Effect of point-moxibustion on ghrelin and GHSR-1a expressions in lateral septal nucleus of rats with diabetic gastroparesis
2018-09-12LiuMi刘密LiuYi刘祎LiuWeiwei刘薇薇WangChao王超TanChengfu谭成富LiuLi刘丽PengYan彭艳ChangXiaorong常小荣LiJing李璟YanJie严洁
Liu Mi (刘密), Liu Yi (刘祎), Liu Wei-wei (刘薇薇), Wang Chao (王超), Tan Cheng-fu (谭成富), Liu Li (刘丽),Peng Yan (彭艳), Chang Xiao-rong (常小荣), Li Jing (李璟), Yan Jie (严洁)
Abstract Objective: To observe the effect of point-moxibustion on gastrointestinal motility, mRNA and protein expressions of ghrelin and growth hormone secretagogue receptor 1a (GHSR-1a) in lateral septal nucleus of rats with diabetic gastroparesis (DGP),and to investigate the central regulatory mechanism of DGP treatment with point-moxibustion.Methods: Forty SPF-grade Sprague-Dawley (SD) rats were randomly divided into a blank group, a model group, an electroacupuncture (EA) group and a point-moxibustion group, with 10 rats in each group. A DGP rat model was established by intraperitoneal injection of 2% streptozotocin (STZ) with 8-week irregular high-sugar and high-fat diet in the model group,the EA group and the point-moxibustion group; and rats in the blank group were injected intraperitoneally with 0.1 mmoL/L(pH 4.5) citric acid-sodium citrate buffer with 8-week normal diet. Eight weeks later, rats in the EA group were treated by EA at Zusanli (ST 36), Liangmen (ST 21) and Sanyinjiao (SP 6); while rats in the point-moxibustion group were treated by point-moxibustion at Zusanli (ST 36), Liangmen (ST 21) and Sanyinjiao (SP 6) for successive 15 d. Rats in the blank group and the model group were fixed as the control without intervention. After treatment, intestinal propulsion rate and gastric emptying rate were measured. The mRNA and protein expressions of ghrelin and GHSR-1a in the lateral septal nucleus were detected by real-time polymerase chain reaction (RT-PCR) and Western blot (WB).Results: Compared with the blank group, the intestinal propulsion rate and gastric emptying rate of the model group were significantly lower (both P<0.01); compared with the model group, the intestinal propulsion rate and gastric emptying rate of the EA group and the point-moxibustion group increased significantly (all P<0.05). The mRNA and protein expressions of ghrelin and GHSR-1a were lower in the model group than those in the blank group (all P<0.01). The mRNA and protein expressions of ghrelin and GHSR-1a were significantly higher in the EA group and the point-moxibustion group than those in the model group (all P<0.05). There were no statistically significant differences between the EA group and the point-moxibustion group (all P>0.05).Conclusion: Point-moxibustion at Zusanli (ST 36), Liangmen (ST 21) and Sanyinjiao (SP 6) can increase the intestinal propulsion rate and gastric emptying rate of DGP rats, and promote the mRNA and protein expressions of ghrelin and GHSR-1a in the central nervous system. The mechanism may be related to the activation of ghrelin pathway in hypothalamic arcuate nucleus-lateral septal nucleus.
Keywords: Acupuncture Therapy; Electroacupuncture; Moxibustion Therapy; Ghrelin; Receptors, Ghrelin; Diabetes Complications; Gastroparesis; Rats
Diabetes mellitus (DM) has become the third largest non-communicable disease following cardiovascular disease and cancer[1]. The global prevalence of DM has shown a significant increasing trend[2], and patients with diabetic gastroparesis (DGP) account for half of those with DM[3]. Ghrelin is a brain-gut peptide hormone widely expressed in various tissues and a natural ligand of endogenous growth hormone secretagogue receptor(GHSR). Studies have found that ghrelin participates in regulation of gastric motility by binding to peripheral and central receptors[4], and one is the central ghrelin pathway promoting gastric effects in the hypothalamic arcuate nucleus-lateral septal nucleus[5]. A large number of studies have confirmed the therapeutic efficacy of acupuncture for DGP[6]. Therefore, based on our previous studies[7-9], the electroacupuncture (EA) group was selected as the control in current study, and a DGP rat model was established to observe the effect of point-moxibustion on the small intestinal propulsion rate, gastric emptying rate, and ghrelin and GHSR-1a mRNA and protein expressions in lateral sepal nucleus of DGP rats, thus to investigate the central regulatory mechanism of point-moxibustion for DGP.
