Anti-epileptic effect of morin against experimental pentylenetetrazol-induced seizures via modulating brain monoamines and oxidative stress
2018-08-02AmitKandhareAnweshaMukherjeeSubhashBodhankar
Amit D. Kandhare, Anwesha A. Mukherjee, Subhash L. Bodhankar
Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Erandwane, Paud Road, Pune-411 038, India
Keywords:Brain GABA Epilepsy Morin Nitric oxide Pentylenetetrazol Oxidative stress Xanthine oxidase
ABSTRACT Objective: To evaluate the protective effect of morin against pentylenetetrazol (PTZ)-induced tonic-clonic convulsions in mice. Methods: Swiss albino mice (18-22 g) was used to induce convulsions by intraperitoneal (i.p.) administration of PTZ (90 mg/kg). Mice were either pretreated with morin (10, 20 and 40 mg/kg) or vehicle (distilled water, 10 mg/kg) 45 min before PTZ administration. Various behavioral and biochemical parameters were assessed. Results:PTZ administration resulted in significant production (P<0.001) of tonic-clonic conclusion and mortality in mice. PTZ-induced increase in the duration of convulsion, onset of convulsion and mortality was inhibited significantly by morin (20 and 40 mg/kg) administration. The PTZ-induced decrease in brain GABA, dopamine and Na+K+ATPase levels and increase in xanthine oxidase activity were inhibited significantly by morin (20 and 40 mg/kg) treatment. The increased levels of malondialdehyde and nitric oxide level were significantly decreased by morin(20 and 40 mg/kg) treatment. Also, reduced levels of superoxide dismutase and glutathione were increased significantly by morin treatment. Conclusions: Results of the present study indicate that morin showed its anti-convulsant effect via modulating the levels of brain GABA,Na+K+ATPase, and oxido-nitrosative stress. Thus, morin can be a potential candidate for further clinical evaluations as an anti-epileptic agent.
1. Introduction
Epilepsy is a chronic disease of neurobiological system, affecting approximately 50/100 000 individual while 100/100 000 individual in developed and developing countries, respectively[1]. It has been reported that epilepsy prevalence in India is about 1/18th of the world population which is about 5.5-7.9/1 000 individual[2]. The children below 7 years-of-age and person of above 55 years have the higher epilepsy incidence[2].
Research carried out over past decades indicated that there are various mechanisms responsible for triggering neuronal injury[3-5].This mechanism includes an imbalance between the neurotransmitter system such as glutamate-GABA (gamma-Aminobutyric acid) or impairment in voltage- and receptor-gated ion channels which plays a decisive role in the neuronal cell death. This resulted in various disorders of neurobiological system such as epilepsy, Alzheimer’s disease, parkinsonism and ischemia[6,7]. The increase in the consequence of epileptic seizures led to the development of many antiepileptic drugs for ameliorating the neuronal damage[8].
Currently available synthetic antiepileptic drugs such as oxcarbazepine,vigabatrin, topiramate, zonisamide, levetiracetam,etc. provide the symptomatic relief in epileptic seizures in the fraction of the patients.Moreover, a combination of antiepileptic drugs is associated with side effects and may increase the complications in the clinical management of epilepsy[9]. Additionally, the treatments for epilepsy have not progressed substantially in recent years. Hence, alternative treatment strategies are required.
Numerous evidence suggested that oxidative stress is most evaluated mechanisms of epilepsy along with other[4]. Increased oxidative stress is associated with elevated levels of Reactive Oxygen Species(ROS). This ROS leads to damage to the neurons which may have the minimal regenerative capacity[10-13]. Hence, drugs that alleviate or terminate ROS damage in brain serve to slow down or reduce aggravations of the disease condition. Research carried out over past decade investigated the application of traditional medicines for the management of epilepsy[14,15]. Many herbal isolated moieties from plant origin have shown promising efficacy in clinical and preclinical settings for the treatment of epilepsy[14,15]. These facts and figures support the research for the selection of moieties from the plant origin for epilepsy treatment.
