Use of Bivalirudin for Anticoagulation in lnterventional Cardiovascular Procedures
2018-09-17ZhenGeMDJayaChandrasekharMBBSMSandRoxanaMehranMD
Zhen Ge, MD, Jaya Chandrasekhar, MBBS, MS and Roxana Mehran, MD
1The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Abstract Anticoagulation is imperative to reduce the incidence of thrombotic complications in patients undergoing percutaneous interventional cardiovascular procedures; however, this is at the expense of increased risk of bleeding. The optimal anticoagulation strategy for these procedures remains unclear. Unfractionated heparin is the most commonly used anticoagulant during interventional procedures, but has several limitations, such as relatively high incidence of bleeding events, occurrence of heparin-induced thrombocytopenia, and a paradoxical thrombotic effect. Contemporary studies have demonstrated that bivalirudin decreases the occurrence of bleeding complications, but potentially increases the risk of acute stent thrombosis. This review discusses the pharmacology of bivalirudin and its current clinical application in patients undergoing percutaneous coronary intervention and transcatheter aortic valve replacement procedures.
Keywords: bivalirudin; anticoagulation; interventional cardiovascular procedures
lntroduction
Use of anticoagulation is necessary during percutaneous interventional cardiovascular procedures to decrease the risk of thrombotic events, at the expense of some increase in bleeding complications, which in turn can contribute to significant morbidity and mortality [1, 2]. Thus to gain net clinical benefit,both ischemic and bleeding risks must be considered. Although unfractionated heparin (UFH) has been the main anticoagulant used in interventional procedures, recently bivalirudin has been increasingly used. Indeed, guidelines recommend this to be a more suitable anticoagulant for patients at high risk of bleeding [3, 4]. Nevertheless, although several clinical trials have compared the safety and efficacy of bivalirudin versus heparin, the optimal anticoagulation strategy remains unclear [5–7].This review summarizes the current clinical evidence for the application of bivalirudin in percutaneous coronary intervention (PCI) and transcatheter aortic valve replacement (TAVR).
Pharmacology
