The Use of Direct Oral Anticoagulants for Prevention of Stroke and Systemic Embolic Events in East Asian Patients with Nonvalvular Atrial Fibrillation
2018-09-17ChangShengMaMD
Chang-Sheng Ma, MD
1Department of Cardiology, Beijing AnZhen Hospital, Capital Medical University, Beijing, China
Abstract As patients in East Asia age, the prevalence of age-related and chronic disease, including nonvalvular atrial fibrillation,may increase. Although warfarin has been the primary choice of anticoagulant for the prevention of stroke and systemic embolic events, the use of direct oral anticoagulants (DOACs) is increasing. DOACs do not require monitoring of the international normalized ratio to determine the optimal dose, and have a lower potential for food and drug interactions,improved benefit-risk profiles, and a quicker onset and offset of action relative to warfarin. The pivotal phase 3 trials for each of the DOACs – dabigatran, rivaroxaban, apixaban, and edoxaban – included at least some East Asian patients.Additionally, several clinical trials were conducted specifically for East Asian patients. This review discusses patterns and predictors of anticoagulant use in East Asian patients with nonvalvular atrial fibrillation, summarizes current guideline recommendations for East Asian patients, details the primary results demonstrating the safety and efficacy of DOACs in East Asian patients relative to non–East Asian patients, provides real-world data supporting the phase 3 testing results, and addresses the clinical profile of DOACs in East Asian populations, including patients at high risk of stroke.
Keywords: anticoagulant; direct oral anticoagulant; East Asia; stroke
lntroduction
The prevalence in East Asia of nonvalvular atrial fibrillation (NVAF) may increase as the population continues to age [1–3]. Although the prevalence of atrial fibrillation (AF) is lower in East Asia than in the United States, variability between studies in the way that patients are selected and AF is diagnosed make comparisons difficult [4, 5]. In a 2012 literature review, the prevalence of AF was 0.6–1.6%in Japan, 0.7% in South Korea, 1.1% in Taiwan,and 0.8–2.8% in China [4]. Patients with AF are at high risk of stroke and systemic embolic events(SEEs), and oral anticoagulants (OACs) are recommended for the prevention of stroke and SEEs in these patients [6–9]. However, throughout Asia, as in the Western world, anticoagulants are underused for the prevention of stroke and SEEs in AF patients[10–15]. Furthermore, warfarin tends to be underused in patients at high risk of stroke and overused in those at low risk of stroke.
Compared with warfarin, direct OACs (DOACs)have no need for monitoring of the international normalized ratio (INR) to optimize the dose, lower potential for food and drug interactions, an improved benefit-risk profile, and a quicker onset and offset of action [16]. The aim of this review is to examine patterns and predictors of anticoagulant use in East Asian patients with NVAF and current guideline recommendations, discuss primary results demonstrating the safety and efficacy of DOACs in East Asian patients relative to non–East Asian patients, and outline the clinical profile of DOACs in East Asian populations.
Guidance for Treatment of NVAF in East Asia
As in European and US guidelines [6, 9, 17], OACs are recommended for stroke prevention in moderate- to high-risk patients with AF by the Japanese Circulation Society and Korean Heart Rhythm Society guidelines [7, 8]. When anticoagulants are indicated, DOACs are the recommended first-line therapy for stroke prevention in NVAF patients in the European, US, and Japanese guidelines [6, 7, 9],and are an acceptable first-line option in the Korean guidelines [8]. The Korean guidelines recommend continuation of the use of warfarin for NVAF patients who are already receiving it and have a well-controlled INR and no significant bleeding,but DOACs may be considered in other patients,except those with severe renal dysfunction [8].
When warfarin is used, most guidelines recommend dose titration to a target INR of 2.0–3.0 [8,18, 19]; in the Japanese, Asia Pacific Heart Rhythm Society, and Korean guidelines, a target INR of 1.6–2.6 is suggested for patients aged 70 years or older[7, 8, 20]; however, the risk of SEEs is increased with an INR below 2.0, as in Western patients [21].This suggests the lower limit of anticoagulation for Japanese patients may require further study [21].
