Male zebra fish(Danio rerio)odorants attract females and induce spawning
2018-08-10JieLiPeterHubbrdAdelinoCnrio
Jie Li,Peter C.Hubbrd,Adelino V.M.Cnário,b
aCollege of Fisheries and Life Science,Shanghai Ocean University,Shanghai 201306,China
bCentro de Ci^encias do Mar,Universidade do Algarve,Campus de Gambelas,8005-139,Faro,Portugal
Keywords:Pheromone Zebra fish Reproduction Behavior Olfaction Spawning
A B S T R A C T In zebra fish,the chemical identity and biological roles of reproductive pheromones have yet to be clarified.The current study assessed the role(s)of male-released odorants in a reproductive context,and evaluated the possible involvement of steroids and their metabolites.Females were placed in chemical and/or visual contact with males.In chemical contact,females ovulated as frequently whether they could see the males or not.Conversely,females with visual contact alone with males ovulated as frequently as isolated females.Male-released odorants attracted females;visual contact with males attracted females,but to a lesser extent;the two effects were additive.The olfactory potency of solid-phase extraction of male-and female-conditioned water was assessed by the electro-olfactogram;males released more potent odorants than females,but the most potent extract came from water in which males and females interacted freely.HPLC fractionation of these extracts revealed that,while in all samples some fractions contained odorants,male-specific activity was contained in the first four fractions,suggesting that the compounds involved are relatively hydrophilic.Finally,the olfactory potency of some steroids,previously suggested to have a pheromonal role,was assessed.However,it is unlikely that these steroids contribute significantly to the odor of fish-conditioned water.
1.Introduction
Chemical communication is animportant facet of reproduction in teleost fishes.The zebra fish(Danio rerio)is an important model species for developmental,genetic and behavioral studies.Yet the chemical identities of its reproductive pheromones remain unknown.Pioneer work in the Netherlands suggested that steroid glucuronates,produced by the testes,are capable of attracting females(Van Den Hurk&Resink,1992;Lambert,Van Den Hurk,Schoonen,Resink,&Van Oordt,1986;Van Den Hurk,Schoonen,Van Zoelen,&Lambert,1987);however,these were never tested individually,or assessed for olfactory potency.On the other hand,4-pregnen-17,20β-diol-3-one 20-sulfate(17,20β-P-sulfate),one of the female-released steroid pheromones identified in the gold fish(Carassius auratus;like the zebra fish,acyprinid)(Dulka,Stacey,Sorensen,&Van Der Kraak,1987;Poling,Fraser,&Sorensen,2001;Sorensen,Scott,Stacey,&Bowdin,1995)is a potent odorant for the zebra fish(Belanger,Pachkowski,&Stacey,2010;Friedrich&Korsching,1998).Another female-released gold fish reproductive pheromone,prostaglandin F2αis detected with high sensitivity by zebra fish and mutant male zebra fish deficient in its receptor have impaired courtship towards females(Yabuki et al.,2016).
Here we tested the hypothesis that male zebra fish release odorants that attract females and promote spawning,using a combination of behavioral experiments,electrophysiology and high performance liquid chromatography(HPLC).Firstly,we tested the relative importance of visual and chemical cues from the males on female ovulation and behavior.Secondly,we subjected maleand female-conditioned water to solid-phase extraction and HPLC.We then the assessed olfactory potency of these fractions by the electro-olfactogram(EOG)and compared these responses to those evoked by a range of steroids that may form part of the male pheromone.We conclude that males release powerful compounds to the water that attract females and induce them to ovulate but,unlike previous suggestions,sex steroids and their conjugates do not appear to be a major component of the male-released odorants.Thus,more work is needed to identify the male reproductive pheromone in zebra fish.
2.Materials and methods
2.1.Fish and housing conditions
Wild-type zebra fish of the AB strain three to six months old(3-4 cm standard length,2.0-3.0g)and sexually mature were used in this study.Fish were kept at 26-28°C on a 14:10 light:dark photoperiod and fed twice daily at 11:00 and 18:00 with dried brine shrimp(Artemia salina).As soon as their sex could be determined(three months onwards),males were separated from females and kept in single-sex groups.Seven days before the experiments(the ovulatory cycle is five to seven days)single males and females were mated to ensure that they were reproductively active.Fish that spawned were placed again in tanks of single-sex groups to be used in all experiments described below.Fish used forelectro-olfactogram(EOG)recording were taken from a separate stock;all female and similar age and size as those used in the behavior experiments.