Bivalirudin is a synthetic 20 amino acid peptide, which inhibits both circulating and clot-bound thrombin by binding to the catalytic site and the anion-binding exosite [8]. It is a direct thrombin inhibitor, and does not require binding to antithrombin III to produce an anticoagulation effect [9]. Its unique mechanism of action contributes to a consistent anticoagulation effect, which does not require routine monitoring and dose titration. Bivalirudin reversibly binds to thrombin, and has a short half-life of 25 minutes in patients with normal renal function [10]. Moreover,in patients with normal renal function and mild renal impairment, its total plasma clearance is similar, and in patients with moderate and severe renal impairment, plasma clearance decreases by only 20% [11].
Use of Bivalirudin in ST-Segment Elevation Myocardial lnfarction
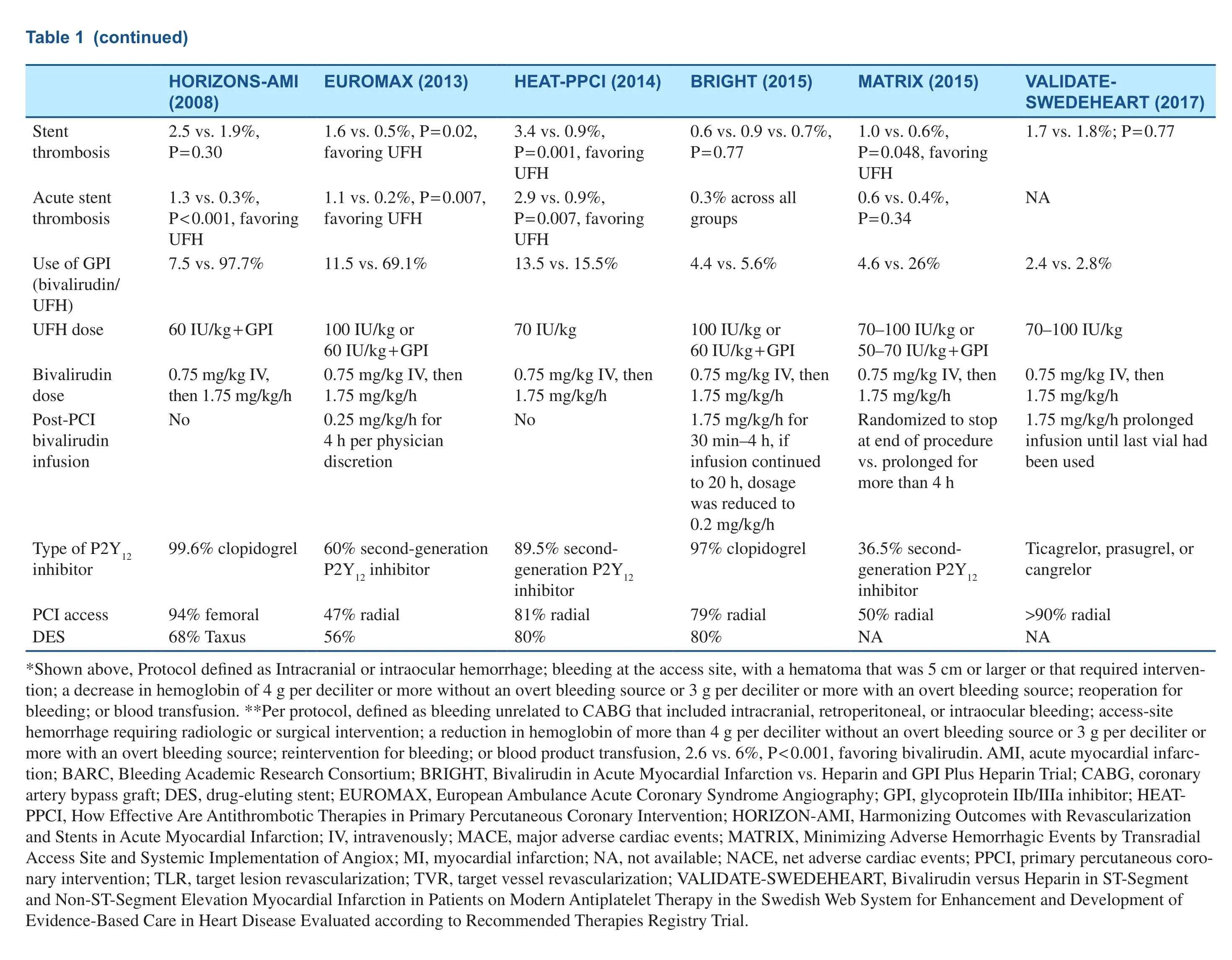
Several randomized controlled trials (RCTs) have investigated the use of bivalirudin in patients with ST-segment elevation myocardial infarction(STEMI) undergoing primary PCI (PPCI), summarized in Table 1 [5–7, 12–14], but the results have been somewhat conflicting. Many factors contribute to the discrepancies in these clinical trial results,such as the use of different bivalirudin maintenance regimens, concurrent use of glycoprotein IIb/IIIa inhibitors (GPI) routinely or in bailout, the dose of UFH in the comparator arm, the use of potent antiplatelet therapy, and rate of radial access use for PCI.The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction(HORIZONS-AMI) trial compared the strategy of bivalirudin therapy alone with UFH therapy plus routine GPI use in 3602 patients who presented with STEMI within 12 hours of symptom onset.At 30 days and 1 year, the rate of net adverse cardiac events (NACE; composite of protocol-defined major bleeding or major adverse cardiovascular events [MACE], including death, reinfarction,ischemia-driven target vessel revascularization[TVR], or stroke) was significantly lower with bivalirudin versus UFH plus GPI (at 30 days, 9.2 vs. 12.1%, P = 0.005; at 1 year, 15.6 vs. 18.3%,P = 0.022). This was mainly driven by reduction in the occurrence of major bleeding (at 30 days, 4.9 vs.8.3%, P < 0.001; at 1 year, 5.8 vs. 9.2%, P < 0.0001).The rate of acute stent thrombosis with bivalirudin therapy was higher than with UFH plus GPI therapy(1.3 vs. 0.3%, P < 0.0001); the rate of subacute stent thrombosis (1.2 vs. 1.7%, P = 0.28) and the rate of 1-year stent thrombosis (3.6 vs. 3.2%, P = 0.53)were similar between the two groups [12, 15].