Use of DOACs in East Asians
Throughout East Asia, the use of anticoagulants,especially DOACs, in patients with NVAF is suboptimal overall, particularly in patients at greatest risk of stroke or SEEs (Table 1) [10–15, 22–37].However, on the basis of data from the Japanese SAMURAI registry, the use of dabigatran, apixaban,and rivaroxaban increased from 29.8 in 2011–2012 to 52.8% in 2013–2014 in NVAF patients [32]. As in non–East Asian populations, DOACs have efficacy similar to that of warfarin in East Asian patients with NVAF, and may be associated with a reduced risk of major bleeding [38–41]. The pivotal clinical trials with DOACs are discussed in the following sections; all of these trials contained a proportion of East Asian patients (Table 2) [38–41]. Additionally,several phase 2 and phase 3 clinical trials were conducted specifically in Asian populations.
Dabigatran
The RE-LY study included patients from mainland China, Hong Kong, Taiwan, Japan, South Korea, India, Thailand, Malaysia, Singapore, and the Philippines [38]. Dabigatran at a dosage of 150 mg twice daily was significantly more effective than warfarin in preventing stroke or SEEs in Asian patients [hazard ratio (HR) 0.45, 95% confidence interval (CI) 0.28–0.72], and there was a trend toward a reduced rate of stroke/SEEs with dabigatran at a dosage of 110 mg twice daily relative to warfarin (HR 0.81, 95% CI 0.54–1.21) [38].There was no difference in the effect of treatment across regions on the rate of ischemic stroke and other outcomes, including hemorrhagic stroke and all-cause death [38]. Both dosages of dabigatran were associated with a significantly lower rate of major bleeding, total bleeding, and hemorrhagic stroke than warfarin in Asian patients [38]. Overall,the net clinical benefit of dabigatran at a dosage of 150 mg twice daily was significantly superior to that of warfarin in Asian patients, but not in non-Asian patients (Table 2).
Rivaroxaban
An analysis of ROCKET AF data from East Asian participants reported that for the primary outcome of ischemic stroke or SEEs in patients with a creatinine clearance (CrCL) of 30–49 mL/min, the event rate was 2.63% per year for patients receiving rivaroxaban once daily (15 or 20 mg) and 3.38% per year for patients receiving warfarin (HR 0.78,95% CI 0.44–1.39). These rates did not differ significantly from those in non–East Asian patients receiving rivaroxaban or warfarin (2.09 and 2.39%per