Morin [2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one], a flavonoid from plant origin, has potent antioxidant property[16]. Morin possesses a plethora of treatment effects such as anti-inflammatory, anti-asthmatic, cardioprotective, anti-cancer,renoprotective, anti-allergic, antihypertensive, antiulcer[17-27].Pentylenetetrazol (PTZ)-induced seizure in animals is widely used and well established experimental model for determination of efficacy of various molecules in generalized tonic-clonic convulsions[3,4,28,29].The researcher showed that PTZ induces convulsions by blocking the picrotoxin-sensitive site of the GABAA receptor[30]. It has also been reported that PTZ induces alterations in membrane phospholipid metabolism which in turn results in the release of free radicals via elevated oxidative stress[31]. Various chemical moieties from the class of flavonoids such as fisetin, quercetin, rutin, baicalein,scutellarein, wogonin, and baicalein have been reported to possess the anticonvulsive potential which was mediated by the GABAergic neuron[30,32]. To the best of our knowledge, the antiepileptic efficacy of morin has not been evaluated yet. Hence, present investigation aimed to assess the anti-epileptic efficacy of morin in PTZ-induced tonic-clonic convulsions in experimental animals.
2. Materials and methods
2.1. Drugs and chemicals
Morin and PTZ (Sigma Chemical Co., St Louis, MO, USA), 1, 1′,3, 3′ tetraethoxypropane, crystalline beef liver catalase, 5,5′-dithiobis(2-nitrobenzoic acid) (S.D. Fine Chemicals, Mumbai, India),sulphanilamides, naphthylamine diamine HCl, and phosphoric acid (Loba Chemie Pvt. Ltd., Mumbai, India) were procured from respective manufacturer. Diazepam was obtained from Symed Pharmaceuticals Pvt. Ltd., Hyderabad, India.
2.2. Experimental animals
Adult male Swiss albino mice (18-22 g) were obtained from the National Institute of Biosciences, Pune (India) and maintained at (24 ± 1) ℃ with a relative humidity of 45%-55% and 12:12 h dark/light cycle. The animals had free access to standard pellet chow (Pranav Agro-industries Ltd., Sangli, India) and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00. The experiment was performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals, Government of India on animal experimentation. Animals were brought to the testing laboratory 1 h before the experimentation for adaptation purpose.All the experimental protocols used in this study were approved by Institutional Animal Ethical Committee (CPCSEA/77/2010). The experimentation was conducted in noise free area.
2.3. Experimental design
PTZ-induced convulsion was induced according to previously reported protocol[3,4,29]. Mice were randomly divided into following groups (n=6) as follows:
Group Ⅰ: Normal (N): Mice were treated with distilled water [10 mg/kg, intraperitoneally (i.p.)] and received normal saline (10 mg/kg,i.p.) 45 min after administration of distilled water.
Group Ⅱ: Pentylenetetrazol control (PTZ control): Mice were treated with distilled water (10 mg/kg, i.p.) and received PTZ (90 mg/kg, i.p.) 45 min after administration of distilled water.
Group Ⅲ: Morin (10 mg/kg) [M (10)]: Mice were treated with morin (10 mg/kg, i.p.) and received PTZ (90 mg/kg, i.p.) 45 min after administration of morin.
Group Ⅳ: Morin (20 mg/kg) [M (20)]: Mice were treated with morin (20 mg/kg, i.p.) and received PTZ (90 mg/kg, i.p.) 45 min after administration of morin.
Group Ⅴ: Morin (40 mg/kg) [M (40)]: Mice were treated with morin (40 mg/kg, i.p.) and received PTZ (90 mg/kg, i.p.) 45 min after administration of morin.
Group Ⅵ: Diazepam (5 mg/kg) [DZP (5)]: Mice were treated daily with diazepam (5 mg/kg, i.p.) and received PTZ (90 mg/kg, i.p.) 30 min after administration of diazepam.
The dose selection for morin (10, 20 and 40 mg/kg) was based on a previous study[18]. The symptoms in mice such onset and duration of clonic-tonic convulsion, as well as incidence (number of mice showing convulsions) and mortality, were observed for next 30 min immediately after PTZ administration.
2.4. Locomotor activity
The percentage reduction in locomotor activity after administration of morin was determined according to previously reported methods by using actophotometer (National Scientific Apparatus Works,India)[3,4,29].
2.5. Biochemical evaluation
2.5.1. Brain GABA and dopamine (DA) estimation
As soon as the onset of convulsions occured, mice were sacrificed,and brain was isolated immediately. Previously reported methods were used for evaluation of brain GABA[33] and DA[34].