1 Materials and Methods
1.1 Animals and grouping
Forty healthy, SPF Sprague-Dawley (SD) rats, half male and half female, weighing (210±10) g, were purchased from Hunan SJA Laboratory Animal Co., Ltd.,China. Animal certificate number: 43004700019099.Rats were housed in the Experimental Animal Center of Hunan University of Chinese Medicine, with the temperature of 22-25 ℃ and the humidity of 40%-60%, and adaptive feeding for 1 week. Rats with normal blood glucose were included after measure of the blood glucose with a blood glucose meter. The rats were divided into a blank group, a model group, an EA group and a point-moxibustion group according to the random number table method, with 10 rats in each group.
1.2 Main reagents and instruments
Hydrated chloral (batch number: 2015126,Sinopharm Chemical Reagent Co., Ltd., China);streptozotocin (STZ, batch number: S0130-1G, Sigma,USA); phenol red (batch number: SHPBY-50, Tianjin Guangfu Fine Chemicals Institute, China); sodium citrate(batch number: 20140905, Sinopharm Chemical Reagent Co., Ltd., China); citric acid (batch number:20140619, Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd., China); reverse transcription kit(Beijing Kangwei Century, China); trizol (Invitrogen, USA);ghrelin antibody (cat number: ab95250, Abcam, UK);GHSR-1a antibody (cat number: Bs-11529R, Beijing Biosynthesis Biotechnology Co., Ltd., China); self-made high-fat and high-sugar diet (ordinary feed, cooked lard,cane sugar, milk powder and egg at 58:15:20:5:2);β-actin (cat number: 60008-1-Ig, Proteintech, USA); HRP goat anti-mouse immunoglobulin G (IgG, Proteintech,USA); developer solution (Wellbiology, China); primer[GenScript (Nanjing) Co., Ltd., China]; SYBGreen polymerase chain reaction (PCR) mix (Invitrogen, USA);BCA protein quantitative kit (Wellbiology, China).
OneTouch UltalEasy blood glucose meter and test strips (Johnson & Johnson, USA); urine glucose test strips (Guangzhou Huadu Gaoerbao Biological Technology Co., Ltd., China); Hwato Brand SDZ-V EA apparatus (Suzhou Medical Appliance Factory Co., Ltd.,China); Hwato Brand acupuncture needle (specification:0.30 mm in diameter and 13 mm in length, Suzhou Medical Appliance Factory Co., Ltd., China); Qianqiu Brand Zhou’s all-purpose moxibustion pen (specification of drug pen: 115 mm × 4 mm, specification of drug paper: 125 mm × 20 mm (Shoumin Moxibustion Factory,Tianchang City, Anhui Province, China); shaker (model:TS-92, Jiangsu Qilin Beier, China); bench-top refrigerated centrifuge (model: TGL-18R, Eppendorf, Germany);electrophoresis apparatus (model: 164-5050, Bio-rad,USA); electrophoresis tank (model: DYCZ-24EN, Beijing Liuyi Instrument Factory, China); transmembrane apparatus (model: DYCZ-40A, Beijing Liuyi Instrument Factory, China); fluorescent quantitative PCR instrument(model: PIKO REAL 96, Thermo, USA); fluorescent PCR plate (model: SPL0960, Thermo, USA); brain stereotaxic apparatus (Chengdu Instrument Factory, China).
1.3 Modeling method
Rats were divided into a blank group (n=10) and a DGP model group (n=30) by the random number table.
