Succinylated whey protein isolate as a sustained-release excipient of puerarin derivative oral tablets:Preparation,optimization and pharmacokinetics
2018-09-05RuiZhngYuZhngYueWuJunLiuTintinYeShujunWng
Rui Zhng,Yu Zhng,Yue Wu,Jun Liu,Tintin Ye,∗,Shujun Wng,∗
aDepartment of Pharmaceutics,College of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
bCollege of Traditional Chinese Medicine,Beijing University of Chinese Medicine,Beijing 100029,China
cChinese Medicine(Traditional Chinese Medicine Preparation Direction),College of traditional Chinese Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
dDepartment of Pharmacy,China Pharmaceutical University,Nanjing 210009,China
Keywords:Whey protein isolate Succinylation Puerarin 5 Sustained drug delivery Pharmacokinetics
ABSTRACT This work was done to investigate succinylated commercial whey protein isolate(S-WPI)as an oral sustained-release delivery carrier for puerarin 5(PR-5).The succinylation conditions were established for S-WPIs by optimization of single factor study and Box–Beehnken design.The effect of succinylation degree on S-WPIs solubility was evaluated.Physicochemical properties of S-WPIs dried by different three methods on their flow ability,particle size,morphology and in vitro release behavior were studied.After preparing PR-5 sustained release protein tablets with S-WPIs as the carrier by direct powder compression method,the drug release were studied in vitro and the oral pharmacokinetics and bioavailability was evaluated using in vivo dog model.It was observed that concentration of substrate has a significant effect on succinylation.Release behavior in vitro showed spry dried S-WPIs with 100%succinylation rate and 30%drug loading would be applied to the preparation of PR-5 sustained-release protein tablets based on the swelling mechanism(protein loss).Compared with PR-5 conventional tablet with oral administration,Tmaxvalue of PR-5 sustained-release protein tablets was approximately 1.58 fold greater than those of the conventional tablets as further evidenced by the significantly prolonged MRT and T1/2.The findings demonstrated that spray-dried S-WPIs has potential as a promising functional excipient for the design of PR-5 oral sustained-release tablets which can fully improve sustained-release effect and oral bioavailability.
1. Introduction
Biocompatible materials are becoming a research hotspot in recent years,especially proteins.Because of functional properties such as emulsion and gelation capacity,proteins are widely used in formulated foods.Furthermore,recently,more and more studies have examined their use in terms of pharmaceutical applications,such as designing drug delivery systems including tablets[1]and microspheres[2,3,4].
Due to its spherical structure and good function,whey proteins isolate(WPIs),as a protein complex derived with a higher proportion of lactoglobulin content,have been recently shown to be very promising as excipients for manufacturing direct compression tablets of active compounds such as probiotics[1,5].
Succinylation is one of the frequently used chemical modification technique used to improve different functional properties of proteins[6].It is a chemical reaction that adds succinic acid to protein amino groups by nucleophilic attack and thus replaces positively charged lysine ε-amino groups with negatively charged carboxyl groups.Succinylation has been shown to be capable of decreasing protein solubility and charge density at acidic pH and increasing protein solubility and zeta potential at alkaline pH[7].Previous study shows that the solubility of succinylated whey protein concentrate increased at alkaline pH but decreased at acidic pH values as compared to native whey protein concentrate[8].Further,succinylated β-lactoglobulin as a single component of WPIs was applied as the excipient in novel delayed release tablets and the protection of vitamins[5].Although six commercial WPIs showed good compactibility as well as compressibility and flowability via in vitro tabletability studies[1],there are few studies about affect of succinylation on WPIs as a sustained release carrier to apply in the area of oral administration.
Puerarin(PR),isolated from isoflavones which is extracted from the dried root of Pueraria lobate,is a major active isoflavone(Willd.)[9].However,PR is poorly water-soluble leading to low bioavailability[10].In order to improve its solubility and pharmacological efficacy,we modified the chemical structure of puerarin and synthesized a derivative named puerarin 7,4-di-succinic acid mon-ester-O-ethoxy(PR-5)by Wang’s method of making derivative of daidzein,DZ5[11].The solubility of PR-5 in water was 33.62 mg/ml which was improved compared with 0.89 mg/l of PR solubility in water.Our pharmacological efficacy studies show that the protective effects of PR-5 on inhibiting calcium influx,reducing insulin resistance,counteracting cell death,inhibiting alcohol intake,enhancing myocardial contractility,protecting cardiomyocytes,lower blood pressure and anti-platelet aggregation[12].
This study selected Provon 190 which was commercial WPIs with good tabletability shown in the previous study[1].The aim of the present study was to apply succinylated Provon 190 as an excipient to loading PR in the oral tablets and investigated in vitro and in vivo release properties,and mainly contains the following aspects of research:(i)to optimize the level of reactants(succinic anhydride and protein)to achieve the optimal degree of succinylation of whey protein for oral release of PR-5 and(ii)to determine the effect of succinylated whey protein on in vitro and in vivo functional properties of PR-5 tablets in comparison to unmodified(native)whey protein tablets.To achieve this goal,this study was intended to design and optimize the succinylation on process of whey protein,especially drying methods.In vitro drug release properties of PR-5 tablets with succinylated whey protein as carriers were studied in gastric and intestinal simulated fluids.In vivo pharmacokinetics and bioavailability in beagle dogs were evaluated afterwards.
2. Materials and methods
2.1. Materials
Provon 190 which was whey protein isolate(containing 92.6%protein content)was obtained from Glanbia Nutritionals,Inc.(Carlsbad,CA,USA).The model drug PR-5(Puerarin 5,purity 99.5%)was synthesized by our laboratory.Succinicanhydride,Sodium dodecyl sulfate(SDS)and Na2B4O7·10H2O were obtained from BodiChem.Company(Tianjin China);2,2′-Biquinoline-4,4-dicarboxylic acid disodium salt(BCA),Dithiothreitol(DTT)were purchased from Shanghai yuanye Bio-Technology Co.,Ltd.1,2-Phthalic dicarboxaldehyde(OPA)was obtained from best-reagent company(Chengdu,Sichuan,China).
