Moderating effect of synthesized docosahexaenoic acid-enriched phosphatidylcholine on production of Th1 and Th2 cytokine in lipopolysaccharide-induced inflammation
2018-08-02ChoiJYBaekParkSYLim
M. Choi, JY. Baek, I. Park, SY. Lim✉
1Department of Mircrobiology, Kosin University College of Medicine, Busan, Korea
2Division of Marine Bioscience, Korea Maritime and Ocean University, Busan, Korea
Keywords:Docosahexaenoic acid Phosphatidylcholine Cytokines Interleukin-2 Interleukin-6 Interleukin -12/IL-23(p40)
ABSTRACT Objective: To evaluate effects of docosahexaenoic acid-enriched phosphatidylcholine (DHAPC) on cytokine production induced by lipopolysaccharide (LPS). Methods: The culture supernatants of splenocytes exposed to DHA-PC along with LPS were harvested to determine the production of Th 1 (IFN-γ and IL-2) and Th2 [IL-4, IL-5, IL-6 and IL-12/IL-23(p40)]cytokines. Cytokines were measured using ELISA. Results: Co-administration of DHAPC with LPS resulted in significantly lower IL-2 expression compared to that observed with administration of only LPS (P<0.01). Treatment with DHA-PC and LPS significantly increased IL-5 expression (P<0.01). Moreover, co-administration of DHA-PC with LPS significantly decreased IL-6 and IL-12/IL-23(p40) expressions compared to that observed with administration of only LPS (P<0.01). Conclusions: Our results suggest that DHA-PC inhibits pro-inflammatory cytokines [IL-2, IL-6 and IL-12/IL-23(p40)] expression on induction of inflammation.
1. Introduction
Phosphatidylcholine (PC) is an essential component found in every cell of the human body. PC is consisted of a choline head group, glycerophosphoric acid, and several fatty acids.Polyunsaturated fatty acids (PUFAs) are common constituents of the diet and have many functionally physiological roles[1-3].Docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid(EPA, 20:5n-3) are major n-3 PUFAs obtained from diet. Calder[2]suggested that an incrmented intake of EPA and DHA resulted in an incremented incorporation of n-3 PUFAs in membrane phospholipids of immune cells. Since dietary n-3 PUFAs have significant immunologically inhibitory and anti-inflammatory properties, they conduct protective effects on inflammatory diseases and immune-mediated diseases[4-6]. Studies demonstrated that both EPA and DHA might control the functional responses of immune cells to stimulation by decreaing the production of pro-inflammatory mediators[7]. In autoimmunity[8] and clinical trials in patients with rheumatoid arthritis[9], intake of fish oil including EPA and DHA revealed significant protective effect against inflammatory activity.Intake of PC also possesses a positive effect on anti-inflammation[10].An animal model study suggested that oral PC supplementation reduced lipopolysaccharide (LPS)-induced plasma tumor necrosis factor-α (TNF-α)[11]. However, there are less studies on the effect of DHA containing PC on moderation of cytokine production.
There are two distinct patterns of cytokine generation in helper T-cells. Human type 1 helper (Th1) cells generate interleukin-2 (IL-2),interferon-γ (IFN-γ) and tumor necrosis factor-β (TNF-β), whereas human type 2 helper (Th2) cells generate IL-4, IL-5, IL-6 and IL-10.Other cytokines including TNF-α, IL-3 and granulocyte macrophagecolony stimulating factor are generated by both the Th subsets[12].Different cytokine profiles are associated with strikingly dramatic functions of the two types of Th cells. In general, Th2 cells mediate B cell immunoglobin (Ig) secretion, especially of the IgE class[12].The helper function of Th1 cells is more uncertain since they have been shown to provide antigen-specific presentation in only fewin vitrosystems[13]. Under certain circumstances, Th1 cells can also assist to B cells, however, one of their major functions is to lead to delayed type hypersensitivity response[12]. Therefore, Th1 cells may control more effectively with intracellular pathogens, whereas Th2 cells may handle extracellular organisms and their secreted products by inducing strong antibody reactions. In the present study, we synthesized didocosahexaenoyl PC (DHA-PC) and investigated its effects on the expression of Th1 (IL-2 and IFN-γ) and Th2 [IL-4,IL-5, IL-6 and IL-12/IL-23(p40)] cytokines.