DGP model was established in the DGP model group based on the literatures[6-9]. After fasting for 12 h, rats were injected with 2% STZ at a concentration of 55 mg/(kg·bw) in the left lower abdominal cavity, which was prepared with 0.1 mmol/L (pH 4.5, 4 ℃) citric acid-sodium citrate buffer just before use. Rats in the blank group were injected intraperitoneally with equal volume of 0.1 mmoL/L citric acid-sodium citrate buffer.After 72 h, blood was drawn from tail vein for blood glucose test, and rats with immediate blood glucose≥16.7 mmol/L were included. Rats in the blank group were regularly fed with normal diet; rats in the DGP model group were irregularly fed with high-sugar and high-fat diets (ordinary feed, cooked lard, cane sugar,milk powder and eggs at 58:15:20:5:2) (in the morning of odd-numbered days and in the afternoon of even-numbered days) for 8 weeks. The criteria of successful rat DGP model establishment: blood glucose≥16.7 mmol/L; characteristics of spirit, behavior, coat color and stool were significantly different within the 8 weeks of feeding versus those in the blank group; small intestinal propulsion rate and/or gastric emptying rate were significantly different from those in the blank group. The successful DGP rat models were randomly divided into a model group, an EA group and a pointmoxibustion group using the random number table method, with 10 rats in each group.
1.4 Acupoint positioning
Acupoint positioning: Referring to common animal acupoint positioning in the Experimental Acupuncture Science[10].
Zusanli (ST 36) is located at the posterolateral side of the knee joint, approximately 5 mm below the capitulum fibulae.
Liangmen (ST 21) is located at the intersection of the horizontal line of the midpoint between xiphosternal synchondrosis and navel with the midclavicular line.Navel is at the intersection of the lower 1/4 and the upper 3/4 of the line between xiphosternal synchondrosis and pubic symphysis.
Sanyinjiao (SP 6) is located at 10 mm directly above the medial malleolus tip of the hind limb.
1.5 Interventions
Rats in the blank group and the model group were fixed for 20 min, same as those in the other groups,without acupuncture or moxibustion, once a day for 15 d. Rats in the EA group were fixed on a rat plate in a supine position, and received perpendicular needling at monolateral Zusanli (ST 36), Liangmen (ST 21) and Sanyinjiao (SP 6) to a depth of 0.3 cm. The points respectively 2 cm straight below the three acupoints were needled perpendicularly to a depth of 0.2 cm,taken as the attachment points for EA. The Hwato Brand SDZ-V type EA instrument was attached to the three acupoints and the matched attachment points.Sparse-dense wave (10 Hz/50 Hz) was applied with the intensity causing slight trembling of rat’s lower extremities, 20 min/time, once a day, for successive 15 d. Bilateral acupoints were used alternately.
Rats in the point-moxibustion group were fixed on the rat plate in a supine position, and monolateral Zusanli (ST 36), Liangmen (ST 21) and Sanyinjiao (SP 6)were selected. Hair around the acupoints (0.4 cm in diameter) was shaved. The drug-containing side of the drug paper in the Zhou's all-purpose point-moxibustion pen set was flatly and closely attached to the designated acupoints. Lighted point-moxibustion pen was used to press 5 times at each acupoint at 0, 10 and 20 min after the fixing, once a day for successive 15 d.Bilateral acupoints were used alternately.
All rats were fed with normal diet during the treatment.
1.6 Observed items and detection methods
1.6.1 Determination of gastric emptying rate[11-12]
Rats in each group received intragastric administration of 2 mL phenol red solution before removal of the stomach and were sacrificed 20 min later.The separated stomach was cut along the arcus major ventriculi to flush the stomach contents with distilled water to a volume of 20 mL. Added 20 mL of 0.5 mol/L NaOH solution and mixed by stirring, standing by 60 min. Protein was removed by adding 0.5 mL of 20%trichloroacetic acid in 5 mL of supernatant and centrifuged at 3 500 r/min for 10 min. The optical density (OD) was measured with a spectrophotometer at a wavelength of 560 nm. The rat stomach emptying rate was calculated according to the following formula:Gastric emptying rate = (1 – Measured phenol red OD value ÷ Standard phenol red OD value) × 100%.
1.6.2 Small intestinal propulsion rate[11]
The removed small intestine was spread on white paper, and the length from the pylorus to the ileocecal part (full length of the small intestine) and the distance from pylorus to phenol red (the migration distance of phenol red in the small intestine) were measured. The following formula was used to calculate the rate of small intestinal propulsion. The small intestinal propulsion rate = Migration distance of phenol red in small intestine ÷ Full length of small intestine × 100%.