2.5.2. Brain oxido-nitrosative stress, Na+K+ATPase and xanthine oxidase (XO) estimation
The contents of superoxide dismutase (SOD), glutathione (GSH),malondialdehyde (MDA), nitric oxide (NO content), Na+K+ATPase and XO were estimated in the brain according to previously reported methods[35-42].
2.6. Statistical analysis
Data were expressed as mean ± standard error mean (SEM). The data were analyzed using one-way analysis of variance (ANOVA),Dunnett’s multiple range test was applied forpost hocanalysis. Data of ‘incidence of convulsion’ was analyzed by theChi-square test.Data of ‘mortality’ was analyzed by Fisher’s exact test. The statistical analysis was performed using GraphPad Prism 5.0 (GraphPad, San Diego, USA). TheP<0.05 was considered as statistically significant.
3. Results
3.1. Effects of morin on duration and onset of tonic-clonic convulsion
Administration of PTZ caused hind-limb and tonic-clonic seizure whereas morin (20 and 40 mg/kg) pretreated significantly (P<0.05 andP<0.001, respectively) delayed the onset of convulsion when compared with PTZ control mice. When compared with PTZ control mice, administration of morin (20 and 40 mg/kg) significantly(P<0.001) decreased tonic-clonic convulsion duration. Diazepam (5 mg/kg) treated mice also showed significant (P<0.001) attenuation in the PTZ-induced onset of convulsion and duration of tonic-clonic seizure as compared to PTZ control mice (Figure 1).

Figure 1. Effects of morin and diazepam on PTZ-induced duration and the onset of tonic-clonic convulsion in mice.
3.2. Effects of morin on mortality
Administration of PTZ caused mortality in mice whereas mice which were pretreated with morin (20 and 40 mg/kg) significantly(P<0.01 andP<0.001, respectively) and dose-dependently decreased PTZ-induced mortality in mice. Incidence of convulsion was also significantly decreased (P<0.01 andP<0.001 respectively) by morin(20 and 40 mg/kg). Diazepam (5 mg/kg) treated mice also showed significant (P<0.001) reduction in mortality as well as convulsion incidence as compared to PTZ control mice (Table 1).
3.3. Effects of morin on locomotor activity
The locomotor activity did not differ significantly in the diazepam(5 mg/kg), morin (10, 20 and 40 mg/kg) treated as well as normal mice before treatment. There was significant (P<0.05 andP<0.001)and dose-dependent decrease in locomotor activity that has been observed in morin (10, 20 and 40 mg/kg) post-treatment when compared with normal mice. Diazepam (5 mg/kg) treated mice also significantly (P<0.001) reduced locomotor activity when compared with normal mice (Table 1).

Table 1 Effects of morin and diazepam on PTZ-induced convulsions and locomotor activity in mice.
3.4. Effect of morin on brain GABA and DA level
When compared with normal mice, PTZ control group significantly(P<0.001) reduced brain GABA and DA levels. The brain GABA level was significantly (P<0.05, andP<0.001) and dose-dependently increased by morin (20 and 40 mg/kg) treatment as compared to PTZ control group. It also showed a significant increase (P<0.05) in brain DA level when compared with PTZ control mice. Moreover,compared with PTZ control animal group, a significant (P<0.001 andP<0.01) increase in brain GABA and DA level was observed in diazepam (5 mg/kg) treated animal group (Figure 2).
3.5. Effect of morin on Na+K+ATPase activity
A significant decrease (P<0.001) in Na+K+ATPase activity was found in PTZ control animal when compared with normal mice.Morin (20 and 40 mg/kg) treated mice significantly (P<0.01 andP<0.001) and dose-dependently increased Na+K+ATPase activity when compared with PTZ control mice. Treatment with diazepam (5 mg/kg) also significantly (P<0.01) increased Na+K+ATPase activity when compared with PTZ control mice (Figure 2).
3.6. Effect of morin on XO activity
PTZ administration resulted in a significant increase (P<0.001) in XO activity in PTZ control mice. Mice pretreated with morin (20 and 40 mg/kg) showed significant (P<0.05 andP<0.01) and dosedependent decrease in XO activity when compared with PTZ control mice. Diazepam (5 mg/kg) treated mice significantly (P<0.001)reduced the PTZ-induced increase in XO activity as compared to PTZ control mice (Figure 2).
