纳米氧化铝弥散强化铜的放电等离子体烧结动力学及机制
2018-09-08蒋少文程立金刘耀刘绍军
蒋少文,程立金,刘耀,刘绍军, 3
纳米氧化铝弥散强化铜的放电等离子体烧结动力学及机制
蒋少文1,程立金2,刘耀1,刘绍军1, 3
(1. 中南大学 粉末冶金研究院,长沙 410083; 2. 华中科技大学 材料成形与模具技术国家重点实验室,武汉 430074; 3. 中南大学 深圳研究院,深圳 518057)
利用经典热压模型,系统研究纳米氧化铝颗粒弥散强化铜的放电等离子烧结(SPS)致密化过程与机理。结果表明,放电等离子烧结初期,氧化铝弥散强化铜的致密化过程由晶界滑移和晶界扩散共同控制。随保温时间延长,烧结机制转变为由晶界滑移所主导。烧结后期致密化主要以塑性变形的方式进行。纳米氧化铝颗粒抑制了铜的烧结致密化,导致材料的密度降低。抑制机理为氧化铝颗粒阻碍晶界和位错运动,导致晶界滑移和塑性变形的激活能提高,从而增大致密化抗力。在外力和纳米氧化铝颗粒的共同作用下,塑性变形的主要形式为孪生。
氧化铝弥散强化铜;放电等离子烧结;烧结动力学;孪生;致密化
氧化铝弥散强化铜是一种具有高强高导的高性能复合材料,广泛应用于汽车、航空等领域,受到众多学者的关注和研究[1−3]。在铜基体中添加增强相氧化铝颗粒可使其力学性能极大地提高[4],且纳米氧化铝颗粒的弥散分布对材料的电、热学性能不会有明显的削弱作用[5−6]。铜基体和纳米氧化铝颗粒间的界面结构决定氧化铝弥散强化铜材料的性能[7]。高致密度是弥散强化铜材料中金属与陶瓷颗粒间界面结合良好的保证。然而,纳米氧化铝颗粒对铜的烧结致密化有较强的阻碍作用[8]。一般可通过2种方式来提高氧化铝弥散强化铜的密度,一种是在简单烧结后进行热挤压,使材料发生较大的塑性变形[9−10];另一种是采用特殊烧结工艺来制备弥散强化铜材料,如微波烧结[11]和放电等离子烧结(spark plasma sintering,SPS)等。在过去的几十年,放电等离子烧结技术因集热场、力场和电场于一身,能快速制备高密度的块状金属、陶瓷和复合材料而被关注[12−14]。许多学者已采用SPS制备了性能良好的氧化铝弥散强化铜复合材料[15−17],但关于该材料的SPS致密化机理却鲜有研究。ZHANG等[18]研究纳米晶铜的SPS动力学时发现,低温无宏观压力状态下主要发生蒸发凝聚和烧结颈形成与长大,随后在温度和压力的共同作用下快速完成致密化。LEON等[19]研究了氧化铝弥散强化铜SPS的致密化过程,发现低温阶段主要通过颗粒重排机制实现烧结致密化,400~700 ℃则由塑性变形和扩散机制主导致密化进程。LEON的研究很有意义,但仅通过致密化曲线来判定烧结机理的方法并不严谨,而且纳米氧化铝颗粒对烧结机制和微观组织结构的影响尚未明确。RAJAN等[20]研究了Y2O3颗粒对Fe-9Cr-1Mo铁素体钢的SPS致密化的影响,发现Y2O3颗粒的加入可促进铁素体钢的致密化。