1.6.3 Real-time PCR (RT-PCR) detection
The lateral septal nucleus (0.12-2.04 mm in front of the anterior fontanelle, 0.70-1.35 mm beside the midline, and 5.50-5.80 mm below the skull) was located with a rat brain stereotaxic apparatus after the rats were sacrificed by dislocation, and separated. A total of 1 mL of trizol was added into about 0.02 g of brain tissue to prepare homogenate; trichloromethane was added to the mixture followed by shaking and centrifugation, and then an equal volume of isopropanol was added to the mixture and sit for a while. After centrifugation at low temperature, the supernatant was discarded and the white precipitate was collected, and then ethanol was added to vortex.Centrifuged at low temperature and discarded the supernatant, and added sterile enzyme-free water to dissolve the precipitate. The OD at 260 nm and 280 nm was determined using 2 μL of RNA solution by UV spectrophotometer, and the concentration and purity were calculated. Reverse transcription of cDNA for PCR amplification reaction (actin as an internal reference,primers are shown in Table 1). The cycle threshold (Ct)value was obtained to calculate the 2-△△Ct, which represented the relative expression level of each test gene in the test sample versus the control sample. The average values were obtained from triplication of independent experiments.
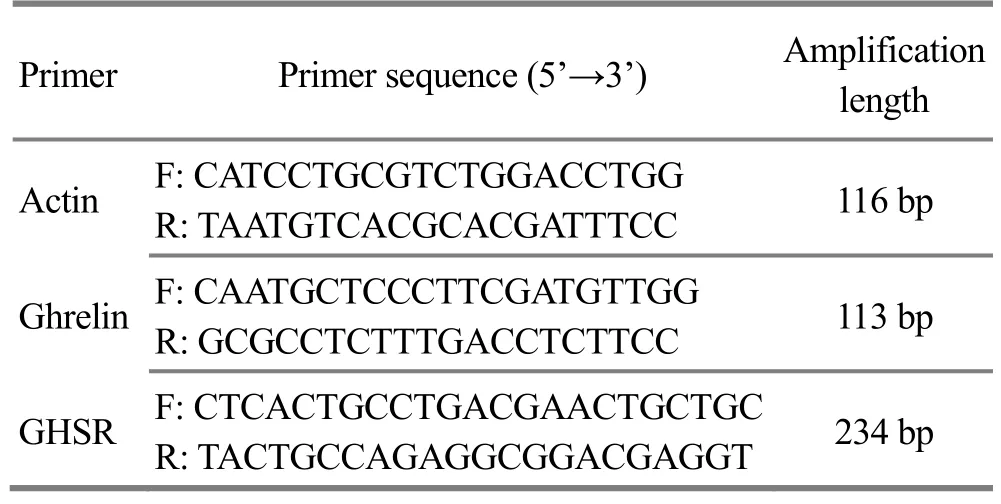
Table 1. Primer sequences and amplification lengths
1.6.4 Western blot (WB) testing
The brain tissue (0.25 g) was homogenated and lysed on ice for 30 min to collect the supernatant for protein(i.e., tissue protein) after centrifugation. The protein concentration was measured using the BCA protein quantification kit. Purified protein was mixed with 5 ×loading buffer and boiled for 5 min, and then separated by SDS-PAGE electrophoresis. The electrophoresis was stopped when the bromophenol blue was electrophoresed to the bottom of the gel. The PVDF membrane and the filter paper were sequentially set and soaked completely in the transmembrane buffer solution, and the proteins in the gel were transferred to the PVDF membrane. After rinsing, the membrane was incubated with diluted primary antibodies (ghrelin at 1:1 500; GHSR-1a at 1:200; β-actin at 1:4 000) and HRP-labeled secondary antibody (1:3 000 dilution) in turn; developed with ECL chemiluminescence solution.The exposed film was scanned and analyzed using quantity one, a professional grayscale analysis software.
