Solid Solution Formation and Densification Mechanism of UO2-ZrO2
2018-08-20,,,,,
, ,, , ,
(1.Department of Mechanical Engineering, Luzhou Vocational and Technical College, Luzhou 6460051, China; 2.Department of Criminal Science and Technology, Sichuan Police College, Luzhou 6460051, China)
【Abstract】 UO2-ZrO2 composite was prepared by Sol-gel. Solid solution formation, microstructure and densification of the composite were investigated and analyzed. X-Ray Diffraction results indicate that the UO2-ZrO2 is composed of both tetragonal ZrO2 solid solution and face-center cubic UO2 solid solution up to 1600℃. As the temperature increases, relative amount of ZrO2 solid solution in the composite decreases gradually while that of UO2 solid solution increase, with 2θ shifting accompanying. Because of the different diffusion rate of uranium and zirconium, zirconium preferentially diffuses into the UO2 phase and the UO2 matrix expands to accept extra zirconium cations, leaving vacancies at the original ZrO2 site. The whole process brings in new pores in the pellets after sintering and results in the decreasing of density. Sintered above 1650℃, the major phase of the matrix is (U,Zr)O2, and The average grain size increases quickly.
【Key words】 solid solution; densification; UO2-ZrO2; grain size
1 Introduction
UO2-ZrO2fuel material attains a burn-up of at least 25×1020fissions/cm3with adequate irradiation stability and corrosion resistance. The advantages of UO2-ZrO2fuel over UO2and other materials have been established after extensive irradiation testing of oxide ceramic fuel materials. Consequently UO2-ZrO2starts to be considered as possible use of nuclear fuel[1-2]. UO2is known to form substitutional solid solution with different metal oxides[3-6]. However, UO2can only form a limited range of solid solution with ZrO2because of crystal structure mismatch and size difference between Zr4+(0.084nm) and U4+(0.10nm)[7]. UO2is face-center cubic structure, and ZrO2is monoclinic at room temperature, and transforms to tetragonal phase at 1170℃ and to cubic (fluorite type) phase at 2170℃[8-9].
In the present work, the processes of solid solution formation and grain growth of UO2-ZrO2during sintering were investigated. Based on the achieved results, the changes of phase, densification and grain growth with temperature are revealed. The present work provides fundamental parameters describing the sintering behavior of UO2-ZrO2in a reducing atmosphere.
2 Experiment
2.1 Microspheres preparation
The UO2-ZrO2materials used in this study were synthesized by a sol-gel processing approach, using UO2and ZrO(NO3)2·nH2O as starting materials. U0.7Zr0.3O2solid solutions and pure uranium and zirconium oxides were synthesized from nitrate precursors by complexing the metal cations with the citrate. The sols were prepared by adding the corresponding alkoxides to an aqueous solution of nitric acid. Subsequently, the suspensions were stirred for 2 h to obtain a complete dissolution of precursors and then cooled at room temperature for about 6 h. The acidic sols were dialyzed to a final pH in the range of 2.5~3, and active catalyst was deposited. The microspheres were dried at 80℃ for 1 h in air. Then, the obtained microspheres were calcined in an open muffle furnace at about 350℃ for 4h at a heating rate of 2℃/min to remove undesirable solvent and organics in part. The calcined microspheres were sintered in a furnace under H2in the range of 1500~1700℃ for 2h.
2.2 Materials characterization
Powder XRD patterns of UO2-ZrO2microspheres sintered at different temperatures (1500, 1600, and 1700℃) were recorded on a Seifert XRD 3000P diffractometer using nickel-filtered Cu Kαradiation. SEM micrographs were taken with a conventional scanning electron microscope (Cambridge Inc.). The grain size of the sintered compact was determined from the obtained SEM photos using the linear intercept method.
3 Results and discussion
3.1 Formation of (U,Zr)O2 Solid solution
Fig.1 shows the X-ray diffraction patterns of the microspheres partially sintered at different temperatures. Both the UO2and ZrO2phases remain up to 1600℃, and the ZrO2phase starts to disappear at 1700℃, indicating that additional phases might form. Accordingly, reaction between UO2and ZrO2starts in the temperature range of 1000~1700℃[10], where UO2-ZrO2microspheres are remarkably densified. Reaction between UO2and ZrO2initially induce the formation of fluorite UO2solid solution and tetragonal ZrO2solid solution. It is still ambiguous that the presence of the tetragonal phase is either due to the dopant effect or the grain size effect. The formation of ZrO2tetragonal solid solution is believed due to the fine grains, and consequent enhanced surface reactivity and diffusion of the atoms at the surfaces.

