for micro-frozen and fresh-keeping to ensure the quality of agricultural products, such as meat products in transportation, the ternary complex composed of C5H7COOK, KCl and H2O was prepared in this article. Considering that the cold storage condition has a great influence on the supercooling degree and cooling rate, -20℃ was selected firstly as the cooling medium temperature in this article via testing the step cooling characteristics of the PCM under different cold storage conditions; the optimal concentration of main base solution was determined by DSC (Differential Scanning Calorimeter) experiment. Then mixing KCl with it, thermodynamic properties and thermal cycle stability of the composite material were studied by Hot Disk thermal constant analyzer, DSC, Agilent temperature time recorder, low-temperature thermostat bath and other equipment. When KCl was added, under concentration of 5 g/L, into the 3 g/L C5H7COOK solution, DSC results indicated that the onset temperature and latent heat were measured as -2.8℃ and 254.6 J/g, which were reduced by -0.3°C and 1.6 J/g respectively. It was indicated that there was no chemical reaction between C5H7COOK and KCl, since there shows a linear relationship between the change of properties and concentration ratio. The thermal conductivity of C5H7COOK/KCl solution was measured as 1.012 W/m·K with an increase of 23.32 %; the specific heat capacity was 1.002 MJ/m3K with a decrease of 50.95 %, and thermal diffusivity was 1.01 mm2/s with an increase of 151.37 %. From the point of view of temperature, the higher the thermal diffusivity, the faster the propagation of internal temperature variation. Thermal cycling test results revealed that the changes of the phase change temperature and latent heat of the C5H7COOK/KCl composite solution were not obvious after 200 freeze-thaw cycles. Under condition of -20℃ in cold storage, the composite PCM added with KCl showed great nucleation, which made the composite PCM has excellent thermal stability. In conclusion, the prepared C5H7COOK solution containing 5 g/L KCl composite PCM can be acted as a potential material for the refrigeration of agricultural products due to the appropriate and acceptable thermal properties, reliable thermal stability, and high thermal conductivity.
refrigeration; phase change materials; thermodynamic properties; stability
10.11975/j.issn.1002-6819.2018.18.034
TM306
A
1002-6819(2018)-18-0277-07
2018-05-28
2018-08-17
国家重点研发项目计划(2018YFD0401300);国家自然科学基金(51376115);上海市科委项目(16040501600)
章学来,教授,博导,研究方向为相变储能技术。
Email:xlzhang@shmtu.edu.cn
章学来,王迎辉,纪 珺,刘俊名,李玉洋, 韩兴超,徐笑锋,周孙希,刘 璐,刘 升. 山梨酸钾/氯化钾复合相变材料制备及热物性分析[J]. 农业工程学报,2018,34(18):277-283. doi:10.11975/j.issn.1002-6819.2018.18.034 http://www.tcsae.org
Zhang Xuelai, Wang Yinghui, Ji Jun, Liu Junming, Li Yuyang, Han Xingchao, Xu Xiaofeng, Zhou Sunxi, Liu Lu, Liu Sheng.Preparation and thermal performance analysis of C5H7COOK/KCl composite phase change material[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(18): 277-283. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2018.18.034 http://www.tcsae.org