year, respectively, HR 0.89, 95% CI 0.75–1.05,Pint= 0.666) [40]. East Asian patients were significantly more likely to develop major or clinically relevant nonmajor (CRNM) bleeding than those from other regions, irrespective of treatment (P< 0.0001);however, use of rivaroxaban and use of warfarin did not differ in bleeding rates [40].
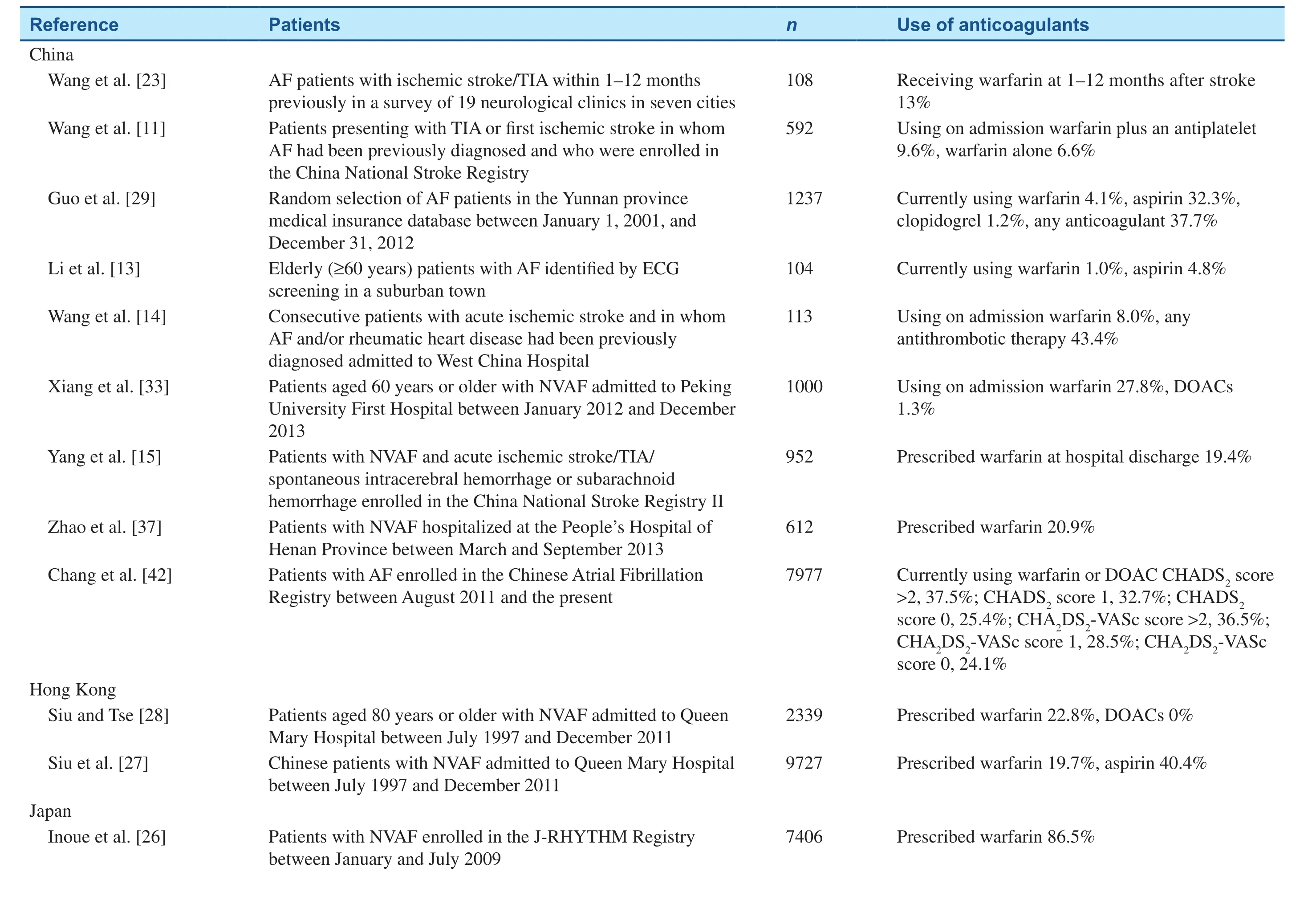
Table 1 Use of Anticoagulants in Patients with Nonvalvular Atrial Fibrillation (NVAF) in East Asia.
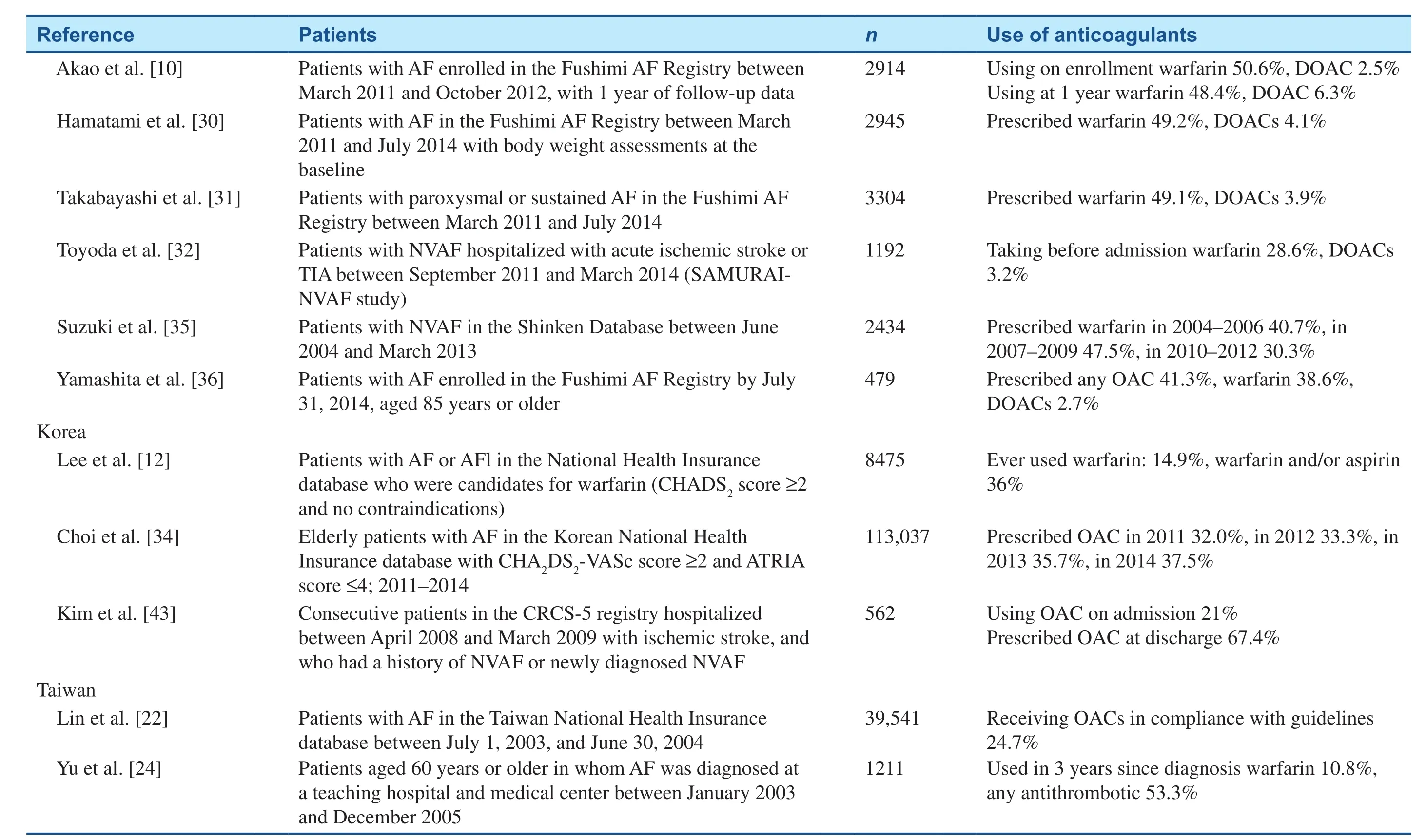
Table 1(continued)
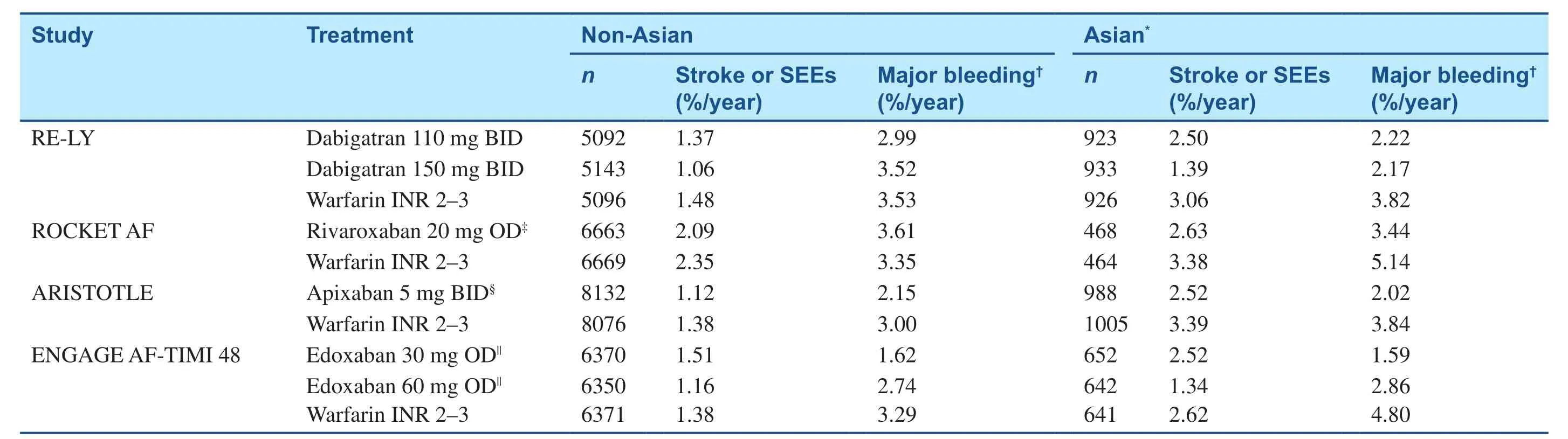
Table 2 Primary Efficacy and Safety End Point Data in the Pivotal Direct Oral Anticoagulant Studies in which Data in Asian Subgroups were Compared with Data in Non-Asian Subgroups [38–41].
In J-ROCKET AF, rivaroxaban once daily (15 mg or 10 mg in those with renal impairment) was noninferior to dose-adjusted warfarin for major and CRNM bleeding (18.04% vs. 16.42%, HR 1.11,95% CI 0.87–1.42); the study was not powered for efficacy analysis [44]. Following a prespecified analysis, the rate of stroke or SEEs was 1.26%per year for patients receiving rivaroxaban versus 2.61% in patients receiving warfarin (HR 0.49, 95%CI 0.24–1.00,P= 0.050) [44].