2.2. Methods
2.2.1. Succinylation of whey protein isolate
The modification of WPI was simply carried out as follows.Firstly,the WPIs were formulated into solutions with different concentrations.Then,succinic anhydride was gradually added into WPI solution(W/V)maintaining the pH value between 8.0 and 9.0(alkaline pH)with 1 or 2 M NaOH using the pH meter and the temperature was 37± 0.5°C regulated by thermostatic water bath with magnetic stirring.Once all the succinicanhydride was added and pH value was stabilized at 8–9,the solutions were dialyzed overnight at 4 °C using 1 kDa dialysis semipermeable membranes before drying.
2.2.2. Determination of the degree of succinylation
The OPA(ortho-phthaldialdehyde)method was used to determine the extent of succinylation which expressed as re-acted lysineε-amino groups.160 mg OPA reagent was dissolved in 4 ml methanol,mixed with 7.620 g sodium borate buffer,and 200 mg sodiumdodecyl sulfate in 150 ml distilled water,then added 176 mg DTT and set resultant solution into 200 ml flask.Be noted that,the OPA should be prepared daily.Samples to be tested(modified and unmodified WPI)were redissolved at appropriate concentration in deionized water and the absorbance of solution was read at 340 nm in a spectrophotometer against a reagent blank.The specific method as shown in the literature[13],differently,we choose the L-Lysine as standard in this study.It standards from 50 to 250 μg/ml as well as a blank were prepared identically.Succinylation degree was calculated as followed:
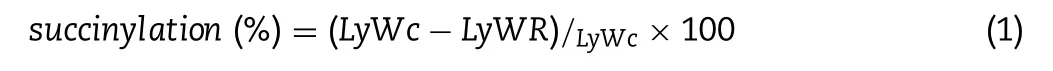
LyWCis the concentration(in free L-Lysineequivalents,from the OD340standard curve)of reactive lysine in the solution of unmodified WPI and LyWRis the residual concentration in the solutions of modified WPI.
2.2.3. Single factor experiments of whey protein isolate succinylation
Study on effect of reaction time:8%of WPI concentration and 10%of anhydride amount were selected,and pH value was stabilized at 8.0–9.0.15,30,45 and 60 min of the reaction time were taken to examine the extent of WPI succinylation.
Study on effect of reaction temperature:8%of WPI concentration and 10%of anhydride amount were selected,and pH value was stabilized at 8.0–9.0.25,37,50 and 70 °C of the reaction temperature were taken to examine the extent of WPI succinylation.
Study on effect of substrate concentration:10%of anhydride amount were selected,and the pH value and temperature were stabilized at 8.0–9.0 and 37 °C.3%,5%,8%,10%and 15%of WPI concentration were taken to examine the extent of WPI succinylation.
Study on effect of reaction pH:8%of WPI concentration and 10%of anhydride amount were selected,and the reaction time was stabilized at 30 min.7.5,8.0,8.5,9.0 and 10.0 of the reaction pH values were taken to examine the extent of WPI succinylation.
2.2.4. Box–Behnken experimental design
Experimental design:A three-factor,three-level BBD was employed to generate quadratic response surface and second order polynomial models to quantify and thereby to optimize succinylation of WPI using the statistical software Design-Expert 8.0.5 software,which required a total of 17 experimental runs.Based on preliminary studies(L9(34)orthogonal tests),temperature(X1),time(X2)and concentration(X3)were chosen and evaluated at three different levels(Table 1).RSM based on the Box–Behnken design of experiment was used to identify the relationship among the independent factors and the dependent variable(response).The three independent factors studied are as follows in Table 2.All experiments were performed in randomized manner to eradicate possible sources of bias.
Response surface methodology:Using the obtained results,the response functions(Y)were used to perform regression analyses and analyses of variance(ANOVA)for the regression.It waspossible to determine the second-order polynomial model and regression coefficients.The generalized second-order polynomial model proposed for the response surface analysis was given as followed:

Table 1–Independent variables and levels investigated in the BBD.
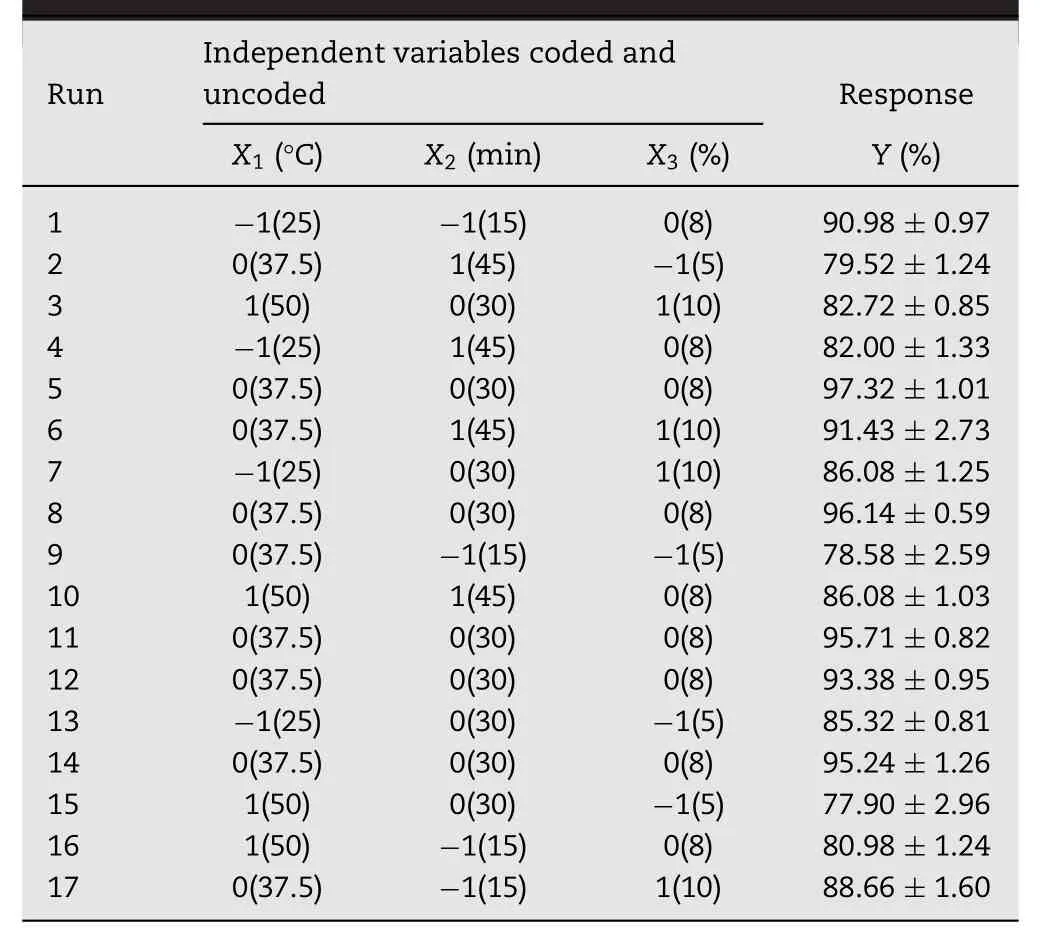
Table 2–Experimental parameters of BBD and the degree for succinylation.