2. Materials and methods
2.1. Materials and cell culture
Six-week-old male C57BL6 mice were purchased from Daehan BioLink (Eumsong, Chungcheongbukdo, South Korea). The animal experiment was approved by Institutional Animal Care and Use Committee (GNU-171116-M0051) in Gyungsang National University(Gyungsang, South Korea). LPS, RPMI 1640, fetal bovine serum(FBS), phosphate buffer saline (PBS) and dimethylsulfoxide (DMSO)were obtained from Sigma-Aldrich (St. Louis, MO, USA). DHA-PC were synthesized according to the method of Borsottiet al.[14]. Mouse enzyme-linked immunosorbant assay kits purchased from Biolegent(San Diego, CA, USA) were used to measure the levels of cytokines[IL-2, IL-4, IL-5, IL-6, IL-12/IL-23(p40), IFN-γ)].
2.2. Spleen cell culture supernatants
Spleen is an organ where immune responses against pathogen can be regulated[15] and thus murine splenocytes have been used for immune studies[16,17]. Cell counts within spleen cell suspensions were adjusted to 2 × 106cells/mL using RPMI 1640 medium with 10% FBS, and the adjusted suspensions were aliquoted into 24-well tissue culture plates (Costar, Cambridge, MA, USA) at 1 mL/well.The cells were treated with sample and set at 37 ℃ in 5% carbon dioxide condition. Concomitant treatment with sample and LPS also was conducted. As a control, cells were treated with 0.01% DMSO.Cells were incubated for 6, 24, 48, 72 h before collection. The suspensions were centrifuged at 300 ×gfor 10 min, followed by additional centrifugation at 1 000 ×gfor 30 min. The supernatants were stored at -70 ℃ until use in analysis of cytokine expression[18].We measured the expression of IL-2, IL-4, IL-5, IL-6, IL-12/IL-23(p40) and IFN-γ in samples collected from 6, 24, 48, and 72 hours of treatments.
2.3. Measurement of cytokines
Capture antibodies for IL-2, IL-4, IL-5, IL-6, IL-12/IL-23(p40)and IFN-γ were diluted with coating buffer, loaded into 96-well plates at 100 μg/well, and incubated at 4 ℃ overnight. The wells were washed with washing buffer 4 times, and 200 μL of assay diluents was supplemented to each well. The plates were then set at room temperature for 1 h. The wells then were washed with washing buffer 4 times again, and 100 μL of the sample was supplemented to each well. The plates were then set at room temperature for 2 h. After washing 4 times, 100 μL of the detection antibody for the appropriate cytokine was supplemented to each well, and the plates were set at room temperature for 1 h. The wells were washed with washing buffer 4 times, 100 μL of avidin-horseradish peroxides was supplemented to each well, and plates were set at room temperature for 30 min. After washing 5 times with washing buffer, 100 μL of substrate fluid containing tetramethylbenzidine was supplemented,and the plates were set at room temperature for 20 min. One hundred μL of stop solution was supplemented to stop reaction, and the optical density was assessed at 450 nm by ELISA reader (Model 550 microplate reader, Bio-Rad, Richmond, VA, USA)[16].
2.4. Statistical analysis
Data were presented as mean ± standard deviation. To determine normal distribution, Kolmogorove-Smirnov test was done. Significance of differences observed between the control and experiment groups using Student’sttest was set atP<0.05. Analyses were conducted using STATISTICA package.