In an exploratory analysis of J-ROCKET AF, the adjusted net clinical benefit of rivaroxaban [the composite of stroke, non–central nervous system SEEs, all-cause death, myocardial infarction (MI),and major bleeding] was not significantly favorable to that of warfarin [45]. The composite event incidence rate was 4.97% per year with rivaroxaban and 6.11% per year with warfarin, for a difference in the incidence rate of −1.14 percentage points (95% CI–3.40 to 1.12 percentage points) [45].
Apixaban
The ARISTOTLE trial included 1622 patients from East Asia (China, Taiwan, Japan, Hong Kong, and South Korea) and 371 patients from Southeast Asia(Singapore, Malaysia, and the Philippines) [39]. In East Asian patients, apixaban at a dosage of 5 mg twice daily (2.5-mg reduced dose) reduced the rate of stroke or SEEs (HR 0.74, 95% CI 0.50–1.10) and major or CRNM bleeding (HR 0.49, 95% CI 0.35–0.67) versus warfarin (Table 2). The rate of stroke or SEEs in East Asian patients was more than twice the rate in non–East Asian patients, regardless of treatment; additionally, East Asian patients had a significantly higher rate of intracranial hemorrhage (ICH)compared with non–East Asian patients (1.27% per year vs. 0.48% per year,P< 0.0001) [39].
In a phase 2 study, ARISTOTLE-J, the composite major and CRNM bleeding rate was 1.4% (95%CI 0.1–6.9%) for apixaban at dose of 2.5 mg, 1.4%(95% CI 0.1–7.0%) for apixaban at a dose of 5 mg,and 5.3% (95% CI 1.8–12.7%) for warfarin [46].The study was not powered to assess efficacy; however, no incidences of stroke, SEEs, MI, or all-cause deaths occurred in either group receiving warfarin,but three patients receiving warfarin experienced stroke.
Edoxaban
The ENGAGE AF-TIMI 48 study included 1943 patients from Japan, China, Taiwan, South Korea,the Philippines, and Thailand [41]. Patients in this study were randomized to receive warfarin, edoxaban at a dosage of 60 mg once daily, or edoxaban at a dosage of 30 mg once daily. Patients with low body weight, with renal impairment (CrCL = 30–50 mL/min), or taking a strong P-glycoprotein inhibitor received a 50% dose reduction.
The higher-dose edoxaban regimen, but not the lower-dose regimen, compared with warfarin significantly reduced the rate of stroke or SEEs in East Asian patients (HR 0.53, 95% CI 0.31–0.90,P= 0.02; Table 2). Both regimens of edoxaban were associated with a significantly reduced rate of major bleeding relative to warfarin in patients from Asia, although bleeding tended to be higher in Asian patients than in non-Asian patients [41]. The net clinical benefit, encompassing both efficacy and safety end points, significantly favored both regimens of edoxaban in Asian patients [41].
In an ENGAGE AF-TIMI 48 subanalysis of regional differences in Asian patients, non-Japanese East Asian patients (China, South Korea, or Taiwan)treated with higher-dose edoxaban had a significantly lower rate of stroke/SEEs relative to non-Japanese East Asian patients treated with warfarin(HR 0.31, 95% CI 0.14–0.68,P= 0.004), whereas in Japanese patients, the event rate for higher-dose edoxaban was similar to that for warfarin (HR 0.95,95% CI 0.44–2.09,P= 0.91); multivariate analysis showed an interaction of 0.052 [47]. Similarly,non-Japanese East Asian patients receiving higherdose edoxaban had less major bleeding relative to non-Japanese East Asian patients receiving warfarin (HR 0.39, 95% CI 0.21–0.71,P= 0.002), while there was no significant difference in the Japanese patients (HR 0.84, 95% CI 0.51–1.40,P= 0.51). In Japanese patients the time in the therapeutic range(TTR) for the INR was greater than that in other East Asian patients. However, the Japanese cohort was older, contained fewer females, and had a greater number of patients with a history of stroke or transient ischemic attack relative to the non-Japanese East Asian cohort. Additionally, more patients in the Japanese cohort were warfarin naïve, receiving aspirin and amiodarone, were at greater risk of a bleeding event, and required a dose reduction more frequently relative to the non-Japanese East Asian cohort.