2.2.Ovulation experiment
Twenty females were placed in one side of a 24cm×10cm×10cm Perspex tanks separated into two by a division,each division with 1l of water.The division could be transparent or opaque without or with 1mm in diameter perforations per cm2.For the control group ‘no vision,no olfaction’,20 females were placed in one side of the tank and 20 males in the other separated by an opaque division.For the'vision only'group,20 females were placed in one side of the tank and 20 males in the other separated by a transparent division without perforations.For the'olfaction only'group 20 females were placed in one side of the tank and 20 males in the other separated by an opaque division with perforations.Finally,for'vision and olfaction'group,20 females were placed in one side of the tank and 20 males in the other separated by a transparent division with perforations.The 20 males and females were placed in their respective compartments at 18:00 the previous day.The next morning,30 min after'lights on'(10:00 a.m.)the females were checked for ovulation by applying gentle pressure to the abdomen(without anesthesia,but under water);those which released eggs were removed from the experimental tank.The procedure was repeated three times at 30 min intervals.The experiment was repeated six times independently with different fish(i.e,six times with 20 fish in each treatment group).The data were compared by one-way ANOVA followed by Tukey'spost hoctest(data were normally distributed with equal variance).
2.3.Attraction experiment
The attraction experiment was carried out in 24cm×5cm×10 cm Perspex tanks with divisions as in the ovulation experiment,each half containing 0.5l water.A 3cm wide region on the female side,contiguous to the middle division,was defined as the attraction zone(Fig.1).One female and one male zebra fish were placed separately either each side of the division,opaque or transparent,with or without holes as above;the control group"no vision,no olfaction"had an opaque division with no holes,the"vision only"group had a transparent division,the"olfaction only"group had an opaque division with holes,the"olfaction and vision"group had a transparent division with holes.Experiments started at 30 min after lights on(peak time of day for spawning activity)and the fish was filmed for 30 min by a digital camera(Sony Handycam FDRAXP55).Each treatment was repeated six times(independently with different fish each replicate).The videos were analyzed by Ethovision XT software(version 11.5,Noldus Information Technology,Wageningen,the Netherlands).The proportion of time that the female spent in the attraction zone was measured.The data were arc-sign transformed and compared by One-Way ANOVA followed by Tukey'spost hoctest(data were normally distributed with equal variance).
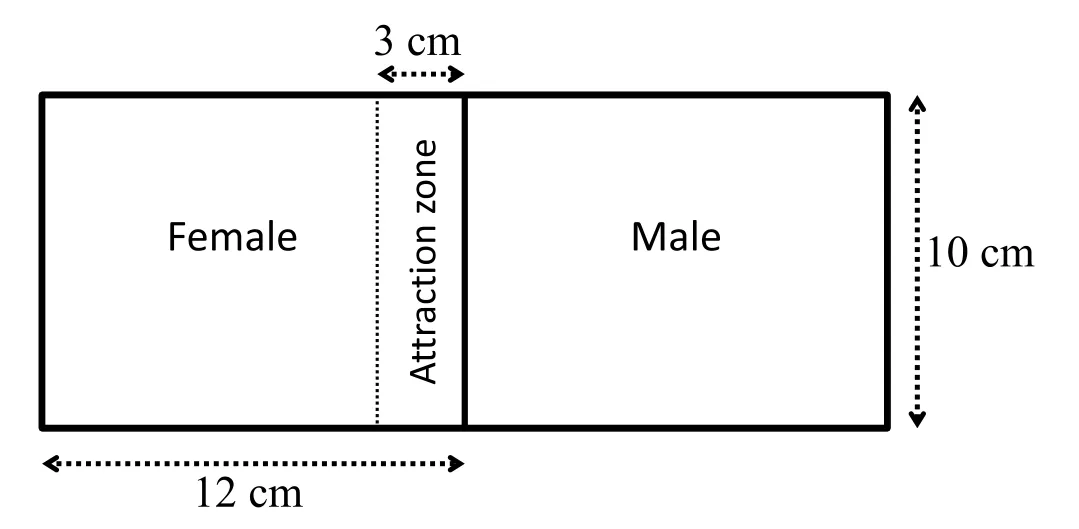
Fig.1.Schematic front view of tanks used in the behavioral experiments.Each tank was divided by a partition separating the male from the female side.The partition could be either transparent without holes(vision only),transparent with a 2 mm diameter perforation per cm2(vision and olfaction),opaque without holes(neither vision nor olfaction),or opaque with a 2 mm diameter perforation every cm2(olfaction only).The 3 cm wide rectangular region on the female side(broken line)next to the partition was defined as the"attraction zone".