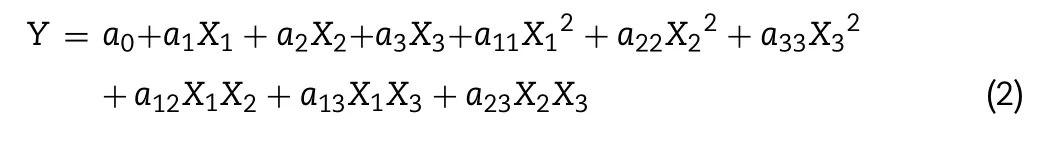
where Y(response function),X1,X2and X3(coded variables)
Response surface graphs and desirability parameters were generated.All executed analysis,desirability and response surfaces were performed by Design-Expert 8.0.5 software.
2.2.5. Effect of succinylation degree on WPI solubility
125 mg of the samples were dispersed in 25 ml distilled water and the solution pH was adjusted to 2–10 using either 0.5 mol/l NaOH or 0.5 mol/l HCL.The slurry was mixed for 1 h at 37± 0.5°C using magnetic bar before centrifuging at 1200 g for 20 min at 4°C.The supernatant was filtered to obtain a clear solution.Protein content in the supernatant was determined by kjeldahl method[14,15].Triplicate determinations were carried out and the solubility profile was obtained by plotting averages of protein solubility(%)against pH:where S was the solubility,A1was the amount of protein in the supernatant,and A2was the amount of the protein in the sample.

2.2.6. Different drying methods of S-WPIs solution
We prepared succinylated WPIs(S-WPI)dried powder by three different drying methods.For freeze drying,protein solution was first frozen for 24 h at-4°C.Frozen samples were then freeze-dried(EYELA,FDU-1100,Tokey RIkakikaiCo.,Ltd.Japan)and were finally grounded to a fine powder using a mortar.Spray drying of S-WPI solution was carried out on a bench-top spray dryer(Mini Büchi B-290,Büchi Labortechnik AGt,Flawil,Switzerland).The outlet and inlet temperature were maintained at 80 and 160°C,respectively,during spray drying.The pump fluid rate of the feed solution was maintained at 12%.Blast drying methods was performed at 50°C for 48 h.S-WPI solution was poured into a glass tray to about 10 mm depth.The dried sample was collected and subsequently pulverized into fine powder in an electric grinder.The properties of SWPIs dried powder were compared.Before the preparation of tablets,all the powders were sieved through 100 mesh sieve.
2.2.7. Characterization of S-WPIs dry powders
Bulk density of each powder sample was determined using a tap device(BT1001-Intelligent Powder Characteristics Tester,Bettersize Instruments Ltd.Dandong,China).The particle size distribution of WPI and S-WPIs dry powders for wet analysis was measured by laser diffraction(Beckman Coulter,LS320,America).The surface morphology of S-WPI prepared by different drying methods were examined using SEM(Hitachi Limited,S-3000N,Tokyo,Japan).The surface morphology of S-WPI dry powders prepared by different drying methods was examined using microscope(Olympus,CX23,Japan).
2.2.8. Preparation of PR-5 sustained release protein tablets
PR-5 sustained release protein tablets with an average weight of 500 mg were prepared by direct powder compression of the mixture of S-WPIs dry powders and PR-5 thoroughly in a mortar.All tablets were made using a single punch Carver press(Jielong,Inc Shanghai),equipped with flat faced punches with a diameter 13 mm.Drug loading of formulations containing 10%,20%,30%,40%and 50%(w/w)PR-5 was determinated according to the dissolution profile.
2.2.9. In vitro drug release study
In vitro drug release study of PR-5 sustained release protein tablets was carried out using a Chinese Pharmacopoeia Apparatus II dissolution bath.The media used was 0.1 M HCL(pH=1)and phosphate buffer(pH=6.8)to simulate conditions in the stomach and colon,respectively.Briefly,the tablets were put in 750 ml SGF(0.1 M HCL)without pepsin for 120 min at 37°C under constant agitation of 100 rpm.Then the tablets were transferred to 1000 ml SIF till totally dissolved.After each appropriate time interval,aliquot 5 ml media was withdrawn and filtered with a 0.45 μm syringe driven filter unit and measured by HPLC.The experiments were carried out in triplicate.The effect of succinylation degree(Native WPI,S-WPIs with 35%,55%,70%,85%and100%succinylation degree,as main excipients,respectively)on drug release was studied with tablets(500 mg)at 10%loading rates.
Moreover,the morphology was observed for the dried residue of S-WPIs tablets with 55%and 100%after drug release to investigate the erosion and swelling in simulated gastricfluid(SGF)and simulated intestinal fluid(SIF).
2.2.10.In vivo pharmacokinetic study
Pharmacokinetic study of PR-5 sustained release protein tablets was carried out in beagle dogs(3 males and 3 females,11–14 kg)after oral administration and compared with a self made tablets.The protocol of the studies was performed according to the Guidelines for the Care and Use of Laboratory Animals that was approved by the Institutional Animal Ethics Committee of Animal Experimentation of Shenyang pharmaceutical university.Beagle dogs did not receive food but had free access to water for 12 h before and after drug administration[16].