Fig.1 XRD pattern of sintered UO2-ZrO2 microspheres
The XRD results in Fig.1 show that peaks of UO2solid solution shifts with the increasing formation of UO2solid solution as the temperature increases, while ZrO2solid solution decreases and its 2θ shifts little. It means that UO2solid solution forms much more easily than ZrO2solid solution during sintering since size of the metal ion, oxidation state, electronegativity and structure are the important factors which determine the solubility of metal oxide[11]. However, ZrO2forms a limited range of solid solution with UO2because of mismatch in ionic sizes of Zr4+(0.084nm) and U4+(0.10nm) and difference in the structures of UO2and ZrO2[7]. The lattice of UO2contracts due to forming substitutionnal solid solution. The lattice parameter evolves linearly with the ZrO2content.
Since the different formation rates of solid solution, ZrO2solid solution exists temporarily, and Zr4+substitutes U4+gradually, and the composition of microspheres finally fits (U,Zr)O2. This fact suggests that the diffusion of Zr ions in UO2is much more favorable in forming a (U,Zr)O2sold solution rather than the reciprocal diffusion. The solubility difference between UO2and ZrO2suggests that Zr ions can diffuse directionally into UO2, at least in an initial stage of (U,Zr)O2formation.
3.2 Microstructure and densification
Fig.2 is the microstructure of UO2-ZrO2sintered at different temperature. It shows that pores grow as temperature increasing. These large pores are due to the difference in sintering rates between UO2-UO2particles and UO2-ZrO2particles, it is proposed that the formation of large closed pores generated by local nonuniform volume variations caused by the difference in the densification rate resulting from the reactions between UO2-UO2particles and UO2-ZrO2particles. These large pores are formed at high temperatures by reacting UO2-ZrO2when the pore structure is already essentially closed and are therefore difficult to eliminate. The pores remain as closed pores in the following sintering cycle and are the direct cause of the decreased final density of the system[12]. This region originally consists of ZrO2particles below 1600℃. It is supposed that new pores are produced during the formation of (U,Zr)O2in the region where ZrO2particles were originally placed.
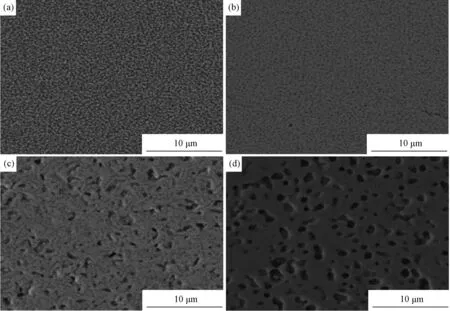
Fig.2 SEM of UO2-ZrO2 sintered at different temperature (a) 1500℃; (b) 1600℃; (c) 1650℃; (d) 1700℃
In this work, the interdiffusion studyon UO2/ZrO2couples revealed that the penetration of zirconium into UO2is considerably more favorable than the penetration of uranium into ZrO2. This observation confirms that the process of material transport is unbalanced during solid solution formation while sintering UO2-ZrO2fuel prepared by sol-gel, where ZrO2agglomerates are dispersed in a UO2matrix before sintering. The zirconium cations diffuse more quickly into the UO2phase than the opposite, and a higher flow of zirconium derived from the ZrO2agglomerates in the direction of the UO2phase is established, when compared with uranium flow in the direction of the interior of the ZrO2agglomerates. In this situation, the occurrence of the Kirkendall effect is probable. The UO2rich phase expands to receive the extra zirconium cations and a void is generated at the site of the original ZrO2agglomerate. This phenomenon is commonly observed in mixed powders systems where an unbalanced diffusivity or solubility between the powder exists[13-15].
Fig.3 is the relation between temperature and density. Fig.3 shows that the density of UO2-ZrO2increases before 1600℃, and then decreases as the temperature further increases. Before the formation of (U,Zr)O2, the densification could take place mainly by sintering between UO2and ZrO2particles, and the diffusion of ions may be mainly dependent on temperature. As discussed above, the formation of (U,Zr)O2involves the directional diffusion of Zr ions into UO2and the formation of new pores, which results in the decrease of density as the temperature increases because of the growth of new pores.

Fig.3 Relation between temperature and density
Fig.4 is the optical microscopy of UO2-ZrO2microspheres sintered at different temperature. Fig.4 shows that ZrO2is uniformly distributed and that it diffuses into UO2zone as the temperature increased. Microstuctural examinations of the samples show a significant development of the UO2-ZrO2fuel matrix polycrystalline structure. Fig 4 illustrates this evolution with average grain sizes larger than 5μm in the sintering at 1650℃. The grain sizes of both phases are described in nano-meters, nano-composites, resistant to grain growth at high temperatures. In addition to the microstructural examination, the different phases present in the fuel variants. It was observed that microsphere sintered at 1650℃ is mainly composed of (U,Zr)O2solid solution. However, many free ZrO2areas are present below 1550℃. It can be assumed that ZrO2is dissolved or dispersed in the fuel matrix.

Fig.4 Optical microscopy of UO2-ZrO2 microspheres (a) 1550℃; (b) 1650℃
The way of ions diffusion effects the grain growth[16-17]. In the solute-drag effect, the grain-boundary mobility depends on lattice diffusion (LD) of solute atoms segregated along the grain boundaries. The segregated Zr4+ions in UO2have the effect that enhances grain-boundary diffusion (GBD). At sintering temperatures above 1650℃, the predominant effect that enhances grain growth is clearly caused by the effect of segregated Zr4+.
4 Conclusion
1.Zirconium prefers diffusing into UO2to uranium into ZrO2. ZrO2solid solutions disappear gradually and UO2solid solutions predominate as the temperature increases because of the imbalance in material interdiffusion transport solid solution.
2.Based on the Kirkendall effect, densification during sintering occurs simultaneously with the formation of pores at sites where ZrO2originally presented. As soon as their formation, it is difficult to eliminate these pores in the subsequent sintering process. The pores remain in the sintered pellet and cause the low densities.
3.The grain size of UO2-ZrO2fuel increases quickly sintered above 1650℃, where the major phase of matrix is face-center cubic (U,Zr) O2.