因此,正确理解氧化铝颗粒弥散强化铜的SPS烧结致密化过程以及纳米氧化铝颗粒对烧结机制的影响,对改善烧结工艺具有重要的指导作用。同时,氧化铝弥散强化铜作为一种典型的金属基复合材料,其SPS烧结动力学以及研究动力学的方法对其它金属基复合材料的相关研究也有很好的参考意义。本文作者采用SPS制备纳米氧化铝弥散强化铜,利用经典热压模型研究烧结过程中的烧结动力学问题,重点研究纳米氧化铝颗粒对铜烧结动力学及机制的影响。
1 实验
1.1 样品制备
实验采用SPS工艺制备氧化铝质量分数为0.65%的弥散强化铜复合材料。所用原料为雾化铜粉(纯度>99.7%,平均粒径约74 μm)和纳米氧化铝粉(纯度>99.9%,平均粒径约30 nm)。具体过程如下:首先按比例称量铜粉和氧化铝粉末,利用行星球磨机球磨混合12 h。球磨介质为无水乙醇,球磨转速为300 r/min。混合后的浆料经干燥后,在氢气气氛中还原2 h,还原温度250 ℃。为了对比纯铜和弥散强化铜SPS致密化的差别,将纯铜粉在同样条件下进行球磨、干燥和还原处理。将还原后的粉末装入石墨模具内,在SPS装置(FCT Systeme GmbH)中进行烧结,得到纯铜和氧化铝颗粒弥散强化铜复合材料样品。烧结温度分别为475,500和525 ℃,压力30 MPa,真空环境下保温15 min。
1.2 性能检测
采用阿基米德排水法测定所有样品的密度。利用球差透射电镜(TECNAI G2 60-300, FEI)表征其微观结构,透射电镜分析样品经机械预减薄至约30 μm后,进行小角度离子减薄得到薄区。
SPS装置能监测烧结过程中样品的即时高度变化。为排除升温过程中模具膨胀对结果的影响,设置一组空烧实验来获取升温状态下模具的膨胀量。因此,升温过程中样品的即时高度可用式(1)计算。
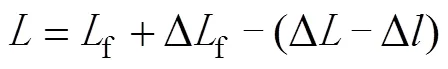
式中:f是样品的最终高度,f是烧结结束时样品的高度变化值。为了分析整个烧结过程中每个时刻的致密化行为,需要得到样品在烧结时的即时相对密度。假设烧结过程中石墨模具的径向截面面积不变,则样品的即时相对密度与最终相对密度f之比等于样品的即时高度与最终高度f之比,整理可得到下式[21]:

采用排水法测定烧结样品的最终密度,计算出最终相对密度f,依据式(2)即可计算出烧结过程中样品的即时相对密度。
2 结果与讨论
通常,常见的烧结过程可根据烧结行为的不同分为2个阶段:烧结致密化阶段和晶粒长大阶段[22−23]。在烧结致密化阶段,随烧结时间延长,烧结体的密度不断提高,不会发生明显的晶粒长大现象。当达到一定密度时,烧结致密化速率变慢,晶粒开始长大,烧结进入晶粒长大阶段。本文以研究烧结致密化阶段为主,依据升温过程中样品的致密化速率变化曲线来大致区分烧结过程的2个阶段。图1所示为400~800 ℃升温过程中氧化铝弥散强化铜的相对密度及致密化速率随温度的变化曲线。由图可知,温度低于400 ℃时致密化过程已经开始。随温度升高,样品的密度不断提高。值得注意的是,致密化速率在525 ℃附近出现最大值,表明在此温度下致密化速率最快,即525 ℃为致密化和晶粒长大2个阶段的临界温度点,在低于525 ℃温度下样品主要处于致密化阶段,在高于525 ℃时晶粒长大现象较明显。因此本文选取致密化阶段的475,500和525 ℃这3个温度点研究弥散强化铜的放电等离子烧结动力学。