Meta-analyses, Postmarketing, and Real-World Analyses in East Asians
As described earlier, Japanese guidelines recommend DOACs as the preferred first-line therapy in patients with AF who require anticoagulation to prevent stroke [7]. The 2014 recommendations were based on phase 3 trial results, and are supported by a 2015 meta-analysis of the dabigatran,rivaroxaban, and apixaban randomized controlled trials [48]. Here, Japanese patients randomized to receive DOACs had a decreased risk of stroke and SEEs [relative risk (RR) 0.45, 95% CI 0.24–0.85,P= 0.01], and showed a nonsignificant trend toward a lower risk of major bleeding (RR 0.66, 95% CI 0.29–1.47), intracranial bleeding (RR 0.46, 95%CI 0.18–1.16), and gastrointestinal (GI) bleeding(RR 0.52, 95% CI 0.25–1.08) relative to Japanese patients randomized to receive warfarin [48].
Researchers in Taiwan using data from the National Health Insurance Research Database examined real-world use of dabigatran and rivaroxaban in patients with NVAF [49, 50]. Compared with warfarin, rivaroxaban and dabigatran were associated with reduced risk of ischemic stroke or SEEs, ICH, and all-cause death [49, 50]. The rates of MI and major GI bleeding did not differ from those with warfarin use [49, 50]. These data are all from observational studies and may be influenced by unknown confounders.
In a single-center observational study of Chinese NVAF patients, the annual incidence of stroke was 1.89% in patients receiving dabigatran at doses of 75, 110, or 150 mg, 1.93% in patients receiving rivaroxaban at a dose of 20 mg, 6.74% in patients receiving rivaroxaban at a dose of15 mg, and 4.95%in patients receiving warfarin who achieved good anticoagulation control [51]. Good INR control was achieved by only 16.3% of patients receiving warfarin. The annual incidence of ICH was 0.89% for patients receiving warfarin and 0.46% for patients receiving a DOAC; most of the incidences of ICH (88%) occurred in patients with TTR of less than 65%. The incidence of ICH was 0.95% for patients receiving warfarin with TTR of less than 65%, 0.58% for patients receiving warfarin with TTR of 65% or greater, 0.52% for patients receiving rivaroxaban, and 0.39% for patients receiving dabigatran.
A postmarketing surveillance study of dabigatran in Japanese NVAF patients (n= 6148) included patients starting dabigatran (110 or 150 mg) treatment between December 2011 and November 2013[52]. Of these patients, 1701 (27.7%) switched from warfarin and 4407 (71.7%) were OAC naïve[52]. During follow-up (duration 498 ± 259 days),five MIs (0.06 per 100 patient-years), 46 serious hemorrhages (0.55 per 100 patient-years), and 11 nonhemorrhagic GI disorders (0.13 per 100 patientyears) occurred [52]. Overall, 15 patients had fatal adverse events (0.18 per 100 patient-years): four patients had a fatal hemorrhage, two patients had a cerebral infarction, one patient had pneumonia,one patient had anoxic encephalopathy, and seven patients died of unknown causes [52]. Although 10.3% of dabigatran recipients received concomitant antiplatelet therapy, its impact on outcomes was not specifically assessed. In a nationwide retrospective cohort study conducted in Japan, 21.5%of patients who developed an ICH during DOAC treatment were taking antiplatelet therapy [53]. Of the 130 patients with ICH during DOAC therapy,87 had cerebral hemorrhages. However, the incidence of hematoma expansion and death was lower with DOACs (14 and 17%, respectively) than with warfarin on the basis of historical Japanese data (26 and 35%, respectively) [53].
Treatment persistence is an important consideration in the real-world efficacy of anticoagulants. In a retrospective study of Japanese NVAF patients who had newly started DOAC or warfarin therapy, 28% of patients (113 of 401) receiving dabigatran, rivaroxaban, or apixaban, and 17% of patients (33 of 200) receiving warfarin discontinued treatment within 22–24 months [54]. During the first 12 months of treatment, a larger proportion of patients discontinued rivaroxaban or apixaban therapy versus warfarin therapy; the discontinuation rate was significantly higher for dabigatran therapy (34%) versus warfarin therapy (17%) (HR 2.19, 95% CI 1.44–3.34,P< 0.01) [54]. Common reasons for patient discontinuation of anticoagulation therapy were drug adverse events, worsening renal dysfunction, and patient choice [54].Similarly, in a Japanese study conducted at a single hospital enrolling AF patients with a CHADS2score greater than 1 and CrCL > 30 mL/min who were prescribed dabigatran (n= 177) or rivaroxaban(n= 179), a greater proportion of patients receiving dabigatran discontinued treatment relative to those prescribed rivaroxaban (27.7% vs. 13.4%,P< 0.001) [55]. Compared with patients receiving dabigatran, patients receiving rivaroxaban were older, had higher CHADS2and HAS-BLED scores,and were less likely to receive a dose reduction [55].Bleeding occurred at a similar rate for rivaroxaban(15.1%) and dabigatran (15.8%). Dyspepsia was more frequent with dabigatran relative to rivaroxaban (18.6% vs. 2.8%,P< 0.001), and was the most common cause of discontinuation in the dabigatran group (33 patients), whereas only five patients who discontinued rivaroxaban therapy cited dyspepsia as the cause. The most common reason for discontinuation of rivaroxaban therapy was subcutaneous bleeding (n= 13), which was reported by 11 patients[55]. No independent risk factors were identified for bleeding in the dabigatran group, but use of antiplatelet therapy was an independent risk factor for bleeding with rivaroxaban [55].