The European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial enrolled 2218 patients with STEMI and compared bivalirudin therapy with heparin therapy (UFH or low molecular weight heparin), with optional GPI use. Anticoagulation was initiated in the ambulance or non-PCI hospital. More than 60% of patients received novel P2Y12inhibitors. Although GPI use was at physician discretion, it occurred more often in patients who received heparin than in patients who received bivalirudin (11.5 vs. 69.1%).Bivalirudin was administered as a standard intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg per hour during PCI, and continuous infusion at a reduced dose of 0.25 mg/kg per hour(77.5% of patients) to a full dose of 1.75 mg/kg per hour for at least 4 hours after PCI. The 30-day primary composite end point of death or major bleeding was significantly lower with bivalirudin than with heparin (5.1 vs. 8.5%, P = 0.001), which was mainly driven by a lower rate of major bleeding (2.6 vs. 6.0%, P < 0.001). The rate of acute stent thrombosis was higher in the bivalirudin group (1.1 vs. 0.2% P = 0.007), and there was no difference in the rates of subacute stent thrombosis (0.5 vs.0.4%, P = 0.75) [13].
The How Effective Are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention(HEAT-PPCI) trial was a single-center STEMI trial,and 1812 patients were randomly assigned to receive UFH (70 IU/kg) or bivalirudin without a post-PCI infusion. Provisional GPI use was permitted in both groups. In comparison with previous trials, in this study, a novel P2Y12inhibitor was used in 89.4% of patients, radial access in 81%, and a drug- eluting stent in 80%. At 28 days, the primary efficacy end point, a hierarchical composite of the rate of allcause death, cerebrovascular accident, reinfarction,or unplanned target lesion revascularization, was higher with bivalirudin (8.7 vs. 5.7%, P = 0.01),which was driven by the higher incidence of reinfarction or unplanned target lesion revascularization. The incidence of major bleeding was similar in both groups (3.5 vs. 3.1%, P = 0.59). The incidences of 28-day stent thrombosis and acute stent thrombosis were significantly higher with bivalirudin than with UFH (3.4 vs. 0.9%, P = 0.001; 2.9 vs. 0.9%,P = 0.007) [7]. Notably, this trial was conducted in a single center, limiting generalizability of findings.
Furthermore, anticoagulation in both groups was considered to be inadequate, particularly with lower-dose heparin use in the UFH group and lack of prolonged bivalirudin infusion after PCI in the bivalirudin group, influencing the relatively higher occurrence of acute stent thrombosis in both arms.

Table 1 Characteristics of Clinical Trials Comparing Bivalirudin with Heparin in Patients with ST-Segment Elevation Myocardial Infarction (STEMI)/Non-STSegment Elevation Myocardial Infarction (NSTEMI) Undergoing Percutaneous Coronary Intervention (PCI).
?
Use of Bivalirudin in ST-Segment Elevation Myocardial lnfarction or Non-ST-segment elevation Myocardial lnfarction
The Bivalirudin in Acute Myocardial Infarction vs Heparin and GPI Plus Heparin Trial (BRIGHT)included 2194 patients presenting with STEMI or non-ST-segment elevation myocardial infarction (NSTEMI) undergoing PPCI randomized to three groups in a 1:1:1 ratio (bivalirudin alone,UFH alone, or UFH plus tirofiban). In the bivalirudin group, all patients received a standard dose of bivalirudin during PCI, followed by post-PCI infusion for 30 minutes to 4 hours at a PCI dose of 1.75 mg/kg per hour, and afterward a reduced-dose infusion of 0.2 mg/kg per hour could be used for up to 20 hours. A bolus dose of heparin (100 IU/kg)was administered in the UFH-alone group, and a dose of 60 IU/kg was administered in the UFH plus tirofiban group. At 30 days, the primary end point of NACE, including death, reinfarction, stroke,ischemia-driven TVR, or any bleeding defined as Bleeding Academic Research Consortium (BARC)type 1–5, occurred in 8.8% of bivalirudin-alone patients versus 13.2% of UFH-alone patients (risk ratio [RR] 0.67, P = 0.008) and 17.0% of UFH plus tirofiban patients (RR 0.52, P < 0.001). The superiority of bivalirudin over UFH alone or UFH plus tirofiban was driven by the lower incidence of any bleeding. With respect to BARC type 3 or 5 bleeding, bivalirudin was still noted to be superior to UFH alone or UFH plus tirofiban (0.5 vs. 1.5 vs.2.1%, respectively). There were no significant differences in the incidence of MACE and its components across the three groups [5]. The incidence of acute stent thrombosis or subacute stent thrombosis with bivalirudin therapy was similar to that with UFH therapy alone or UFH plus tirofiban therapy,which was different from the findings of previous trials. Furthermore, the incidence of acute stent thrombosis was only 0.3% in the bivalirudin group,which was much lower than in the HEAT-PPCI trial. In the EUROMAX trial, prolonged bivalirudin infusion was allowed after PCI, but 77.5% of patients were given a reduced dose of 0.25 mg/kg per hour, which was different from that in BRIGHT,which might explain the relatively higher incidence of acute stent thrombosis in the EUROMAX trial.
The Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX) trial enrolled 7213 patients with STEMI or non-ST-segment elevation acute coronary syndrome (NSTEACS),who were randomly assigned to receive bivalirudin or heparin. Provisional GPI use was permitted in the two groups. A total of 3610 patients in the bivalirudin arm were randomly allocated to receive prolonged bivalirudin infusion after PCI or no infusion after PCI. In the prolonged infusion arm, the patients were given either a full-dose infusion of 1.75 mg/kg per hour for up to 4 hours or a reduced dose of 0.25 mg/kg per hour for at least 6 hours at the discretion of the treating physicians. At 30 days the primary end point of NACE (composite of all-cause death, myocardial infarction, stroke, or BARC type 3 or 5 major bleeding) was 11.2% in the bivalirudin arm versus 12.4% in the heparin arm (RR 0.89, P = 0.12). The rate of major bleeding was lower (1.4 vs. 2.5%, RR 0.55, P < 0.001) but the rate of definite stent thrombosis was higher with bivalirudin versus heparin (1.0 vs. 0.6%, P = 0.048).Curiously, the incidence of type 3 or 5 major bleeding was lower in the prolonged bivalirudin infusion subgroup versus the no bivalirudin infusion subgroup (1.0 vs. 1.8%, P = 0.03). There was no significant difference in the rate of definite/probable stent thrombosis or acute stent thrombosis between the two infusion subgroups (1.5 vs. 1.1%, P = 0.29; 0.6 vs. 0.6%, P = 0.99) [6].
The Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation Myocardial Infarction in Patients on Modern Antiplatelet Therapy in the Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated according to Recommended Therapies Registry Trial(VALIDATE-SWEDEHEART) [14] is the latest registry-based RCT on this subject. A total of 6006 STEMI or NSTEMI patients were randomized to bivalirudin therapy or intra-arterial heparin therapy with bailout GPI therapy. Radial access was used for PCI in 90.3% of patients, and patients were mainly treated with potent P2Y12inhibitors (ticagrelor in 94.9%, prasugrel in 2.1%, or cangrelor in 0.3%).In the bivalirudin group, bivalirudin infusion after PCI was recommended until the last vial had been used. In the heparin group, the recommended dose was 70–100 IU/kg. At 30 days and 180 days, there were no differences in the primary composite end point of all-cause death, myocardial infarction, or major bleeding (at 30 days, 7.2 vs. 8.0%, P = 0.21; at 180 days, 12.3 vs. 12.8%, P = 0.54). The incidence of stent thrombosis was also similar between the two groups. Furthermore, the incidence of ischemic events was relatively lower than in previous studies.In this study, bivalirudin was not superior to heparin in reducing the risk of major bleeding and resulted in similar risk of ischemic events, which is probably attributed to high rate of use of potent P2Y12inhibitors and radial access.
In a meta-analysis of 17,294 patients included in six STEMI PPCI trials [16], at 30 days, the incidence of all-cause death or cardiac death was significantly lower with bivalirudin than with heparin (2.28 vs.2.74%, P = 0.03, for all-cause death; 1.68 vs. 2.39%,P = 0.001, for cardiac death). The incidence of major bleeding was lower with bivalirudin (1.92 vs. 2.93%,P = 0.006). However, the incidence of acute stent thrombosis was significantly higher with bivalirudin than with heparin (0.93 vs. 0.33%, P = 0.002), albeit there was no difference when prolonged bivalirudin infusion after PCI was compared with heparin treatment (0.26 vs. 0.33%, P = 0.71). Another meta-analysis excluded NSTEMI patients from BRIGHT, and stratified patients on the basis of the bivalirudin infusion strategy after PCI as receiving full-dose infusion(1.75 mg/kg per hour), low-dose infusion (0.25 mg/kg per hour), or no infusion after PCI. Full-dose infusion after PCI was the most effective strategy for reducing the risk of NACE or cardiac death compared with low-dose infusion or no infusion [17].
We conducted a pooled study-level meta-analysis of seven RCT [5–7, 12–14, 18], including VALIDATE-SWEDEHEART, that showed that bivalirudin is associated with significantly lower risk of NACE (RR 0.81, P < 0.001; Figure 1) and major bleeding (RR 0.71, P < 0.001; Figure 2), but there was no difference in the risk of definite stent thrombosis (RR 1.40, P = 0.247; Figure 3) or death(RR 0.88, P = 0.144; Figure 4).