PR-5 solution(15 mg PR-5/kg)was administered to the Beagle dogs by i.v.injection.PR-5 sustained release protein tablets(15 mg PR-5/kg)and conventional PR-5 tablets(self-made using the MCC as direct excipient)were oral administrated.A randomized,two-period cross-over design was employed to study the pharmacokinetics in beagle dogs.Approximately 2.0 ml blood samples were obtained from anterior limb vein before dosing and at 0.083,0.167,0.25,0.5,1,1.5,2,3,4,6,8,10 and 12 h post-administration.The washout period between consecutive treatment schedules was one week.Blood samples were transferred to heparinized tube and centrifuged at 3000×g for 10 min.The supernatant layer of plasma was separated by micropipette.50 μl plasma sample was mixed with 10 μl of internal standard in a 2 ml tube.The mixture was vortexed for 30 s.The sample was extracted with 200 μl acetonitrile by vortex-mixing for 3 min,and then centrifuged at 13 000 rpm for 10 min.The supernatant was transferred to another clean tube and then evaporated to dryness under nitrogen at 37 °C.The residue was reconstituted in 50 μl mobile phase,vortexed for 1 min and centrifuged at 13 000 rpm for 10 min.An aliquot of 20 μl of sample was injected into the HPLC system for analysis.
Analysis of PR-5 in plasma samples was carried out by reverse phase HPLC(Shimadzu,LC-15C,Japan).The chromatography was carried out using C-18 column(250 mm×4.6 mm,5 μm)at 249 nm wavelength(λ)with a mobile phase of MeOH:Water(0.1%Phosphoric acid)(60:40)at flow rate of 1 ml/min.The injection volume was kept constant at 20 μl and run time was maintained for 15 min.Non-compartmental pharmacokinetic analysis of concentration-time data was performed using DAS2.0 software(Mathematical Pharmacology Professional Communities of China,Shanghai,China).Standard methods were used to calculate the pharmacokinetic parameters such as area under the drug-concentration time curve(AUC),half-life(t1/2)and mean residence time(MRT).The maximum plasma concentration of drug(Cmax)and time to reach Cmax(Tmax)were directly computed from the plasma concentration vs.time plot.
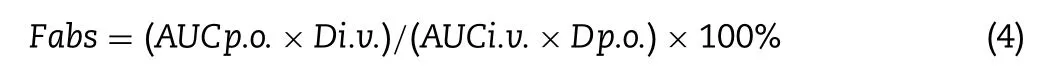
where Fabs was absolute bioavailability,p.o.and i.v.,respectively,express oral and intravenous administration,and D is the dosage of administration.

Fig.1–The succinylation of WPI following the amount of the succinic anhydride.
2.2.11.Statistical analysis
The experimental data were presented as the mean±S.D.Statistical comparisons of the experimental results were performed by one-way analysis of variance(ANOVA)at an alpha level of 0.05 and 0.01.
3. Results and discussion
3.1. Succinylation extent of S-WPIs
The amount of modified lysine group in WPI was dependent on the amount of succinic anhydride added.The results in Fig.1 revealed that the degree of modification was not linearly related to succinic anhydride/WPI ratio(%,g/g).When the succinic anhydride/WPI ratio increased from 3%to 20%,the degree of succinylation increased from 35.64%to 100.00%.With the increasing succinic anhydride/WPI ratio added,the degree of succinylation gradually increased which is consistent with the general rule of chemical reaction.When the succinic anhydride/WPI ratio added to 10%,the degree of succinylation did not change significantly and reached to around 100%,which indicated the reaction had already achieved completely.These results were comparable to the results obtained with succinylated soy protein isolates,spray-dried egg white and whey protein isolates[17].While previous study reported that the succinylation degree respectively increased from 34.62%and 38.23%to 99.09%and 87.16%in yak and cow caseins with the increase of succinic anhydride/protein ratio%from 4%to 60%(g/g)[18].Different steric and conformational constraints of proteins could affect the availability of free amino groups to succinylation.
3.2. Effect of single reaction factor on succinylation extent
Fig.2 demonstrated the effect of reaction time,temperature,pH and the concentration of substrate and the time on the succinylation extent.In Fig.2A,the degree of succinylation increased with the reaction time(up from 0 min to 30 min).When the reaction time continues from 45 min to 60 min,the degree of succinylation keeps stable around 100%,implying that the reaction went to completion.As shown in Fig.2B,the degree of acylated WPI keeps growing with the increase of reaction temperature in a certain temperature range.The reaction gradually loses its activity when the reaction temperature is more than 37°C.Fig.2C shows that the degree of succinylation increased within 5%–8%of substrate concentration range.The succinylation degree appears decrease when substrate concentration is more than 8%.In Fig.2D,when pH values range is from 7.5 to 10,and the degree of succinylation is a nonlinear function.The degree of the succinylation appear slightly increase at pH value 8–9.The results show that reaction time,temperature and substrate concentrations have a significant impact on succinylation degree of S-WPIs.
3.3. Succinylation extent optimization
The regression coefficients were determined by employing the least squares technique.Final Equation in Terms of Actual Factors[19]:

Analysis of variance(ANOVA)was calculated to test significance of regression.As shown in Fig.3,the concentration had a significant effect on the succinylation when compared with temperature and time.ANOVA revealed that the X3(C:concentration)had a very low P-value(0.0089<0.01),whereas the values of X1(A:temperature)and X2(B:time)were higher(0.0579>0.05 and 0.9623>0.05).The ranking of the impact of various factors is:C≫A>B,which is the concentration of substrate≫temperature>time.Namely,the optimal conditions obtained with the second-order polynomial equation of the response surfaces were 8%concentration of substrate,37°C reaction temperature and 30 min reaction time.Because the pH level had little effect on the degree of succinylation,the pH could be sustained between 8 and 9.
3.4. Impact of succinylation extent on WPI solubility
The impact of succinylation on whey protein solubility as a function of pH was studied.As expected,succinylation appeared to increase the nitrogen solubility index at pH above the isoelectric point and gradually up to 100%,while decrease to below 50%with pH value less than 4.5.After succinylation,pH with minimal solubility changed from 4.5 to 3.The chemical modification shifts isoelectric point to lower pH value that is why minimum solubility is shifted to lower pH values[20].By promoting unfolding,reducing protein–protein aggregation,decreasing hydrogen bonding,increasing dissociation of subunits from quaternary structure,reducing isoelectric point to lower value,and increasing protein–water interactions,succinylation increases protein solubility[21].
3.5. Impact of drying method on physicochemical properties of S-WPIs
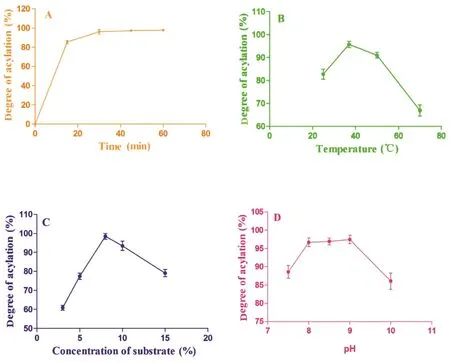
Fig.2–The effect of reaction time(A),temperature(B),substrate concentration(C),and pH(D)on the extent of succinylation.