2.4.Fish-conditioned water
To extract and,eventually,identify odorants-putative pheromones-released by zebra fish, fish-conditioned water was prepared by using a similar experimental approach outlined above for the ovulation experiment with 500 mL water on each side of the division:i) five males and five males that could not see each-other but with chemical contact;ii) five males and five females with both visual and chemical contact.The fish were placed in the tanks at 17:00 of one day until 11:00 the following morning(18 h)to allow plenty of time for odorants to be released.The fish were then removed and the water extracted through a 500 mg C18 solidphase extraction column(Cronus,SMI-LabHut Ltd.,Gloucester,UK)using methanol to prime the cartridges and to elute the retained compounds.The methanol was evaporated to dryness(under nitrogen)and the pellet re-dissolved in 100μL HPLC grade methanol.
2.5.High performance liquid chromatography(HPLC)of water extracts
20μL of the solid-phase extract of fish-conditioned water(described in the previous section)was injected ontoaHPLC system(Smart line KNAUER,Berlin,Germany)with aC18column(3.9mm×300 mm;4μm particle size;Nova-Pak,Waters).HPLC conditions were as follows:mobile phase was pure water(MilliQ)and methanol(HPLC-grade),both containing 0.001%FA;0-4min isocratic at 15%methanol,5-91 min linear gradient from 15%to 100%methanol,91-96 min isocratic at 100%methanol; flow-rate:0.7mL/min,the same conditions used to isolate the steroid glucoronate pheromone from urine of male tilapia(Keller-Costa et al.,2014).The column eluate was first routed to a diode array UV detector(Smartline 2600,KNAUER,Germany),then split off and half routed to an evaporative light scattering detector(Varian 380-LC ELSD,Polymer Laboratories)and the other half collected by an Advantec CHF100SA fraction collector into 32 fractions,each 3min(i.e.2.1 mL).ELSD conditions were as follows:nitrogen carrier gas(≥98%purity,1.6 SLM),65°C nebulization temperature,110°C evaporation temperature.
2.6.Recording the electro-olfactogram(EOG)
The EOG is a robust and sensitive method for assessing olfactory potency of a given compound or biological sample.In the current study,it was used to screen the HPLC fractions and steroids(and some conjugates)for olfactory activity.The EOG was recorded from female zebra fish as previously described for gold fish(Hubbard,Barata,&Canário,2002)with the following modifications.Zebrafish were anesthetized by immersion in water containing 250 mg/L 3-aminobenzoic acid ethyl ester(MS222;Sigma-Aldrich)but not given any neuro-muscular blocker.They were maintained alive by aerated,anesthetic-containing water flowing over the gills(by gravity)at a flow of 2-3 mL/min,and odorant-containing water was delivered to the olfactory epitheliumviaa thin glass tube(under gravity)at a flow of around 1 mL/min;no surgery was necessary to expose the olfactory rosette.The EOG was recorded using borosilicate glass micropipettes filled with 1 mol/L NaCl in 2%agar bridged to the D.C.amplifier with Ag/AgCl pellets in 3mol/L KCl.Pulses(4s)of odorant-containing solution were introduced into the flow of water over the olfactory rosetteviaa computer operated solenoid valve.The optimal position for the recording electrode was found using 10-5ML-serine as a stimulus;this was also used to normalize the response amplitude and check the stability ofthe preparation through out the recording period(approximately every 12 min),usually evoking a response between-1 and-2mV(-1.46±1.02 mV,N=11).HPLC fractions were diluted 1:100 in water(i.e.odorants would be at approximately the same concentration as in the original water sample).Pure steroids were all given at 0.5 mg/L(approximately 10-6M).Data are shown as arithmetic mean±standard deviation(SD).
