Heterogeneous genetic architecture by gender for precision medicine of cardiovascular disease
2018-08-09ChaeyoungLee
Chaeyoung Lee
School of Systems Biomedical Science, Soongsil University, Seoul, Republic of Korea
Keywords: Cardiovascular disease; Genetic heterogeneity; Genome-wide association study; Mixed model; Sex difference
It is well-known that gender differences exist in the onset,progression, and prognosis of cardiovascular diseases(CVDs),[1]and that risk factors such as high blood pressure and lipid profiles vary between men and women.[2]Currently, sex differences are stressed as important variables to take into account when examining the etiology of CVD.Genome-wide association studies of CVD have employed the sex as a covariate in their analytical models, but generally disregarded potential genetic heterogeneity (GHS) attributable to sex. Spurious or biased genetic effects can be produced by ignoring various types of GHS for CVDs (Figure 1). Only sex-limited CVDs (e.g., gestational hypertension as a female-specific disease) for which genetic factors are analyzed for one sex are free from such biases. The bias might become more serious for the elderly, because genetic effects contribute to more variations in blood pressure and lipid profiles in older people.[3]
Various sources of information can be utilized with respect to the GHS for CVD. Information on both population risk variants and individual risk values is important to clinical applications regardless of the corresponding reflection of the risk variants on the risk values (Figure 2). The information on the significant variants is often more important for treatment, and the pooled risk assessment is often more important for prevention. It might be essential to provide not only GHS of CVD itself, but also the GHS of its risk factors in understanding the mechanism of GHS (Figure 2). Specific risk factors and standards should be determined for each CVD.
Various analytical methods for explaining GHS were discussed with the corresponding statistical models in a previous study.[4]It was suggested that a sex-influenced disease should be treated as two different diseases (or phenotypes)based on sex in determining genetic factors and in estimating genetic effect size. That is, the genetic analysis should be separately conducted by sex, or a two-disease model could be utilized to treat female data as one disease and male data as another, employing a genetic covariance structure between men and women. Naturally, careful attention should be paid to analysis of the data to avoid confounding genetic effects. When a genetic effect is estimated and/or a genetic association is determined, other genetic factors and environmental factors could be appropriately explained by the analytical model. CVDs are typical complex diseases,inevitably influenced by many genetic factors. Genetic factors can be identified by the Bayesian approach, employing a Markov chain Monte Carlo method to avoid violating transgressive assumptions for genetic analysis of sex-influenced diseases.[4]Risk values can be estimated by the best linear unbiased prediction using genetic covariance among individuals.[5]All of these analyses are based on a mixed model approach that can reflect polygenic effects by examining genetic relationships among individuals.[6–8]They also control population stratification and thus reduce false positive genetic associations.[9]
Although the GHS is reflected in genetic analysis for CVD, a guarded evaluation on the resulting genetic factors is necessary, because they are moving targets produced from a variety of invisible interactions. For example, dimorphic genomic components originated from different structures of the genome (e.g. sex chromosomes) and different pressures of natural selection based on sex (e.g. sexually antagonistic selection)[10]may interact with environmental factors and/or other genetic factors (especially, sex hormone-responsive elements and genes). Thus, it is hard to reflect these hidden conditions in every individual. To reliably estimate the stochastic risk of an individual, a high priority has to be placed on finding a population that shared similar genetic and environmental effects to the individual undergoing examination.

Figure 1. Schematic concepts for genetic heterogeneity of cardiovascular disease based on sex. The difference in genetic variance (Vg)between women and men is negligible (A) or significant (B). Genetic variance is limitedly defined for only one sex (C).
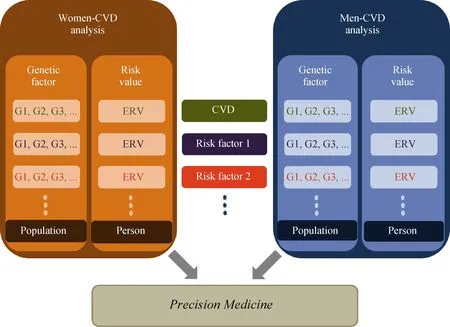
Figure 2. Strategic information of genetic heterogeneity by sex for CVD. G1, G2, and G3 are genetic factors with their effect sizes for CVD or its risk factors in females (or males), and ERV is genetic risk value estimated for an individual. CVD: cardiovascular disease; ERV: .
Most genome-wide association studies for CVD have included fixed sex effects as a covariate in analytical models and neglected GHS. These analyses might have failed to identify many sex-specific genetic associations with CVD.Accuracy in the genetics of CVD has emerged as more of a concern than ever with rapid changes of technology that can dramatically reduce the cost of genomic nucleotide sequencing. Reflecting on the GHS in estimating genetic architecture for CVD, is considered as one of the urgent tasks in the discipline of geriatric cardiology, and it may take us a step further to precision medicine for customized treatment of CVD. Knowledge on the GHS will shed new light on understanding complex genetics and the underlying mechanisms for CVD.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Echocardiography in centenarians: characteristics, utility and follow-up
- Sphingosine 1 phosphate receptor-1 (S1PR1) signaling protects cardiac function by inhibiting cardiomyocyte autophagy
- Association between baseline platelet count and severe adverse outcomes following percutaneous coronary intervention
- Optimal timing of staged percutaneous coronary intervention in ST-segment elevation myocardial infarction patients with multivessel disease
- Predictors of long-term outcome in patients with biopsy proven inflammatory cardiomyopathy
- Persistent ductus arteriosus in old patient with atrial fibrillation