1.7 Statistical analysis
The SPSS version 17.0 software for Windows was used for data analysis. The measurement data were first tested for normal distribution and homogeneity of variance, and those who met normal distribution were expressed as mean ± standard deviation (±s), and multiple comparisons were performed using one-way ANOVA. The least significant difference (LSD) method was used for homogeneity of variance, and the Tamhane method was performed when the variance was heterogeneity. P<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Comparison of rat’s blood glucose
After 8-week modeling, compared with the blank group, the blood glucose levels of the model group, the EA group and the point-moxibustion group were significantly increased (all P<0.01), indicating the successful modeling. After treatment, compared with the model group, the blood glucose levels of the EA group and the point-moxibustion group were significantly decreased (P<0.01, P<0.05), indicating that both EA and point-moxibustion treatment were effective. There was no significant difference in blood glucose level between the EA group and the point-moxibustion group (P>0.05), (Table 2).
Table 2. Comparison of blood glucose (±s, mmol/L)

Table 2. Comparison of blood glucose (±s, mmol/L)
Note: Compared with the blank group, 1) P<0.01; compared with the model group, 2) P<0.05, 3) P<0.01
Group n After modeling After treatment Blank 10 6.09±1.00 5.61±1.24 Model 10 31.17±1.981) 28.40±2.481)EA 10 30.58±2.281) 23.15±3.231)3)Point-moxibustion 10 30.60±1.741) 24.65±2.831)2)
2.2 Comparisons of the small intestinal propulsion rate and gastric emptying rate
Compared with the blank group, the small intestinal propulsion rate and gastric emptying rate of the model group were significantly decreased (both P<0.01),indicating that the gastrointestinal dysfunction appeared in the diabetic model rats, and the DGP model was successfully prepared. Compared with the model group, the intestinal propulsion rate and gastric emptying rate of the EA group and the pointmoxibustion group were significantly increased (all P<0.05). There were no significant differences in small intestinal propulsion and gastric emptying rates between the EA group and the point-moxibustion group(both P>0.05), (Table 3).
Table 3. Comparisons of small intestinal propulsion rate and gastric emptying rate (±s, %)
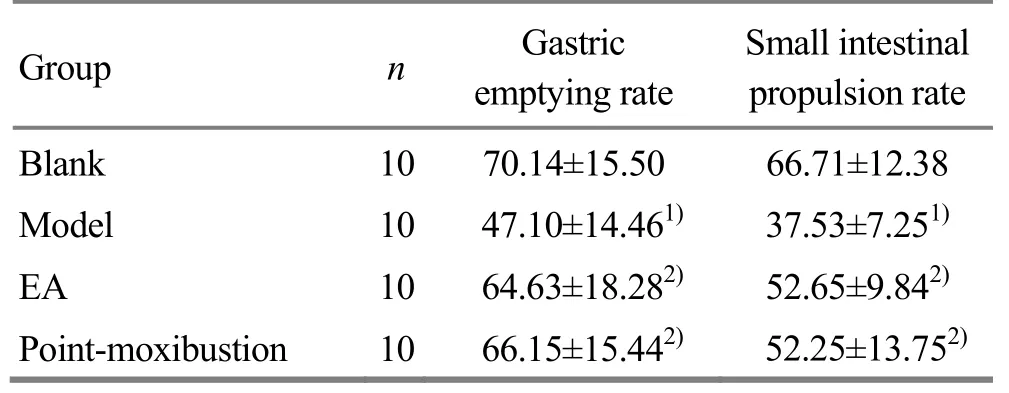
Table 3. Comparisons of small intestinal propulsion rate and gastric emptying rate (±s, %)
Note: Compared with the blank group, 1) P<0.01; compared with the model group, 2) P<0.05
?
2.3 Comparison of ghrelin and GHSR-1a mRNA expressions in rat’s lateral septal nucleus
Compared with the blank group, the mRNA expressions of ghrelin and GHSR-1a in the model group were significantly lower (both P<0.01). Compared with the model group, the mRNA expressions of ghrelin and GHSR-1a in the EA group and the point-moxibustion group were significantly increased (all P<0.05). There were no significant differences in ghrelin and GHSR-1a mRNA expressions between the point-moxibustion group and the EA group (both P>0.05), (Table 4).