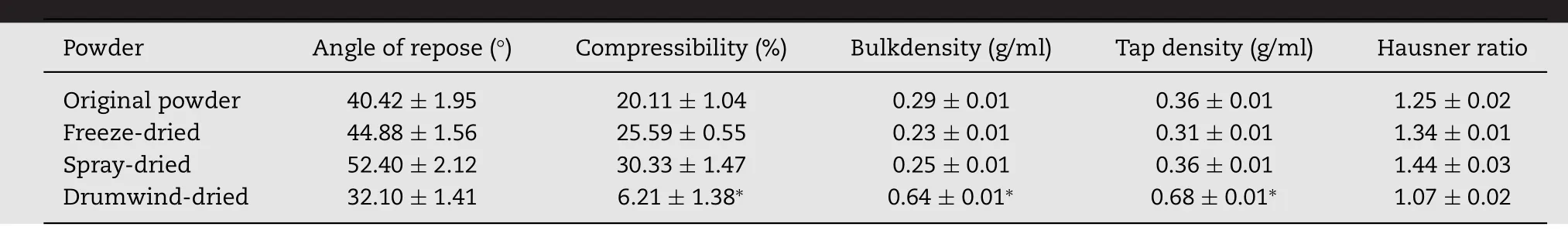
Table 3–Comparison of the properties of the acylated whey protein powder which prepared by different drying methods.
As shown in Table 3,bulk densities ranged from 0.23(Freezedried)to 0.64 g/ml(Drum wind-dried).The bulk density for drum wind-dried powder was the highest amongst these three.From these density values,flow ability of S-WPIs was measured by calculating Hausner ratio(HR).HR was greater than 1.5,the powder is a powder adhesive,poor flow ability and filling;less than 1.2 indicates that the powder has good flow ability and filling[22].Drum wind powder showed“excellent”flow ability(HR=1.07);the native S-WPIs would be classified in the “fair” category(HR=1.25)while freezedried and spray-dried S-WPIs may be classified in the“passable”category(HR=1.34 and 1.44).While all the powders have shown flow ability complying with industry standard in generally.
The particle size distribution of three samples was significantly different(P<0.05).Mean diameter for 90%particles of spray dried S-WPIs powder was 55.29 ± 3.24 μm,which is smaller than the freeze-dried(482.03 ± 7.29 μm)and vacuum-dried(555.90 ± 3.69 μm)powders.Similar trends of particle size distribution were reported for spray-dried and freeze-dried lentil protein isolates[23]and faba bean protein[24]powders by Joshiet al.and Cepeda et al.,respectively.The micrographs of S-WPIs powders(Fig.4A and B)reveal that spray-dried S-WPIs appears to be more uniform in particle size distribution and has a spherical appearance,which supports the particle size distribution data obtained through laser diffraction.While the freeze-dried and drum wind dried protein isolates showed an “agglomerated”particle shape.Particle size and shape strongly affects the isolate tabletability.This relationship between particle size,particle shape and compact strength is well established[25],because the lower particle size and more regular shape(almost spherical)lead to an increase in the bridging surface area[26],which caused the formation of tablets presenting the highest mechanical strength.The results should be noted that spray-dried S-WPIs had better powder compactibility compared with freeze-drying and drum wind-drying WPIs.
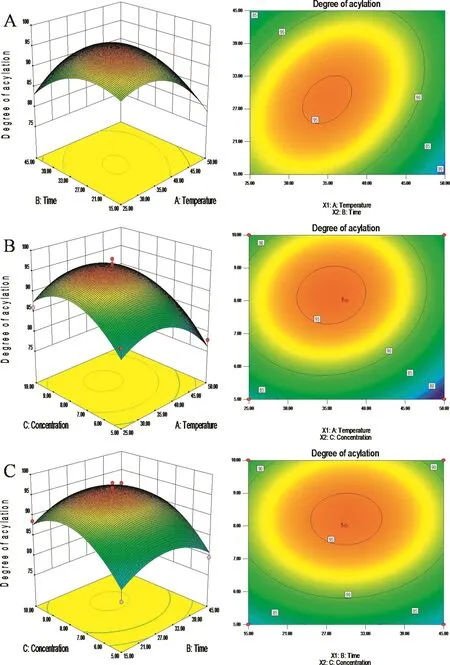
Fig.3–(A)Response surfaces and 2D contour plot with Argument X1(temperature)and X2(time);(B)Response surfaces and 2D contour plot with Argument X1(temperature)and X3(concentration);(C)Response surfaces and 2D contour plot with Argument X2(time)and X3(concentration).