Nevertheless, individual trials have shown that the incidence of acute stent thrombosis is significantly higher with bivalirudin therapy than with heparin therapy. This increased risk with bivalirudin therapy may be attributed to several factors, such as the short half-life of bivalirudin of 25 minutes, variations in post-PCI infusion practices with respect to dosing and duration, and use of clopidogrel or potent P2Y12inhibitors, which can modify this risk in the context of STEMI or NSTEMI presentations [17].
Use of Bivalirudin in Non-ST-segment elevation Acute Coronary Syndrome
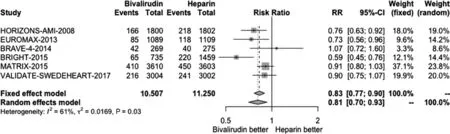
Figure 1 Forest Plot Illustrating the Risk Ratio (RR) of Net Adverse Cardiac Events in Bivalirudin-Treated versus Heparin-Treated ST-Segment Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention.CI, confidence interval.
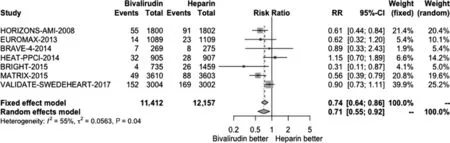
Figure 2 Forest Plot Illustrating the Risk Ratio (RR) of Major Bleeding in Bivalirudin-Treated versus Heparin-Treated ST-Segment Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention.CI, confidence interval.
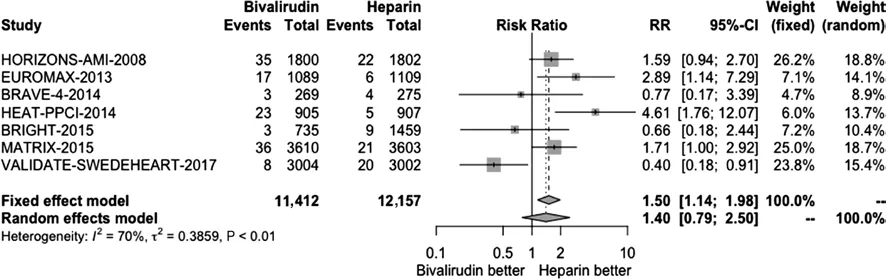
Figure 3 Forest Plot Illustrating the Risk Ratio (RR) of Definite Stent Thrombosis in Bivalirudin-Treated versus Heparin-Treated ST-Segment Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention.CI, confidence interval.
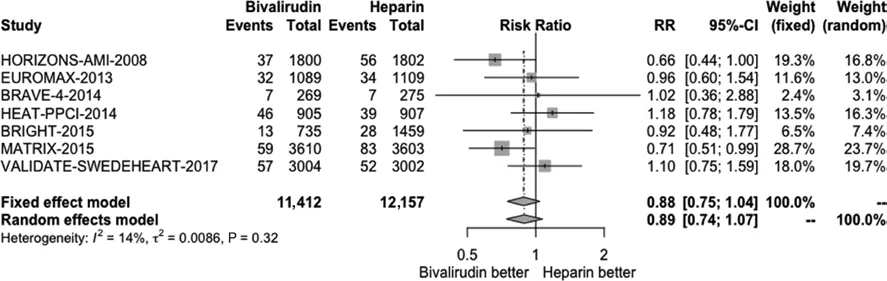
Figure 4 Forest Plot Illustrating the Risk Ratio (RR) of Death in Bivalirudin-Treated versus Heparin-Treated ST-Segment Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention.CI, confidence interval.
The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial [19] randomized 13,819 NSTEACS patients to bivalirudin plus GPI therapy, bivalirudin therapy alone, or heparin plus GPI therapy. At 30 days the incidence of NACE,MACE, or major bleeding with bivalirudin plus GPI therapy did not differ from that with heparin plus GPI therapy; however, bivalirudin therapy alone was associated with lower risk of major bleeding and similar ischemic risk compared with heparin plus GPI therapy. The Intracoronary Stenting and Antithrombotic Regimen Rapid Early Action for Coronary Treatment 4 (ISAR-REACT 4) trial [20]randomized 1721 NSTEACS patients to bivalirudin therapy or UFH plus abciximab therapy. At 30 days the incidence of NACE was similar with bivalirudin and UFH plus abciximab; however, UFH plus abciximab was associated with significantly greater risk of major bleeding.