图2所示为弥散强化铜和纯铜样品分别在475,500和525 ℃这3个温度点的烧结致密化曲线,保温时间为15 min。由图可见,随保温时间延长,样品的密度增加。在保温开始阶段,烧结致密化速率非常快;约3 min后,致密化曲线变得平缓。整个烧结过程中,烧结温度越高,样品密度越高。更重要的是,在相同条件下烧结,纯铜的相对密度显著高于弥散强化铜的相对密度。由此可推断,氧化铝颗粒的加入,阻碍了弥散强化铜烧结致密化的进行,降低了材料的密度。

图1 升温过程中Al2O3弥散强化铜的相对密度及致密化速率随温度的变化曲线

图2 纯铜和Al2O3弥散强化铜在不同温度下的等温烧结致密化曲线
SPS和热压均为有压烧结,外界宏观压力是烧结驱动力的主要来源[24]。而且,SPS和热压的烧结机制相似[25]。由于烧结上的共性,已有不少学者利用热压模型研究SPS烧结机理[26−27]。当宏观压力是主要的烧结驱动力时,烧结致密化速率(dd)用下式表示[28]:


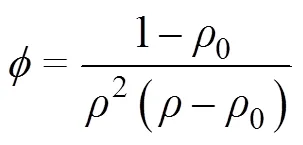
式中:0为样品的初始相对密度。在等温烧结过程中,温度保持不变,且由于烧结时间较短且温度较低,晶粒长大不显著,假设晶粒大小也是常数,式(3)可简化为:
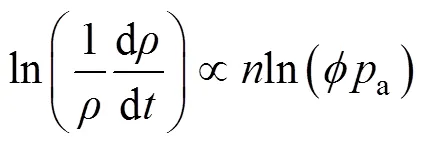


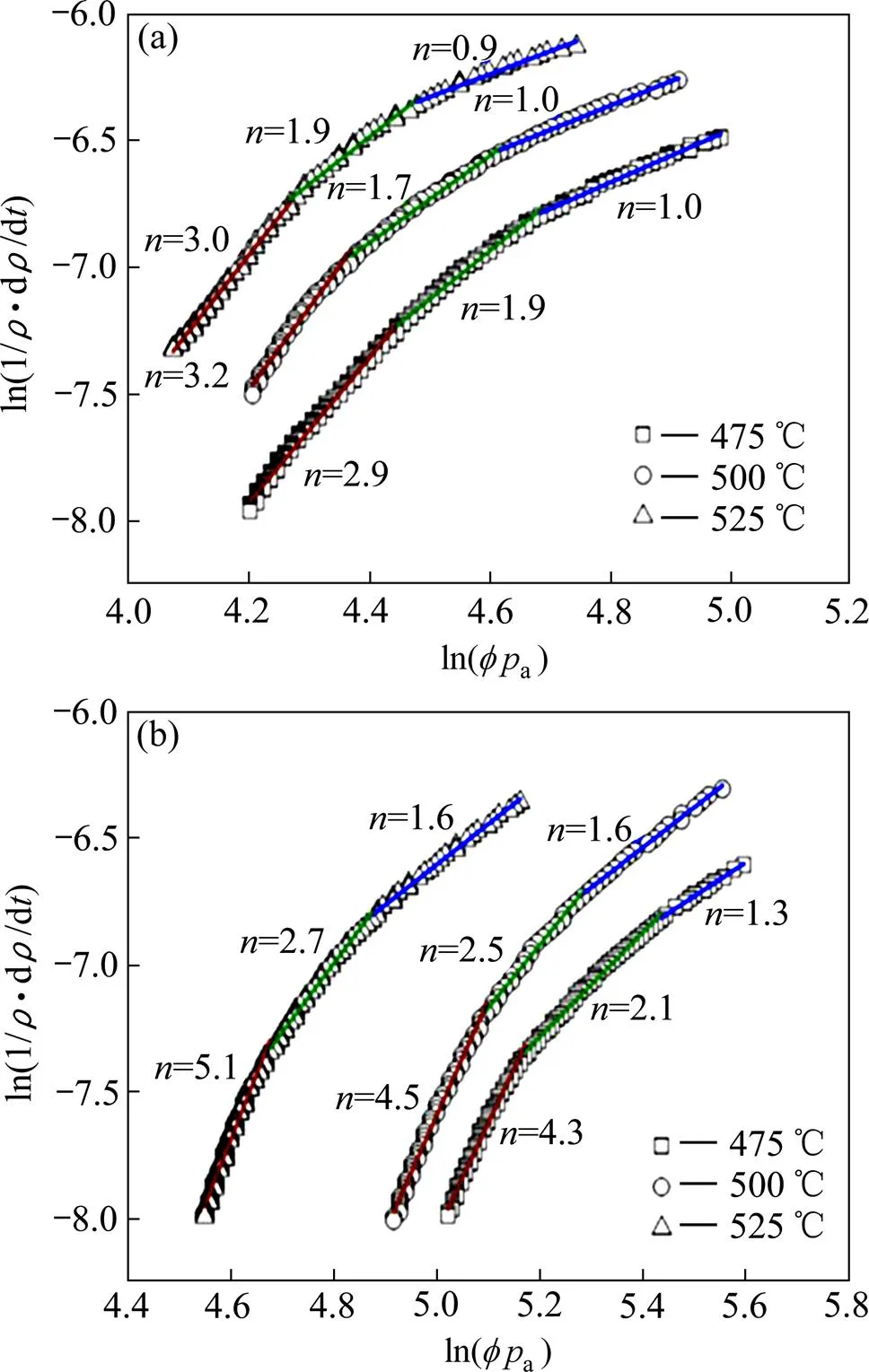
图3 纯铜(a)和弥散强化铜(b)的ln[(1/ρ)∙(dρ/dt)]与ln(pa)关系曲线
根据阿伦尼乌斯方程,致密化速率(d/d)和烧结温度之间存在以下关系[26]:

式中:是理想气体常数;a为表观激活能。


图4 纯铜(a)和弥散强化铜(b)的ln[(1/ρ)(dρ/dt)(pa)−nT]与T−1的关系曲线
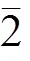

图5 纯铜的暗场像照片和孪晶衍射花样标定
图6所示为525 ℃烧结15 min的弥散强化铜的高角环形暗场像和EDX分析。图6(a)显示弥散强化铜中也分布着与纯铜中相似的孪晶,晶粒尺寸较小,数量较多。除此之外,晶界处还弥散分布着许多纳米级颗粒。EDX分析结果表明,纳米颗粒的主要元素包括Cu、Al和O。可以推断,分布在晶界处的纳米颗粒为氧化铝颗粒。众所周知,纳米氧化铝颗粒通过钉扎晶界而阻碍晶界的移动[31]。另外,位错线移动时无法直接越过第二相颗粒[32−33],但在外力作用下,位错线可以环绕第二相发生弯曲,形成位错环并导致晶格畸变能增加。在这2种机制的共同作用下,弥散强化铜发生晶界滑移和塑性变形所需能量比纯铜高(见图4),烧结致密化过程被抑制。同时,在外力作用和纳米氧化铝颗粒的阻碍下,弥散强化铜塑性变形的主要形式为孪生。