Table 4. Comparison of ghrelin and GHSR-1a mRNA expressions in rat’s lateral septal nucleus (±s)
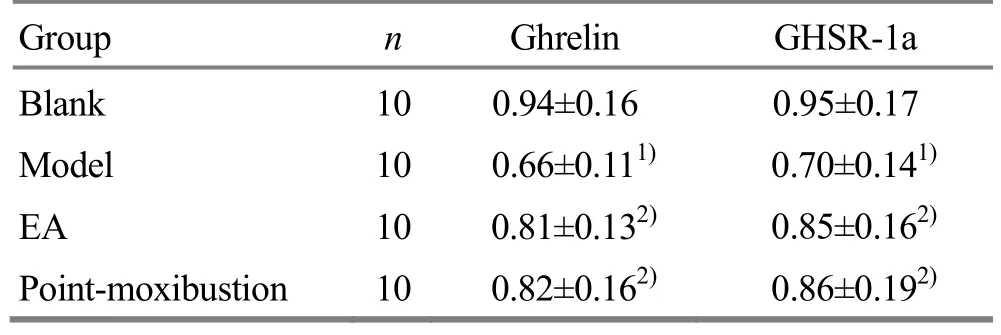
Table 4. Comparison of ghrelin and GHSR-1a mRNA expressions in rat’s lateral septal nucleus (±s)
Note: Compared with the blank group, 1) P<0.01; compared with the model group, 2) P<0.05
Group n Ghrelin GHSR-1a Blank 10 0.94±0.16 0.95±0.17 Model 10 0.66±0.111) 0.70±0.141)EA 10 0.81±0.132) 0.85±0.162)Point-moxibustion 10 0.82±0.162) 0.86±0.192)
2.4 Comparison of ghrelin and GHSR-1a protein expressions in rat’s lateral septal nucleus
Compared with the blank group, the expressions of ghrelin and GHSR-1a proteins in the model group were significantly decreased (both P<0.01). Compared with the model group, the expressions of ghrelin and GHSR-1a proteins in the EA group and the pointmoxibustion group were significantly increased (all P<0.05). There were no significant differences in the expressions of ghrelin and GHSR-1a proteins between the point-moxibustion group and the EA group (all P>0.05), (Table 5 and Figure 1).
Table 5. Comparisons of ghrelin and GHSR-1a protein expressions in rat’s lateral septal nucleus (±s)
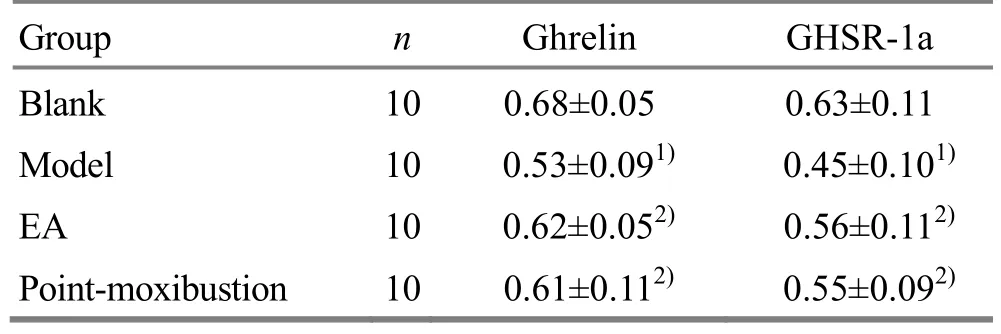
Table 5. Comparisons of ghrelin and GHSR-1a protein expressions in rat’s lateral septal nucleus (±s)
Note: Compared with the blank group, 1) P<0.01; compared with the model group, 2) P<0.05
Group n Ghrelin GHSR-1a Blank 10 0.68±0.05 0.63±0.11 Model 10 0.53±0.091) 0.45±0.101)EA 10 0.62±0.052) 0.56±0.112)Point-moxibustion 10 0.61±0.112) 0.55±0.092)
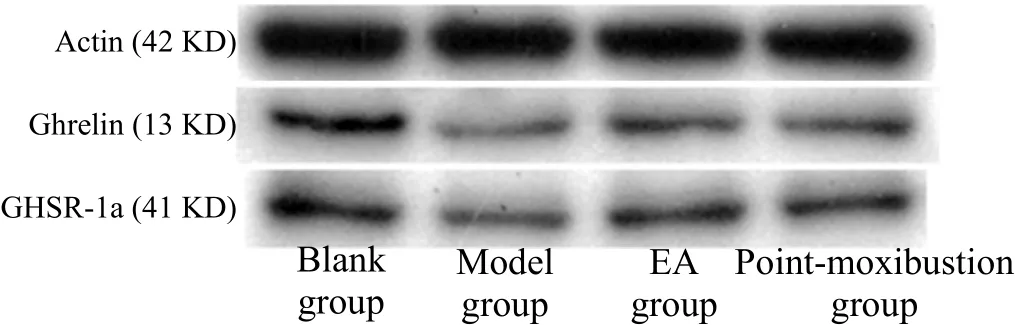
Figure 1. Ghrelin and GHSR-1a protein concentrations in rat’s lateral septal nucleus detected by WB
3 Discussion
Patients with DGP present gastrointestinal symptoms such as belch, postprandial fullness, nausea and vomiting. Therefore, this disease belongs to the vomiting and fullness in Chinese medicine, which is located in the stomach and related to the liver and spleen. It is a syndrome of combined deficiency and excess, which is based on spleen deficiency and dampness retention, liver qi stagnation, and spleen-stomach weakness, while the manifestations are qi stagnation, phlegm stasis and blood stasis[13].Acupuncture can stimulate the spleen and stomach,unblock the meridians and coordinate organs, and is widely used in the prevention and treatment of DGP with reliable clinical efficacy[14-15]. Moxibustion can warm the meridians, regulate the organs with unique effects versus needles and medicines.
Moxibustion with point-moxibustion pen is also known as quick moxibustion of Zhou’s all-purpose point-moxibustion pen. It is a moxibustion method consisting of a drug pen and a drug paper. It is a new type of moxibustion tool that was developed by Professor Zhou Mei-sheng on the basis of ancient moxibustion methods of thunder-fire needling and Yangsui ingot.