Use of Bivalirudin for Elective Percutaneous Coronary lntervention
The role of bivalirudin in elective PCI may be different from that in the setting of PPCI because of different patient clinical presentation and level of risk, as well as other adjuvant treatments.
The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events 2(REPLACE-2) trial was a randomized, doubleblind, active-controlled trial involving 6010 patients undergoing urgent or elective PCI who were assigned to bivalirudin therapy with provisional GPI use or heparin therapy with planned GPI. Use of bivalirudin with provisional GPI was found to be non-inferior to heparin with planned GPI use in reducing the risk of acute ischemic events, and decreased the risk of in-hospital major bleeding [21].
The Intracoronary Stenting and Antithrombotic Regimen Rapid Early Action for Coronary Treatment 3 (ISAR-REACT 3) trial was a multicenter, double-blind, RCT comparing bivalirudin and UFH treatment in 4570 patients with stable(approximately 82%) or unstable angina with negative biomarkers, pretreated with 600 mg clopidogrel and undergoing elective PCI. The primary composite of death from any cause, myocardial infarction,urgent TVR within 30 days, or major bleeding was similar between the two groups (8.3 in the bivalirudin group vs. 8.7% in the UFH group, P = 0.57), but the incidence of major bleeding was significantly lower with bivalirudin than with UFH (3.1 vs. 4.6%,P = 0.008). The rate of stent thrombosis was 0.5% in the bivalirudin group and 0.4% in the UFH group(P = 0.52) [22].
The Novel Approaches in Preventing and Limiting Events III (NAPLES III) trial was a single-center,double-blind, RCT that randomized 837 high bleeding risk patients undergoing transfemoral elective PCI to receive bivalirudin or UFH. No significant difference was found in the primary end point of in-hospital major bleeding between the two groups(3.3% in the bivalirudin group vs. 2.6% in the UFH group, P = 0.54). The rate of access-site bleeding requiring intervention was numerically higher with bivalirudin (1.7 vs. 0.5%, P = 0.1). At 30 days, there were no significant differences in the rates of stent thrombosis and NACE between the two groups [23].
A meta-analysis of 22 RCTs, including elective PCI trials, showed that bivalirudin is associated with lower risk of major bleeding compared with UFH but higher risk of 30-day stent thrombosis and reinfarction [24]. There were no significant differences between bivalirudin and UFH in reducing the risk of death regardless of acute coronary syndrome or elective presentation for PCI.
Guidelines
Table 2 gives the latest American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC)recommendations for bivalirudin use in patients with acute coronary syndrome. The 2013 ACC/AHA guidelines provide a class I, level B recommendation for bivalirudin use in STEMI and NSTEMI and a class IIa, level B recommendation in patients at high risk of bleeding [3, 4].
The latest ESC guidelines have downgraded the recommendation for bivalirudin from class I,level A to class IIa, level A for STEMI PCI [25].Bivalirudin use is still recommended with a class I,level A indication in NSTEMI PCI [26].
Use of Bivalirudin during Transcatheter Aortic Valve Replacement
UFH is mainly used as the anticoagulant of choice during TAVR procedures; however, bivalirudin has been shown to be a suitable alternative in this setting. Kini et al. [27] first reported that use of bivalirudin was associated with a lower rate of major bleeding and NACE (a composite of death,myocardial infarction, stroke, or major bleeding)than UFH in patients with severe aortic stenosisundergoing balloon aortic valvuloplasty. There was no significant difference in the rate of MACE between the two groups. Conversely, Lange et al.[28] reported that use of bivalirudin versus heparin did not result in significantly different rates of lift-threatening bleeding, major bleeding, minor bleeding, all-cause death, and cardiovascular death in patients undergoing TAVR. The Bivalirudin versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement 3 (BRAVO 3) trial [29]is the only RCT to have compared bivalirudin with UFH for procedural anticoagulation in inoperable or high-risk patients undergoing transfemoral TAVR. The first co-primary end point of this trial was BARC type 3b or higher major bleeding within 48 hours or before hospital discharge. The second co-primary end point was NACE within 30 days defined as the composite of MACE (all-cause death, myocardial infarction or stroke) or major bleeding. At 48 hours the rate of major bleeding was 6.9% in the bivalirudin group and 9.0% in the UFH group (RR 0.77, P = 0.27). At 30 days, there were no significant differences in the rate of NACE(14.4% in the bivalirudin group vs. 16.1% in the UFH group, RR 0.89, P = 0.50, Pnoninferiority< 0.01).Major bleeding is a significant concern in TAVR procedures commonly performed via transfemoral access with large sheath sizes. Indeed, TAVR may be associated with 10-fold higher risk of bleeding compared with PCI [30].