3.7. Effect of morin on oxido-nitrosative stress
The SOD and GSH activities were significantly (P<0.001)decreased, and MDA and NO activities were significantly (P<0.001)increased in PTZ control animal as compared to normal mice. A significant (P<0.05,P<0.001 andP<0.001) and dose-dependent increase in SOD level was observed in morin (10, 20 and 40 mg/kg) treated mice when compared to PTZ control mice group,respectively. The GSH activity was significantly (P<0.01 andP<0.001) and dose-dependently increased in morin (10, 20 and 40 mg/kg) treated mice as compared to PTZ control mice. There was significant (P<0.01 andP<0.001) and dose-dependent reduction in MDA level that has been observed in morin (20 and 40 mg/kg) when compared to PTZ control mice. Besides, morin (20 and 40 mg/kg)significantly (P<0.05) decreased PTZ-induced increased NO level as compared to PTZ control mice. Diazepam (5 mg/kg) treated mice also showed significant (P<0.01 andP<0.001) improvement in PTZ-induced alterations in oxido-nitrosative stress when compared with PTZ control mice (Table 2).

Figure 2. Effects of morin and diazepam on PTZ-induced alterations in brain GABA (A), DA (B), Na+K+ATPase (C) and XO activity (D) in mice.
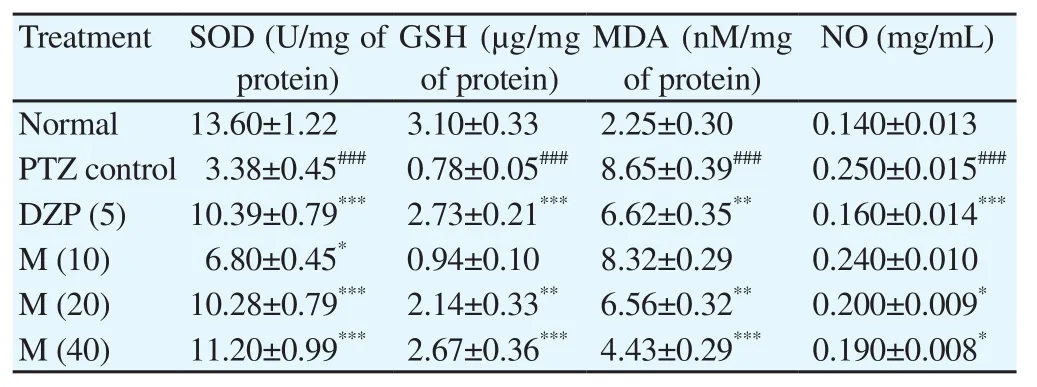
Table 2 Effects of morin and diazepam on PTZ-induced alteration in oxido-nitrosative stress in mice.
4. Discussion
Epilepsy is a chronic neurobiological disease mainly characterized by repeated convulsions due to excessive activation of cerebral neurons. An array of neurotransmitter plays an essential role in the generation and maintenance of convulsions. Thus, reduction in duration, as well as the onset of tonic and clonic seizures by modulating these neurotransmitters, is vital parameter to judge drug efficacyin-vivo. GABA serves as a vital neurotransmitter in a number of neurodegenerative disorders including seizure mainly[43]. Many researchers also reported the role of DA in the pathogenesis of epilepsy besides GABA[44]. Thus, DA agonists such as bromocriptine, lisuride, pergolide, and apomorphine are also potential targets of epilepsy[14,15]. Present investigation evaluated the potential of morin against PTZ-induced convulsions by assessing various behavioral and biochemical parameters. Administration of morin significantly attenuated the PTZ-induced alterations in duration and onset of a tonic-clonic seizure, mortality, locomotor activity, brain GABA and DA levels, Na+K+ATPase and XO activity and oxido-nitrosative stress in mice.
PTZ is a GABAAreceptor antagonist which blocks GABA-mediated Cl-¯influx. Deficiency in GABAergic transmission (through GABAAionotropic receptor) leads to neuronal Cl-deficiency associated with abnormal neuronal discharges of hyperexcitable nature, which otherwise acts through GABAAionotropic receptor[3,29]. This tends to shift the excitatory NMDA (N-methyl-D-aspartate receptor) and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)neurotransmitter pathways on alone discharge state and results in activation of excitable tonic and clonic phases of seizures[45]. It has been well documented that the drugs that activate GABAergic transmissions or influence its physiology influence the induction and onset of convulsions[4]. Furthermore, DA receptor pathway plays a significant role in the determination of the epileptic pathophysiology.Activation of D2receptors resulted in increased brain DA levels that in turn caused anti-epileptic potentials[44]. In the present study, pretreatment with morin showed a significant reduction in the duration and onset of tonic-clonic convulsions which may be due to increase in the activity of GABA and DA levels. These findings suggest that morin may have GABA and DA receptor activation property which contributes to its anti-epileptic potential.