Compared with acupuncture or moxibustion alone,point-moxibustion is a complex therapeutic method including medicine, moxa, warming and pressing. The shape of the point-moxibustion pen is thin with a small contact area tothe acupoint, so that the acupoint positioning is more accurate, and it is convenient for scientific research, especially for rats and other small experimental animals. At the same time, this method has the advantages of simple use, short operation time,safety, basically painless, and flexible selection of acupoints, and the effective time can be maintained for 6-8 h. It is applicable to the diseases of multiple systems[16-17]. It has become a clinical technology promotion project of traditional Chinese medicine for the treatment of fullness[18]. Our previous research showed that EA promoted gastric emptying in DGP rats,and controlled blood and urine glucose[7-9]. Therefore, a DGP rat model was established to observe the effect and mechanism of point-moxibustion on ghrelin and GHSR-1a expressions in lateral sepal nucleus of rats with DGP, with EA treatment as the control.
Ghrelin is produced by gastric mucosa, and is also secreted in the hypothalamus, pituitary gland and other sites. It has been reported that the early diabetesassociated polyphagia is related to increased ghrelin secretion in stomach; lower plasma ghrelin level in late diabetic patients causes a series of symptoms of gastroparesis[19]. GHSR is a receptor of ghrelin and widely distributed in the central and peripheral regions.It is divided into type 1a and type 1b due to the coding sequence of exons. In the periphery, ghrelin achieves contraction of gastrointestinal smooth muscle and promotes gastric motility, by binding to GHSR receptors in gastric antrum smooth muscle cells and inhibiting calcium-dependent potassium channels to increase intracellular Ca2+concentration[20]. In the central nervous system, by binding to its receptor GHSR-1a,ghrelin stimulates nerve impulses in the vagal and cholinergic pathways, promotes gastric acid secretion and gastrointestinal motility, and increases gastric emptying[21]. Experimental studies have shown that DGP gastric dyskinesia is closely related to the decrease of central and peripheral ghrelin gene expression[22].The results of this experiment suggested that gastric dysregulation was associated with a decrease in the expressions of ghrelin and GHSR-1a mRNAs in the central nervous system, which is consistent with previous studies. This study further found that pointmoxibustion at Zusanli (ST 36) and other acupoints promoted gastric movement. The mechanism may be the up-regulated mRNA expressions of central ghrelin and GHSR-1a and binding of ghrelin to its receptors.