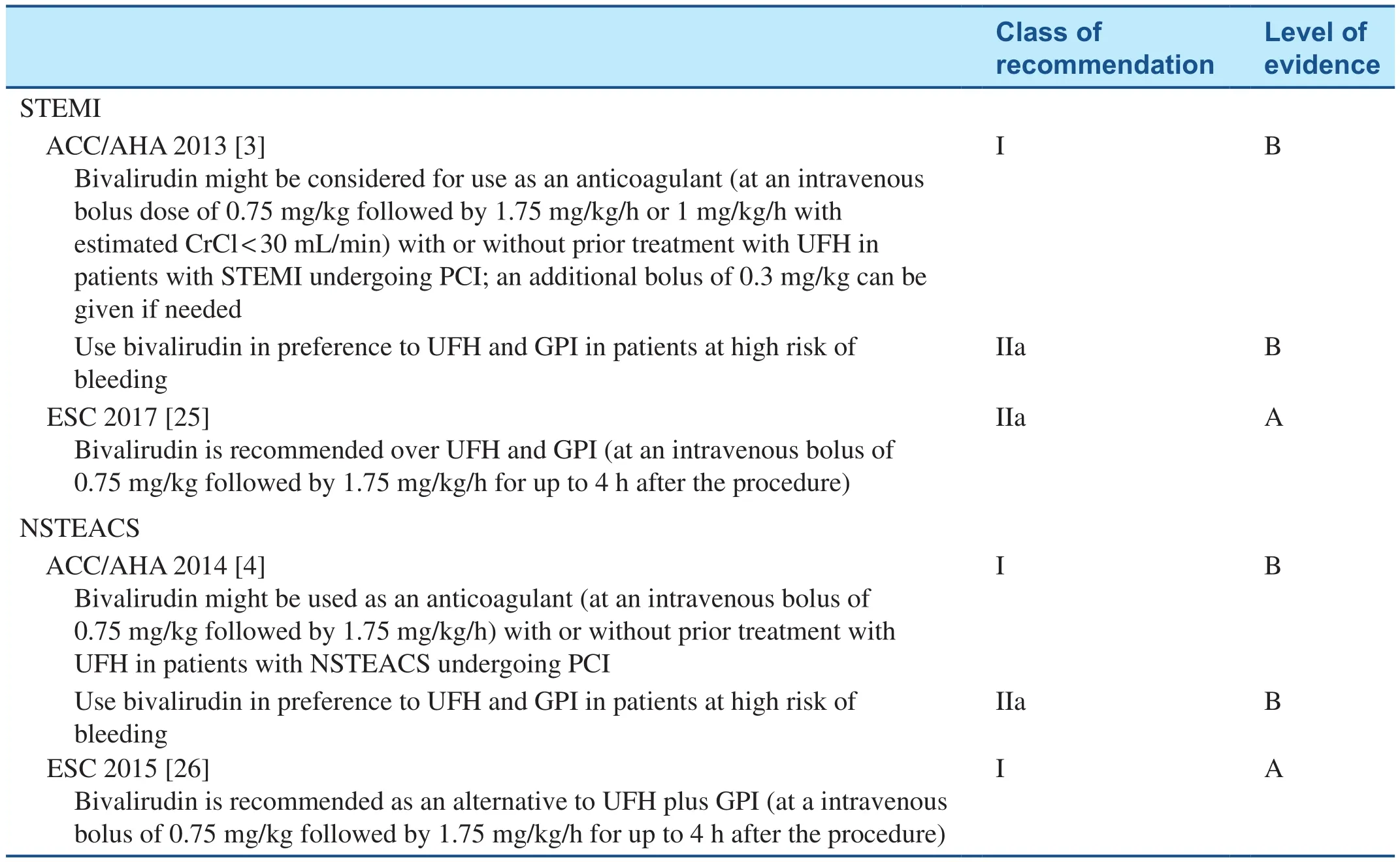
Table 2 Recommendations for Bivalirudin use in Interventions in Acute Coronary Syndrome.