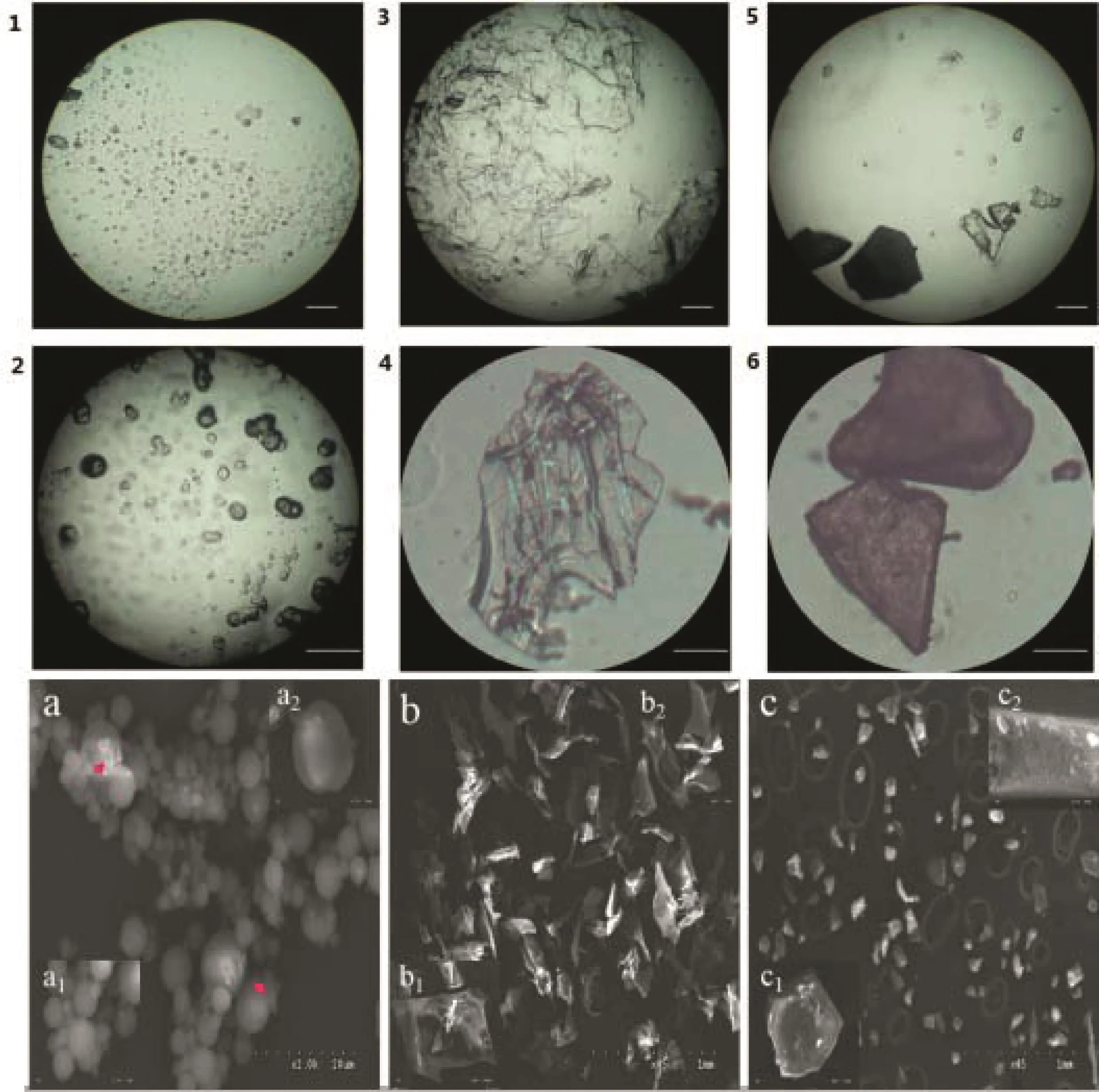
Fig.4–(A)Diagrams of different drying powder under the microscope(Eyepiece 10×;Objective lens 10×,40×).Spray-dried powder:1(10×)and 2(40×);freeze-dried powder:3(10×)and 4(40×);drum wind-dried powder:5(10×)and 6(40×).(B)Whey protein isolates by different drying method particle shape as observed by scanning electronic microscopy(a:spray drying,b:freeze-drying,c:drum wind drying).
All the three dried powders were used to prepare PR-5 tablets;release profiles in the Fig.5A showed the order of in vitro sustained-release properties of PR-5 tablets with S-WPIs via three kinds of drying preparations was:spraydrying>freeze-drying>drum wind-drying.The spray-dried tablets have sustained-release behavior.There was no burst in the initial stage of release in 2 h and the cumulative release reached 60%after 8 h.Although,the drum wind-dried powders show good flow ability,the tablets made by this powder have no sustained-release behavior.Three PR-5 tables with SWPIs via three kinds of drying preparations had not obviously control release effect for PR-5 in the SGF and SIF.Above all,finally the spray-dried method was chosen to dry the S-WPI solution which was applied in this study thoroughly.
3.6. In vitro release of PR-5 sustained release protein tablets
Fig.5B1 illustrated the relationship between the succinylation degree and the sustained-release behavior of PR-5 sustained release protein tablets.It appeared that tablets formulated with 35%to 100%S-WPIs showed sustained-release behaviors.With the increase of the succinylation,sustained release behavior was shown obviously.In addition,between 70%and 85%,the release profiles have no significant differences,85%–100%perhaps accompanied by certain ascension in sustained release.From Fig.5B2,within the scope of the experiments,S-WPIs still have the sustained-release properties until drug loading reached 50%.But when the drug loading reached 40%or above,tablet release had burst release within 2 h.Consequently,based on the release data in vitro,the maximum drug loading of PR-5 sustained release protein tablets should be chosen below 30%for PR-5 as a water-soluble drug.In comparison to unmodified(native)whey protein tablets,S-WPIs have potential to be the sustained release excipient for PR-5 oral administration based on in vitro release results.But succinylated WPI was not obviously able to inhibit releasing during passing through stomach,which seems not to be consistent with the properties of succinylated soy proteins[3]and β-lactoglobulin[11].