The researcher has reported that ionic control is one of the prime importance in epilepsy[46]. It is an interesting fact that when extracellular K+concentration increases,i.e., the transmembrane potential changes from -60 mV, the terminal and neuronal depolarization leading to abnormal neurotransmitter release and action potential discharges, respectively. An abnormality in the electrochemical gradients reflects a Na+K+ATPase pumps abnormality which is present in the plasma membrane. Blockade of this pump is associated with aggravations of epilepsy[46]. In the current investigation, intraperitoneal PTZ administration resulted in decreased activity of Na+K+ATPase significantly, which is in accordance with the findings of previous researchers[47]. Morin treatment enhances the activity of Na+K+ATPase which may lead to decrease in seizures.
It has been suggested that elevated levels of XO associated with increased ROS generation during the epileptic seizures[3,4,29].Activation of XO resulted in neuronal cell apoptosis by degradation of intracellular adenine nucleotides. Studies have reported that administration of antioxidants plays a protective role during convulsion[4,29]. The antioxidants have potential to quench the ROS such as superoxide anion, hydroxyl, and peroxyl radicals thus protected cellular components[48-51]. Administration of morin which is a potent antioxidant showed significant inhibition of XO which may provide the credence to its antiepileptic potential.
Results of previous studies have suggested that there is a correlation between elevated oxidative stress and epilepsy[30,32]. The intracellular antioxidant enzymes include dismutase such as SOD and catalases which are a potent scavenger of toxic O2.that converted to non-toxic H2O2[52-56]. It has been reported that decrease in SOD level manifests neuronal ROS injury[57-59]. Thus, increase in SOD level by means of administration of exogenous antioxidant substance may facilitate termination of free radical activity associated with neuronal damages[60-62]. Reduced GSH is another important cellular defense antioxidant[63-65]. Studies reported that administration of PTZ intraperitoneally caused decreased levels of GSH. However,administration of morin significantly elevated these cellular antioxidant levels. The findings of the present study are corroborated with the results of previous researchers where treatment with morin significantly increased the SOD and GSH levels[22,23,26].
Increased MDA level is an essential hallmark for the increased lipid peroxidation in the neuronal tissue. MDA is associated with neuronal damage by targeting polyunsaturated fatty acids (PUFAs)[66-68].In addition to MDA, increased level of NO plays a decisive role in oxidative damage[69-72]. NO interacts with superoxide to generate the peroxynitrite which leads to neural damage. PTZ induces NO production in the hippocampus of the brain, maybe by increasing glutamate release and subsequently reducing GABA levels in brain[29]. Thus, inhibition of NO may act in line with the GABA facilitation. Morin treatment showed significant inhibition in PTZ-induced increase in MDA and NO levels which in turn may increase GABA levels to exert its anticonvulsant potential.
In conclusion, the findings of the present study demonstrate that morin shows its anti-convulsant effect via modulating the levels of brain GABA, Na+K+ATPase, and oxido-nitrosative stress. Thus,morin can be a potential candidate for further clinical evaluations as an anti-epileptic agent.
Conflict of interest statement
The authors declare there is no conflicts of interest.
Acknowledgments
The authors would like to acknowledge Dr. S. S. Kadam,Chancellor, and Dr. K. R. Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune for providing necessary facilities to carry out the study.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Role of toll like-receptor 2 in inflammatory activity of macrophage infected with a recombinant BCG expressing the C-terminus of merozoite surface protein-1 of Plasmodium falciparum
- Moderating effect of synthesized docosahexaenoic acid-enriched phosphatidylcholine on production of Th1 and Th2 cytokine in lipopolysaccharide-induced inflammation
- Characterization of Cnidoscolus quercifolius Pohl bark root extract and evaluation of cytotoxic effect on human tumor cell lines
- Expression of fluorescent tagged recombinant erythroferrone protein
- Identification of a toxin coding fragment in pBSSB1, a linear plasmid from Salmonella enterica serovar Typhi that can stabilize a multicopy plasmid
- Influence of different cultivars of Phoenix dactylifera L-date fruits on blood clotting and wound healing