3.Results
3.1.Spawning
When female zebra fish could neither see nor smell the males,few of them ovulated(Fig.2).Similarly,when they could not smell the males,but could see them,few females ovulated.However,when the females could smell the males,about half of them ovulated,whether they could see the males or not.This suggests that olfaction of male-released odorants is much more important for female ovulation than visual contact with the males.In all cases,with or without visual and chemical contact,the females ovulated almost entirely within the first hour of the experiment,mostly within the first 30 min(data not shown).
3.2.Attraction
When female zebra fish could neither see nor smell the males,they spent less than 10%of their time in the attraction zone(Fig.3).However,if they had chemical contact with the males but could not see them,they spent significantly more time in the attraction area(approximately 45%)than when in visual contact but without chemical contact(approximately 25%).With both visual and chemical contact with the males,females spent significantly more time(approximately 70%)in the attraction area.Thus,olfaction is more important than vision in this behavior,but both play a role;their effects are additive.
3.3.EOG responses to HPLC fractions
The EOG responses to HPLC fractions proved to be rather variable(only female zebra fish were used for EOG recording),so only qualitative comparisons were made.Nevertheless,several features became clear.Firstly,males released more potent odorants than females(Fig.4).A large part of these male-released odorants exited the column in the first four fractions(0-12 min);the activity in these fractions was much greater when males and females were in chemical contact,suggesting that males may be stimulated to release moreputative pheromones when in the presence of females(it should be noted that spawning occurred in this tank).Similarly,responses to fractions 15 and 20 were greater in water with males and females together than in that of males or females alone.In this case,however,it is less clear which sex released these odorants.Finally,fraction 7 evoked similar amplitude EOGs in water with males and females,and water with males alone;this fraction was less potent in water from females alone.
3.4.EOG responses to steroids and metabolites
Olfactory responses to the steroids(all at 0.5 mg/L;around 10-6mol/L)were also variable,but rarely exceeded the response to 10-5mol/LL-serine(Fig.5).The most potent androgens(C19 steroids)were 4-androstenedione,a steroid previously shown to have a pheromonal role in the gold fish(Sorensen,Pinillos,&Scott,2005),and the structurally related 4-androstene-11β,17β-diol-3-one(11β-hydroxytestosterone)and 11β-hydroxyandrostenedione.5α-androstane-3α,17β-diol was also among the most potent;however,its glucuronated metabolite was without olfactory potency.Among the progestogens(C21 steroids),5α-pregnane-3α,17,20β-triol,5α-pregnane-3α,17-diol-20-one and 4-pregnane-17,20α-diol-3 one were the most potent;more potent than 4-pregnene-17,20β-diol-3-one,the well characterized maturationinducing steroid(Nagahama,1997)and gold fish pre-ovulatory pheromone(Dulka et al.,1987).
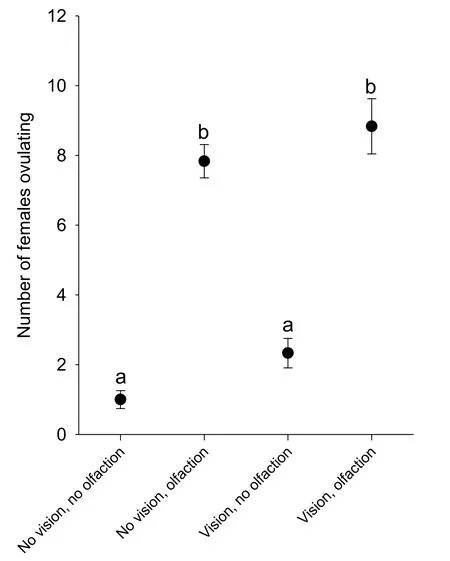
Fig.2.Effect of visual and/or chemical contact(olfaction)with males on spawning in female zebra fish.Numbers of females(out of a total of twenty)that spawned within two hours in the four different treatments(with or without visual and/or chemical contact).Groups with different letters are significantly different from each-other(P<0.05;One-Way ANOVA followed by Tukey's test,F3,23=55.556,N=6 per treatment group).