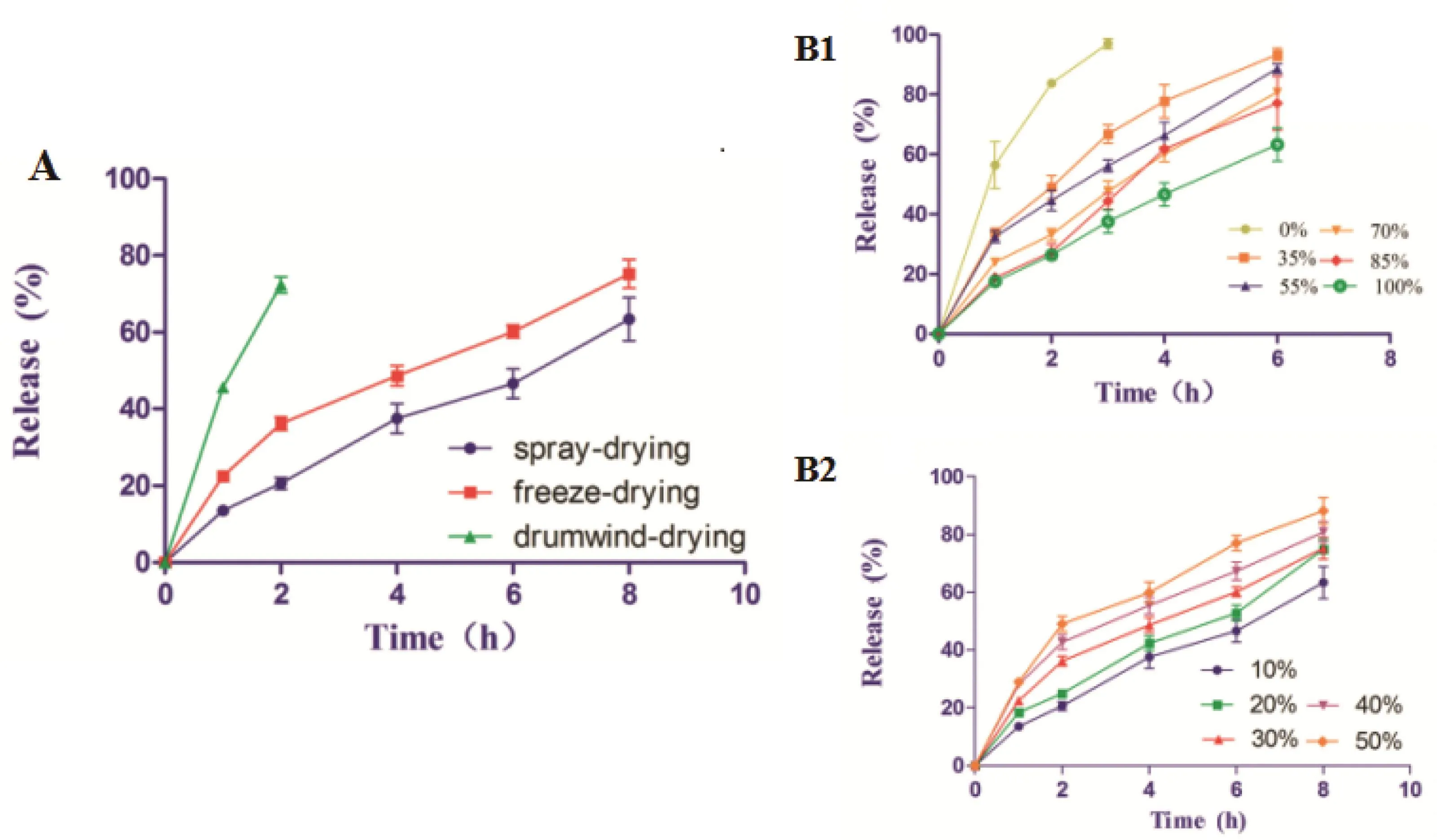
Fig.5–Release profiles of PR-5 sustained release tablets.(A)shows release profiles of PR-5 sustained release tablets with S-WPIs made by different drying methods powder.(B)shows release profiles of PR-5 sustained release tablets.(B1):table formulations(10%drug-loading)with protein succinylation rate 0%,35%,55%,70%,85%and 100%in SGF and SIF;(B2):table formulations with drug loading10%,20%,30%,40%,50%in SGF and SIF.
3.7. In vitro erosion and swelling of PR-5 sustained release protein tablets
The external and internal appearances of the tablets at different stages of incubation are pictured in Fig.6A.To observe the post-effect of drug release on the surface morphology,the SEM images of the dried residue in SGF 2 h to SIF 8 h(totally 10 h)were obtained in Fig.6A.Fig.6A presents that PR-5 sustained release protein tablets with S-WPIs maintained their complete shape throughout the experiment and appears more translucent surface layer,which was the mechanism ensured the sustained release effect of PR-5.The surface of tablets appears more rigid and opaque increasing with the degree of succinylation;while tablets with unmodified WPI as carriers broke down and have a swollen surface within 2 h.Fig.6B1 showed that the surface of tablet formed a network gelatinous layer which delayed drug release after exposuring under SGF 2 h;while in SIF,longer the release time(Fig.6B2–B4),looser the surface of table.So that PR-5 releases quicker in SIF than in SGF.The results appear that physicochemical characteristics of protein,and in particular solubility,drying method and charge density,play a major role in the drug release properties of the tablets[27,28].
3.8. In vivo pharmacokinetic studies of PR-5 sustained release protein tablets
The pharmacokinetics and metabolism of PR-5 in Wistar rats had been investigated in previous study,which presented thatHPLC method was simple and had the necessary precision,accuracy and sensitivity to allow the detection of PR-5 in plasma,urine and feces.The method for analysis of biological samples in present article was referred to earlier studies with the mobile phase adjusted slightly and exhibited good precision and accuracy coupled with a high recovery.Due to the different model of HPLC instruments,the limitation of quantification(LOQ)was 0.04 μg/ml,differing from the previous studies.
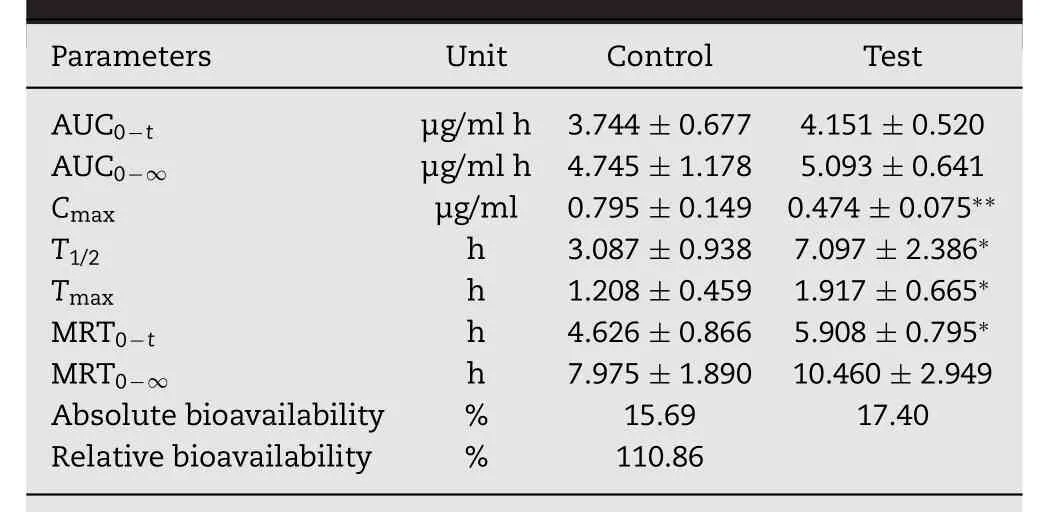
Table 4–The parameters of conventional and sustained tablets in Beagle dogs(Mean±SD,n=6).