Considerations for the Elderly and in Patients with Hypertension or Renal Dysfunction
In a subanalysis of J-ROCKET AF, hypertension,age 75 years or older, and moderate renal impairment (CrCL < 50 mL/min) did not significantly affect the rates of stroke/SEEs [56, 57]. Similarly,hypertension and age did not affect the incidence of the composite of major or CRNM bleeding [57, 58];however, the rate of major or CRNM bleeding was significantly increased in patients aged 75 years or older treated with rivaroxaban (25.05% per year)relative to warfarin (16.95% per year; HR 1.49,95% CI 1.02–2.16,P= 0.04) [56].
A retrospective analysis of 196 Japanese AF patients receiving dabigatran at a dosage of 110 mg twice daily assessed the incidence of bleeding events and anticoagulant activity, measured by activated partial thromboplastin time, in patients with preserved renal function (CrCL ≥ 50 mL/min;n= 127) and moderately impaired renal function(CrCL = 30–49 mL/min;n= 69) [59]. Compared with patients with normal renal function, patients with impaired renal function were older (mean age 76.9 years vs. 67.6 years), were lighter (mean body weight 51.9 kg vs. 64.0 kg), were more likely to have congestive heart failure (38% vs. 17%), and had higher mean CHADS2(2.6 vs. 1.8), CHA2DS2-VASc (4.3 vs. 3.2), and HAS-BLED (2.3 vs. 2.0)scores (allP< 0.01) [59]. Despite these differences,the incidence of major or minor bleeding did not significantly differ between the patients with normal or impaired renal function [59]. Additionally,the anticoagulant effect of dabigatran, as indicated by postadministration activated partial thromboplastin time, was similar in patients with impaired renal function versus patients without impaired renal function [59]. A real-world analysis of the efficacy and safety of dabigatran and rivaroxaban in patients with renal dysfunction (estimated glomerular filtration rate less than 60 mL/min) showed efficacy comparable to that of warfarin and a reduction of rates of major bleeding [60].
Use of DOACs in East Asian Patients Undergoing Ablation
There have been no large phase 3 studies assessing the efficacy and safety of uninterrupted DOAC anticoagulation in East Asian patients undergoing ablation for AF. However, the Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF)national survey examined outcomes in 3373 patients undergoing ablation, of whom 504 patients received dabigatran, 37 received rivaroxaban, 1808 received warfarin, and 1024 received no periprocedural OACs [61]. The incidence of complications was lower in DOAC-treated patients (2.6%;14/541) than in patients receiving uninterrupted warfarin therapy (4.8%,P< 0.05) [61]. Pericardial effusions occurred less commonly following DOAC treatment (0.7%) than in warfarin-treated patients (2.6%,P< 0.05). Cerebral infarction developed in one patient receiving a DOAC, one patient receiving warfarin, and one patient not receiving an anticoagulant [61]. Similar results were seen in a smaller prospective randomized registry of 176 patients with AF [62]. Here, patients taking warfarin on admission continued taking warfarin (n= 70),and patients taking a DOAC were randomized to receive either rivaroxaban (n= 55) or apixaban(n= 51). No patients developed a symptomatic cerebral infarction; however, asymptomatic cerebral microthromboembolism occurred in comparable numbers of patients per group: nine patients receiving rivaroxaban (16.4%), 10 receiving apixaban(20%), and 13 receiving warfarin (18.8%) [62].Hemopericardium occurred in five patients (2.8%):two receiving rivaroxaban, one receiving apixaban,and two receiving warfarin [62].