图6 弥散强化铜的HADF-STEM照片和EDX分析
3 结论
1) 氧化铝弥散强化铜的放电等离子烧结初期,烧结致密化过程由扩散和晶界滑移共同控制。随保温时间延长,烧结机制转变为晶界滑移。烧结后期致密化主要以塑性变形的方式进行。
2) 氧化铝弥散强化铜放电等离子烧结时,晶界滑移和塑性变形所需的激活能分别为115.4和155.6 kJ/mol。
3) 在相同烧结条件下,纳米氧化铝颗粒会抑制铜的烧结致密化,导致样品密度降低。抑制机理为氧化铝颗粒阻碍晶界和位错运动,导致晶界滑移和塑性变形的激活能提高,从而增大致密化抗力。
[1] BESTERCI M, IVAN J. The mechanism of the failure of the dispersion-strengthened Cu-Al2O3system[J]. Journal of Materials Science Letters, 1998, 17(9): 773−776.
[2] KUDASHOV D V, BAUM H, MARTIN U, et al. Microstructure and room temperature hardening of ultra-fine-grained oxide- dispersion strengthened copper prepared by cryomilling[J]. Materials Science & Engineering A, 2004, 387/389(36): 768−771.
[3] PLASCENCIA G, UTIGARD T A. High temperature oxidation mechanism of dilute copper aluminium alloys[J]. Corrosion Science, 2005, 47(5): 1149−1163.
[4] ANDERSON K R, GROZA J R, DRESHFIELD R L, et al. High-performance dispersion-strengthened Cu-8Cr-4Nb alloy[J]. Metallurgical and Materials Transactions A, 1995, 26(9): 2197− 2206.
[5] PARK J Y, OH S J, JUNG C H, et al. Al2O3-dispersed Cu prepared by the combustion synthesized powder[J]. Journal of Materials Science Letters, 1999, 18(1): 67−70.