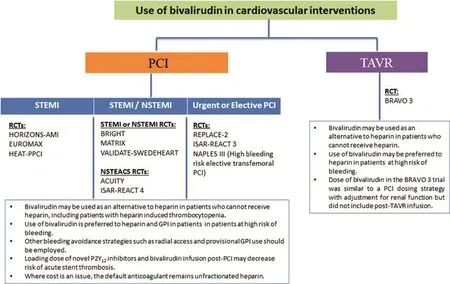
Figure 5 Recommendations for Bivalirudin use in Patients Undergoing Percutaneous Coronary Intervention (PCI) and Transcatheter Aortic Valve Replacement (TAVR) Procedures.GPI, glycoprotein IIb/IIIa inhibitor; NSTEACS, non-ST-segment elevation acute coronary syndrome; NSTEMI, non-ST-segment elevation myocardial infarction; RCT, randomized controlled trial; STEMI, ST-segment elevation myocardial infarction.
Notwithstanding, in the BRAVO 3 trial there were no differences in the rate of major bleeding with bivalirudin therapy as compared with UFH therapy among high-risk or inoperable TAVR patients, which may be a result of the expertise of the participating centers, the limited sample size, and the absence of GPI-driven higher bleeding rates in TAVR patients treated with heparin, as seen in PCI trials. However,the prespecified noninferiority hypothesis was met,demonstrating that bivalirudin was not inferior to UFH for 30-day NACE outcomes. These results were attributed to experienced operators and centers participating in this trial, and advances in TAVR device technology with smaller delivery sheath sizes in newer-generation devices. On the basis of the BRAVO 3 trial results, bivalirudin may be recommended as an alternative anticoagulant in patients who cannot receive UFH for TAVR. In the prespecified sex-based subanalysis, there were numerical reductions with bivalirudin versus UFH in the incidence of 30-day major bleeding (15.4 vs. 8.3%)among women with three or more high bleeding risk characteristics, including age older than 80 years,weight less than 50 kg, history of atrial fibrillation,and chronic kidney disease [31]. A device-based subanalysis noted an interaction between anticoagulation and device type (P = 0.039), with lower risk of major vascular complications in bivalirudin-treated patients receiving non-balloon-expandable TAVR valves [32], which may be linked to differential rates in successful deployment of vascular closure devices or may be a chance finding.
Recommendations
Figure 5 illustrates the recommendations for anticoagulation adapted from the algorithm proposed by Chandrasekhar and Mehran [33] in the context of PPCI, which we extend to include patients undergoing elective or urgent PCI and TAVR. The selection of anticoagulation should be individualized by careful assessment of the risk of bleeding, cost issues,and contraindications.
In PCI procedures, heparin can effectively decrease the risk of ischemic events, although the risk of bleeding remains unavoidable. Heparin is recommended as the principal anticoagulation with weight-based dosing, monitoring of activated clotting time, and provisional rather than routine use of GPI. However, bivalirudin is superior to heparin for reduction of bleeding events, and is therefore recommended in patients with high risk of bleeding or heparin-related contraindications. Where bivalirudin is used for STEMI patients, continuous infusion with full-dose bivalirudin (1.75 mg/kg per hour) up to 4 hours after PCI may be preferred to reduce the risk of acute stent thrombosis.
In TAVR procedures, given the lack of evidence to determine the superiority of bivalirudin compared with UFH, it is recommended as an alternative agent only in patients who cannot receive UFH.However, future trials should examine potential applications of bivalirudin in TAVR patients with very high bleeding risk.
Conclusions
Bivalirudin is associated with decreased risk of major bleeding, at the risk of increasing stent thrombosis in patients undergoing PCI for STEMI or NSTEMI, which may be mitigated with the use of prolonged full-dose bivalirudin infusion after PCI.Bivalirudin is also a suitable alternative anticoagulant in inoperable or high-risk patients undergoing TAVR. The contemporary evidence base suggests that bivalirudin is a suitable anticoagulant for use in most settings for both PCI and TAVR procedures,particularly in patients at high risk of bleeding.Future trials should investigate the role of bivalirudin in TAVR patients deemed to be at the highest risk of bleeding.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Speckle Tracking Echocardiography ldentifies lmpaired Longitudinal Strain as a Common Deficit in Various Cardiac Diseases
- Current Status of Coronary Atherectomy
- The Use of Direct Oral Anticoagulants for Prevention of Stroke and Systemic Embolic Events in East Asian Patients with Nonvalvular Atrial Fibrillation
- Bioresorbable Vascular Scaffold in the Midportion of the Left Anterior Descending Artery for Cardiac Allograft Vasculopathy in a Cardiac Transplant Patient
- The Contemporary Role of Femoral Artery Access
- Cardiovascular Innovations and Applications