Three studies in Japanese patients undergoing ablation have compared outcomes in those receiving warfarin or dabigatran [63–65]: two prospectively studied consecutive patients [63, 64] and one had a randomized controlled design [65]. In one of the consecutive analyses, dabigatran use was discontinued 12–24 hours before the procedure and restarted 3 hours after the procedure [63]. Patients taking warfarin (n= 126) discontinued treatment 3 days before the procedure and were bridged with unfractionated heparin until the procedure, after which they received heparin, in combination with warfarin, which was administered for the first 4 days,from 3 hours after the procedure until a therapeutic INR was reached. Heparin use was then discontinued and patients received warfarin alone thereafter [63]. There was no significant difference in the incidence of major bleeding (three events in the dabigatran group and four in the warfarin group),cardiac tamponade (three cases in each group),intracranial bleeding (one event in the warfarin group and none in the dabigatran group), or minor bleeding (five cases in each group) [63]. Patients receiving dabigatran had a significantly shorter hospital stay than those taking warfarin (7.2 ± 2.0 days vs. 10.3 ± 3.9 days,P= 0.0001), possibly because of the simpler anticoagulation protocol relative to the heparin bridging strategy [63].
The randomized comparison of dabigatran and warfarin in patients undergoing ablation also used a heparin bridging strategy. Patients were randomized to receive dabigatran (n= 45) or warfarin (n= 45)and then underwent transesophageal echocardiographic assessment 3 weeks later in the case of dabigatran or 3 weeks after achieving the target INR in the case of warfarin. Following confirmation of the absence of atrial thrombi, patients were scheduled for ablation. On the morning of the procedure,patients did not take their usual dose of anticoagulant, and received heparin intravenously during the procedure. Because of the need to establish optimal anticoagulation in the warfarin recipients, the mean time from anticoagulant use initiation to ablation was significantly shorter in the dabigatran group than in the warfarin group (43 days vs. 63 days,P< 0.0001) [65]. Rebleeding from the venipuncture site was less common in dabigatran-treated patients than in warfarin-treated patients (20% vs. 44%,P= 0.013), but 7% of patients receiving dabigatran needed to discontinue treatment because of dyspepsia [65].
No heparin bridging strategy was used in a comparison of consecutive patients receiving either dabigatran (n= 110) or warfarin (n= 101) before catheter ablation [64]. In this study, dabigatran use was discontinued on the morning of the procedure, and restarted again 1 day after the procedure,while warfarin was used without interruption [64].No periprocedural deaths or symptomatic thromboembolic complications occurred in this study[64]. Five patients receiving dabigatran (4.5%) and 13 patients receiving warfarin (12.9%) developed bleeding complications (P< 0.05), primarily groin hematoma at the puncture site (five cases in the dabigatran group and 11 in the warfarin group), but two patients in the warfarin group developed cardiac tamponade [64].
Conclusions
Throughout East Asia, OACs are underused in patients with AF, particularly in patients at greatest risk of stroke or SEEs. DOACs have efficacy similar to that of warfarin in East Asian patients with NVAF, and may be associated with a reduced risk of major bleeding. This also appears to be the case in patients undergoing catheter ablation and in subgroups of high-risk patients, although to date the data are limited to studies in Japanese patients,and more data on high-risk patients from other East Asian countries are needed. Nevertheless, despite the efficacy and safety profile of DOACs in East Asian patients, the rate at which patients discontinue use of these agents is still unacceptably high,emphasizing the need for improving patient and provider treatment and education.
Acknowledgments
Writing and editorial support was provided by Terri Schochet of AlphaBioCom LLC, and was funded by Daiichi Sankyo Inc.
Conflict of lnterest
Chang-Sheng Ma has received research grants and speaker fees from Bristol-Myers Squibb,Pfizer, Bayer, Boehringer-Ingelheim, and Biosense Webster, and speaker fees from Daiichi Sankyo Ltd.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Cardiovascular Innovations and Applications
- The Contemporary Role of Femoral Artery Access
- Persisting Angina after Successful Surgical Removal of a Large Coronary Artery Aneurysm Attached to the Proximal Portion of the Left Circumflex Artery: Role of Coronary Artery Spasm
- Speckle Tracking Echocardiography ldentifies lmpaired Longitudinal Strain as a Common Deficit in Various Cardiac Diseases
- Bioresorbable Vascular Scaffold in the Midportion of the Left Anterior Descending Artery for Cardiac Allograft Vasculopathy in a Cardiac Transplant Patient
- Current Status of Coronary Atherectomy