Studies have confirmed that a series of physiological effects caused by central ghrelin are mainly produced in the hypothalamus. Ghrelin immunoreactive neurons express in hypothalamic arcuate nucleus[23-24]. The researchers have found that injection of different doses of ghrelin in the lateral septal nucleus significantly promoted gastric motility[25]. At the same time, it has been found that there is a projection of ghrelin immunoreactive neurons in the arcuate nucleus toward the lateral septal nerve nucleus pathway, and clarify the existence of ghrelin energy nerve pathway in hypothalamic arcuate nucleus-lateral septal nucleus[5].The results of our current study showed that the protein concentrations of ghrelin and GHSR-1a in the lateral septal nucleus of DGP rats were significantly reduced.The ghrelin and GHSR-1a proteins in the lateral septal nucleus were increased by point-moxibustion at Zusanli(ST 36) and other acupoints. The mechanism may be activation of ghrelin pathway in hypothalamic arcuate nucleus-septal septal nucleus, which means that ghrelin is released from ghrelin-positive neurons in the arcuate nucleus, and then transmitted into the lateral septal nucleus to bind its receptor GHSR-1a, thereby promoting gastric motility.
In summary, point-moxibustion therapy can effectively promote the mRNA and protein expressions of ghrelin and GHSR-1a in the central nervous system of DGP rats, thereby improving gastric motility. This further confirms the feasibility of point-moxibustion therapy in the treatment of gastrointestinal diseases,and provides a foundation for application of point-moxibustion pen. At the same time, this also partly shows the mechanism of acupuncture and moxibustion in treating DGP, therefore, provides a theoretical basis for the mechanism of gastroparesis prevention and treatment by acupuncture and moxibustion, and also a reliable evidence for further development of gastroparesis prevention and treatment methods. Clinically, the treatment methods such as EA or point-moxibustion can be flexibly selected according to patient’s acceptance. However, the mechanism of acupuncture and moxibustion on gastroparesis remains unclear, which needs further study. The clinical application of point-moxibustion pen needs to be further explored and generalized.
Conflict of Interest
The authors declared that there was no potential conflict of interest in this article.
Acknowledgments
This work was supported by National Basic Research Program of China (973 Program, 国家重点基础研究发展计划项目, No. 2015CB554502); National Natural Science Foundation of China (国家自然科学基金项目, No.81574082, No. 81774431); Hunan Provincial Natural Science Foundation of China (湖南省自然科学基金项目,No. 2018JJ2295); Fund Project of Hunan Province Education Office (湖南省教育厅科学研究项目, No.14B129); Hunan Provincial Project of Scientific and Technological Ⅰnnovation Platform and Talent Plan:Academician Shi Xue-min Expert Workstation (湖南省科技创新平台与人才计划项目-石学敏院士专家工作站,No.2017RS3052); State Clinical Key Specialty of Tuina Department of Yueyang Hostpital Affiliated to Hunan University of Chinese Medicine (湖南中医药大学附属岳阳医院推拿科国家临床重点专科); Tuina Key Discipline of State Administration of Traditional Chinese Medicine(推拿学国家中医药管理局重点学科).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 8 October 2017/Accepted: 10 November 2017
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Therapeutic efficacy observation on acupuncture for persistent allergic rhinitis
- Effects of acupuncture on ovarian blood supply and pregnancy outcomes in patients receiving assisted reproduction
- Effect of Governor Vessel-unblocking and mindrefreshing acupuncture plus functional training on neural development in infants with brain damage
- Correlation analysis on clinical effects of acupuncture for elderly patients with sensorineural deafness and ear distending sensation
- Observation of therapeutic effects of point application at Shenque (CV 8) plus moxa-salt hot compress for prevention of gastrointestinal adverse reactions after chemotherapy for non-Hodgkin lymphoma
- Application and exploration of suspended magnetic moxibustion cup for obesity