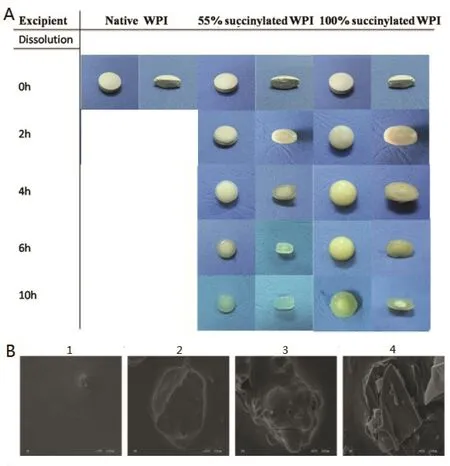
Fig.6–Pictures and micrographs of surface gel of PR-5 tablets.(A)Pictures of PR-5 tablets made of native,55%succinylated or 100%succinylated WPI before and after incubation in simulated gastric fluid without pepsin for 2 h and simulated intestinal fluid 8 h.(B)Micrographs of surface gel of PR-5 tablet with100%succinylated WPI in SGF and SIF by SEM(1:SGF2 h;2:SIF2 h;3:SIF4 h;4:SIF8 h).
The main pharmacokinetic parameters for conventional or sustained release tablet of PR-5 in beagle dogs after oral administration and the absolute bioavailability of PR-5 were summarized in Table 4 and the drug concentration-time curve was shown in the Fig.7.The plasma concentration from the conventional tablets increased quickly and reached the maximum concentration within 1 h,and the drug was more quickly eliminated.PR-5 sustained release protein tablets maintained drug concentration in plasma up to 18 h as compared to the conventional formulation,where the drug levels could no longer be detected after 12 h.It was also observed that MRT of PR-5 sustained release protein tablets(5.90±0.795 h)was 1.2-times higher than that of the conventional formulation(4.626±0.866 h),indicating prolonged residence in the body.In contrast,the Tmax values for the sustained release protein tablets was approximately 1.58 fold greater than those of the conventional tablets as further evidenced by the significantly prolonged T1/2.Thus,significant sustained drug-plasma levels were observed with rate-sustained release of PR-5 from the SWPIs tablets after oral administration when compared to the conventional formulation.
Table 5 displayed the pharmacokinetic parameters for PR-5 after i.v.administration and Fig.7 showed the drug concentration-time curve after intravenous injection.From Table 5 after intravenous administration,plasma clearance was 1.4705±0.268 l/h/kg in dogs,which indicated that PR-5 distributed and eliminated rapidly from the plasma in dogs.The results of calculated bioavailability showed that absolute bioavailability is 15.69%and 17.40%,respectively.Relative bioavailability is 110.86%.It initially proved that PR-5 sustained-release tablets and conventional tablets were bioequivalent.Statistical analysis by ANOVA revealed significant difference between two groups in the values of Cmax,Tmax,AUC,and MRT as revealed by the P value<0.05 or 0.01.
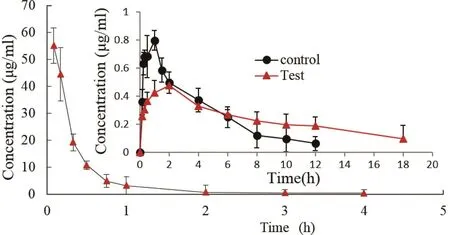
Fig.7–The drug concentration-time curve for PR-5 after i.v.administration and after oral administration of conventional or sustained release tablets in beagle dogs at the dose of 15 mg/kg.Control group:oral administration of PR-5 conventional tablets;Test group:oral administration of PR-5 sustained release tablets.

Table 5–The parameters of injection in Beagle dogs(Mean±SD,n=6).
4. Conclusion
In this study,S-WPIs,a potential excipient for sustained drug delivery system was synthesized through succinylation of whey protein isolate.Following optimization by Response surface methodology based on the Box–Behnken design of experiment,influence of succinylation conditions on the succinylation degree were selected and evaluated which was observed that concentration of substrate as a significant effect on the succinylation.Three drying methods used for preparation of S-WPIs could significantly affect the physicochemical and their functional properties.Therefore,the choice study of the drying method showed spray-dried S-WPIs has potential for the preparation ofPR-5sustained-release tablets according to suitable flow ability,particle size,morphology and in vitro release properties.
Furthermore,this work highlights the potential of S-WPIs as pharmaceutical excipients for the design of sustained release tablets in vitro and especially in vivo using PR-5 as a model drug.It demonstrates that S-WPIs with specific succinylation rate and appropriate drug loading which would be widely applied to the preparation of PR-5 sustained-release protein tablets according to the swelling mechanism shown in Fig.8.As succinylation reduces the PI of whey protein isolate,reducing solubility and increasing viscosity under acidic conditions,succinylated tablets swell in the stomach and form a swelling layer barrier to prevent drug release.After entering the intestine,whey protein isolate dissolves and brokes because of increasing solubility,which leads to drug release.Based on the parameters,in vivo pharmacokinetic study in beagle dogs revealed that tablets made by S-WPI maintained drug concentration in plasma up to 18 h.Statistical analysis by ANOVA revealed significant difference between conventional tablets and PR-5 sustained-release tablets.The developed technology is simple and can be easily scaled up and thus,holds enormous potential for exploitation of pharmaceutical excipients.
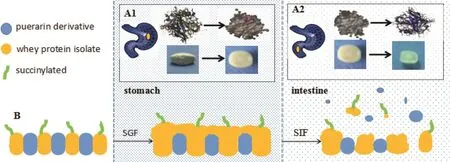
Fig.8–Release mechanism of puerarin derivative tablets with succinylated whey protein isolate as a sustained-release excipient.Fig.6A shows the change of these tablets and succinylated whey protein isolate in tablets from the stomach through the intestine after oral administration.Fig.6B shows release process of these tablets from the stomach through the intestine after oral administration.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Acknowledgment
Declared none.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.04.003.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Evaluation of micelles incorporated into thermosensitive hydrogels for intratumoral delivery and controlled release of docetaxel:A dual approach for in situ treatment of tumors
- Intracellular distribution and internalization pathways of guanidinylated bioresponsive poly(amido amine)s in gene delivery✩
- Development and bioequivalence study of potassium chloride extended release tablets✩
- Creation of an assessment system for measuring the bitterness of azithromycin-containing reverse micelles
- Predicting oral disintegrating tablet formulations by neural network techniques✩
- Enhanced digestion inhibition and mucus penetration of F127-modified self-nanoemulsions for improved oral delivery✩