[6] BOTCHAROVA E, FREUDENBERGER J, SCHULTZ L. Mechanical and electrical properties of mechanically alloyed nanocrystalline Cu-Nb alloys[J]. Acta Materialia, 2006, 54(12): 3333−3341.
[7] VAUCHER S, BEFFORT O. Bonding and interface formation in metal matrix composites[J]. MMC-Assess Thematic Network, 2001, 9(3): 1−41.
[8] 燕鹏, 林晨光, 崔舜, 等. 弥散强化铜合金的研究与应用现状 [J]. 材料导报, 2011, 25(11): 101−106. YAN Peng, LIN Chenguang, CUI Shun, et al. Present status in research and application on dispersion strengthened copper by in-situ methods[J]. Materials Review, 2011, 25(11): 101−106.
[9] LEE D W, KIM B K. Nanostructured Cu-Al2O3composite produced by thermochemical process for electrode application [J]. Materials Letters, 2004, 58(3): 378−383.
[10] NACHUM S, FLECK N A, ASHBY M F, et al. The microstructural basis for the mechanical properties and electrical resistivity of nanocrystalline Cu-Al2O3[J]. Materials Science and Engineering: A, 2010, 527(20): 5065−5071.
[11] ZHONG X L, GUPTA M. Development of lead-free Sn- 0.7Cu/Al2O3nanocomposite solders with superior strength[J]. Journal of Physics D: Applied Physics, 2008, 41(9): 095403.
[12] SASAKI T T, MUKAI T, HONO K. A high-strength bulk nanocrystalline Al-Fe alloy processed by mechanical alloying and spark plasma sintering[J]. Scripta Materialia, 2007, 57(3): 189−192.
[13] CHENG Lijin, JIANG Shaowen, MA Qing, et al. Sintering behavior and microwave properties of dense 0.7CaTiO3- 0.3NdAlO3ceramics with sub-micron sized grains by spark plasma sintering[J]. Scripta Materialia, 2016, 115(7): 80−83.
[14] YANG M J, ZHANG D M, GU X F, et al. Fabrication and properties of SiCp/Al composites by pulsed electric current sintering[J]. Journal of Materials Science, 2005, 40(18): 5029− 5031.
[15] RITASALO R, LIUA X W, SDERBERG O, et al. The microstructural effects on the mechanical and thermal properties of pulsed electric current sintered Cu-Al2O3Composites[J]. Procedia Engineering, 2011, 10(7): 124−129.
[16] DASH K, RAY B C, CHAIRA D. Synthesis and characterization of copper-alumina metal matrix composite by conventional and spark plasma sintering[J]. Journal of Alloys and Compounds, 2012, 516(5): 78−84.
[17] ZEIN Eddine, MATTEAZZI P, CELIS J P. Mechanical and tribological behavior of nanostructured copper-alumina cermets obtained by pulsed electric current sintering[J]. Wear, 2013, 297(2): 762−773.
[18] ZHANG Z H, WANG F C, LEE S K, et al. Microstructure characteristic, mechanical properties and sintering mechanism of nanocrystalline copper obtained by SPS process[J]. Materials Science and Engineering: A, 2009, 523(1): 134−138.
[19] LEON C A, RODRIGUEZ-ORTIZ G, NANKO M, et al. Pulsed electric current sintering of Cu matrix composites reinforced with plain and coated alumina powders[J]. Powder Technology, 2014, 252(1): 1−7.
[20] RAJAN K, SHANMUGASUNDARAM T, SUBRAMANYA Sarma V, et al. Effect of Y2O3on spark plasma sintering kinetics of nanocrystalline 9Cr-1Mo ferritic oxide dispersion- strengthened steels[J]. Metallurgical and Materials Transactions A, 2013, 44(9): 4037−4041.
[21] BERNARD-GRANGER G, GUIZARD C. Densification mechanism involved during spark plasma sintering of a codoped α-alumina material: Part I. Formal sintering analysis[J]. Journal of Materials Research, 2009, 24 (1): 179−186.
[22] ZHANG T S, KONG L B, SONG X C, et al. Densification behaviour and sintering mechanisms of Cu-or Co-doped SnO2: A comparative study[J]. Acta Materialia, 2014, 62(1): 81−88.