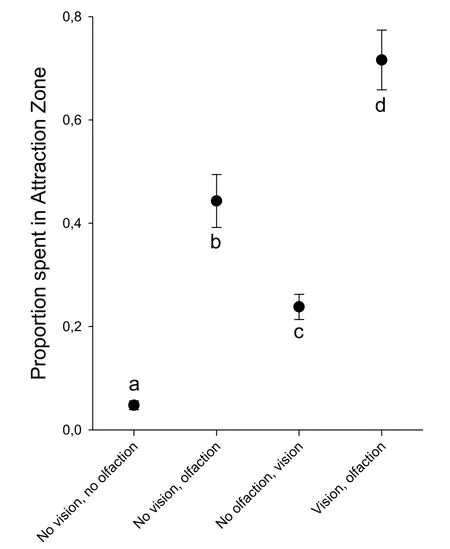
Fig.3.Effect of visual and/or chemical contact(olfaction)with males on attraction in female zebra fish.A.Percentage of time spent by the female in the predefined‘attraction area’adjacent to the male's half of the tank.Groups with different letters(with or without visual and/or chemical contact)are significantly different from eachother(P<0.05,One-Way ANOVA(arc-sign transformed data)followed by Tukey's test,F3,23=50.146,N=6 per treatment group).
4.Discussion
The current study has shown that female zebra fish are attracted to odorants released by males,consistent with earlier studies(Bloom&Perlmutter,1977).Furthermore,it shows that the attraction to odorants is greater than that to the sight of the males,and that these two effects are additive.Male odorants also induce ovulation in females,again consistent with earlier studies(Chen&Martinich,1975;Van Den Hurk&Resink,1992;Van Den Hurk et al.,1987).Furthermore,olfaction is of prime importance;visual contact alone with males does not appear to have any effect,as few females ovulated in visual contact alone as with neither visual nor chemical contact.Thus,putative male zebra fish pheromones have both ‘primer’and ‘releaser’effects on females.
The gold fish(like the zebra fish,a cyprinid)female pre-ovulatory pheromone has been identified as a mixture of the maturation inducing steroid 17,20b-dihydroxy-4-pregnen-3-one and its glucuronate and sulfate metabolites(Dulka et al.,1987;Scott&Sorensen,1994;Sorensen et al.,1995;Van Der Kraak,Sorensen,Stacey,&Dulka,1989).It was proposed that steroid glucuronates also comprise the male pheromone in zebra fish and 7 steroid glucoronates in testis and 5α-androstan-3α,17β-diol and cholesterol glucoronates in male holding water have been identified(Lambert&Resink,1991;Lambert et al.,1986;Van Den Hurk et al.,1987);however,in the present study,the olfactory potency of 5α-androstan-3α,17β-diol was much higher than its glucoronate.In fact,none of the steroid sulfates or glucuronates tested showed sufficient olfactory potency to justify further investigation into their possible pheromonal roles;the concentrations used(around 10-6M)would be extremely high for a pheromone.Nevertheless,that malesarereleasing potent odorants is clear from the EOG responses to the HPLC fractions.
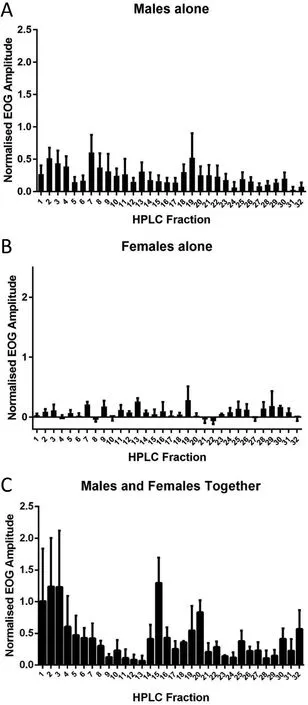
Fig.4.Olfactory responses to fractions of male and female holding water.A.HPLC fractions of male holding water(in visual but not chemical contact with each-other).B.HPLC fractions of female holding water(in visual but not chemical contact with eachother).C.HPLC fractions of holding water with males and females in visual and chemical contact.Data are shown as mean+SD(N=3 or 4).