[23] CHENG Lijin, LIU Liang, MA Qing, et al. Relationship between densification behavior and stabilization of quasi-liquid grain boundary layers in CuO-doped 0.7CaTiO3-0.3NdAlO3microwave ceramics[J]. Scripta Materialia, 2016, 111(2): 102− 105.
[24] LI R T, DONG Z L, KHOR K A. Spark plasma sintering of Al-Cr-Fe quasicrystals: Electric field effects and densification mechanism[J]. Scripta Materialia, 2016, 114(6): 88−92.
[25] RAMOND L, BERNARD-GRANGER G, ADDAD A, et al. Sintering of a quasi-crystalline powder using spark plasma sintering and hot-pressing[J]. Acta Materialia, 2010, 58(15): 5120−5128.
[26] LANGER J, HOFFMANN M J, GUILLON O. Electric field-assisted sintering in comparison with the hot pressing of yttria-stabilized zirconia[J]. Journal of the American Ceramic Society, 2011, 94(1): 24−31.
[27] HU Ke, LI Xiaoqiang, QU Shengguan, et al. Spark plasma sintering of W-5.6Ni-1.4Fe heavy alloys: Densification and grain growth[J]. Metallurgical and Materials Transactions A, 2013, 44(2): 923−933.
[28] RAHAMAN M N. Ceramic Processing and Sintering[M]. 2nd ed. New York: Marcel Dekker, 1996: 514−523.
[29] SAITOU K. Microwave sintering of iron, cobalt, nickel, copper and stainless steel powders[J]. Scripta Materialia, 2006, 54(5): 875−879.
[30] WEN Haiming, TOPPING T D, ISHEIM D, et al. Strengthening mechanisms in a high-strength bulk nanostructured Cu-Zn-Al alloy processed via cryomilling and spark plasma sintering[J]. Acta Materialia, 2013, 61(8): 2769−2782.
[31] LOHMILLER J, KOBLER A, SPOLENAK R, et al. The effect of solute segregation on strain localization in nanocrystalline thin films: Dislocation glide vs. grain-boundary mediated plasticity[J]. Applied Physics Letters, 2013, 102(24): 241916.
[32] PREININGER D. Effect of particle morphology and microstructure on strength, work-hardening and ductility behaviour of ODS-(7-13)Cr steels[J]. Journal of Nuclear Materials, 2004, 329(1): 362−368.
[33] MONNET G, NAAMANE S, DEVINCRE B. Orowan strengthening at low temperatures in bcc materials studied by dislocation dynamics simulations[J]. Acta Materialia, 2011, 59(2): 451−461.
(编辑 汤金芝)
Sintering kinetics and mechanism of nano Al2O3particles dispersion strengthened copper by spark plasma sintering
JIANG Shaowen1, CHENG Lijin2, LIU Yao1, LIU Shaojun1, 3
(1. Powder Metallurgy Research Institute, Central South University, Changsha 410083, China; 2. State Key Laboratory of Material Processing and Die & Mould Technology, Huazhong University of Science and Technology, Wuhan 430074, China; 3. Shenzhen Research Institute, Central South University, Shenzhen 518057, China)
The effects of nano Al2O3particles on Al2O3dispersion strengthened copper prepared by spark plasma sintering (SPS) were studied systematically by using the hot-pressing sintering model. The results show that the densification process is dominated by grain boundary diffusion and sliding in the early stage of sintering, followed by the grain boundary sliding. And the plastic deformation occurs in the last stage of sintering. The density of the copper strengthened by Al2O3particles decreases. The Al2O3particles existing along the grain boundaries can inhibit the densification of copper because the particles can block the movement of grain boundaries and dislocation, which indicates that the densification process required higher activation energy. The deformation mode is mainly twinning, which is resulted from co-existence of the shear stress and the pinning of Al2O3particles.
Al2O3dispersion strengthened copper; spark plasma sintering (SPS); sintering kinetics; twin; densification
TG146
A
1673-0224(2018)04-354-07
深圳市基础研究计划资助项目(JCY201110100,JCYJ20140509142357196)
2017−04−07;
2018−04−24
刘绍军,研究员,博士。电话:0731-88876135;E−mail: liumatthew@csu.edu.cn