Fig.5.Olfactory responses to steroids in zebra fish.Normalized EOG amplitudes(mean+SD,N=3 or 4)to a single concentration(0.5 mg/L;approximately 10-6mol/L)of steroids,and some glucoronate and sulfate metabolites,suspected to play pheromonal roles in the zebra fish.
Firstly,only those odorants retained by the solid-phase extraction(SPE)cartridges can be responsible for the EOG responses seen to the HPLC fractions.SPE is commonly used to extract steroids and their metabolites for measurement(Scott et al.,2008)or identification(Keller-Costa et al.,2014).It is likely,therefore,that any steroids and/or conjugates released by zebra fish would be present in the solid-phase extract.However,it seems unlikely that any of the tested steroids contribute to the olfactory responses to the HPLC fractions of male-conditioned water,as they are insufficiently potent odorants.Nevertheless,we cannot exclude steroidsper se;it is necessary to identify the compounds present in the more olfactory potent fractions.
Secondly,it is clear that male zebra fish release more potent odorants than females;at least,those that are retained on the solidphase extraction cartridges.Although females are thought to release ovarian steroids that play a pheromonal role on males(Van Den Hurk&Lambert,1983),the primacy of male released odorants in reproductive chemical communication in zebra fish is suggested by both physiology and behavior in the current study.That a significant proportion of the odorants are found in the first four fractions( first 12 min)means that they have relatively high polarity and,perhaps,are non-steroidal in nature.Future work needs to clarify this,although odorants eluting later from the HPLC column(e.g.,fractions 15,19 and 20)may well be steroidal compounds.
Thirdly,the larger EOG responses to fractions from water containing both females and males suggests that,when in chemical communication with each-other,one sex is stimulated by the other or that they are mutually stimulated to release odorants.Since water was collected soon after lights on some females ovulated and spawned,prostaglandin F2α,which influences attraction and spawning behavior(Yabuki et al.,2016),could be the female chemical responsible for the activation of the male pheromone release.The large responses to the first four fractions are also seen in the male-alone conditioned water,so it is likely that these odorants are male-released.On the other hand,the odorants in fraction 15 could come from either sex;this odorant does not appear to be present in either female-alone or male-alone conditioned water.Nevertheless,it seems that the smell of the opposite sex can induce odorant-putative pheromone-release in either or both sexes.
In conclusion,the current study supports the role of pheromones in zebra fish reproduction,and indicates the primacy of male-released pheromones-as yet unidentified-on both the physiology and behavior of females.However,the contribution of steroids and/or their metabolites,at least those tested,must be augmented by other male-released odorants;zebra fish pheromones are likely to be complex mixtures.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics statement
The animal experimentation procedures used in this study followed the institutional guidelines for the use of animals in experimentation and were approved by the Ethics Committee of Shanghai Ocean University(behavior experiments)and of the Centre of Marine Sciences(electrophysiology experiments)and by the National Veterinary Authority(DGAV,Portugal;group 1 license).
Acknowledgements
Li Jie was supported by a grant from Shanghai Aquatic Products(grant no.A1-2035-150048C1).This study received national funds from Fundaç~ao para a Ciência e a Tecnologia(FCT;Portuguese Foundation for Science and Technology)through project UID/Multi/04326/2013.
杂志排行
Aquaculture and Fisheries的其它文章
- Prey quality impact on the feeding behavior and lipid composition of winter flounder(Pseudopleuronectes americanus)larvae
- Gladius growth pattern and increment of jumbo squid(Dosidicus gigas)in the tropical Pacific Ocean
- Antibacterial mechanism of Ginkgo biloba leaf extract when applied to Shewanella putrefaciens and Saprophytic staphylococcus
- Dermestes maculatus Degeer infestation impact on market loss of dried fish in Kwara State,Nigeria