3. Results
3.1. Effect of DHA-PC on Th1 cytokine expression
Changes in levels of Th1 cytokines (IL-2 and IFN-γ) were investigated upon treatment of murine splenocyte suspensions with only LPS- or by co-administration of LPS and different concentrations of DHA-PC. Co-administration of DHA-PC (0.3 μg/mL) with LPS significantly reduced the IL-2 expression after 6 and 72 hours of treatment, respectively, as compared to treatment with only LPS(P<0.05) (Table 1). Table 2 showed the modulation of IFN-γ expression induced by DHA-PC. The expression of IFN-γ in spleen cells increased after 48 hours of LPS treatment. However, no such significant difference was observed with DHA-PC treatment.

Table 1 Effect of DHA-PC on production of LPS induced IL-2 at different times in mouse spleen cells.
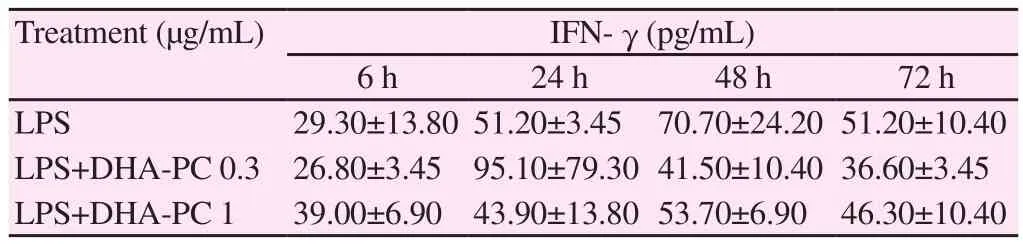
Table 2 Effect of DHA-PC on production of LPS induced IFN-γ at different times in mouse spleen cells.
3.2. Effect of DHA-PC on Th2 cytokine expression
We investigated changes in the levels of Th2 cytokines [IL-4, IL-5,IL-6, and IL-12/IL-23(p40)] upon treatment of murine splenocyte suspensions with LPS, or with co-administration of different concentrations of DHA-PC. Table 3 showed the expression of IL-4 after treatment with DHA-PC. The treatment of spleen cells with only LPS increased the expression of IL-4 after 48 h. However,there were no significant differences in IL-4 expression induced by DHA-PC. Co-administration of DHA-PC (1 μg/mL) with LPS significantly increased the IL-5 expression after 24 hours of treatment, as compared to treatment with only LPS (P<0.05) (Table 4). Table 5 showed the expression of IL-6 induced by DHA-PC.Co-administration of DHA-PC (0.3 μg/mL) with LPS significantly decreased IL-6 expression after 48 and 72 hours of treatment,respectively in comparision with the treatment with LPS alone(P<0.05). As shown in Table 6, co-administration of DHA-PC at concentration of 0.3 μg/mL with LPS significantly decreased IL-12/IL-23(p40) expression after 48 and 72 hours of treatment compared with the treatment with only LPS (P<0.05).

Table 3 Effect of DHA-PC on production of LPS induced IL-4 at different times in mouse spleen cells.

Table 4 Effect of DHA-PC on production of LPS induced IL-5 at different times in mouse spleen cells.
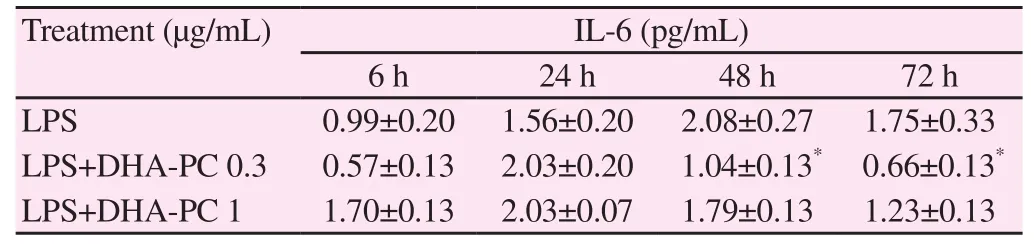
Table 5 Effect of DHA-PC on production of LPS induced IL-6 at different times in mouse spleen cells.
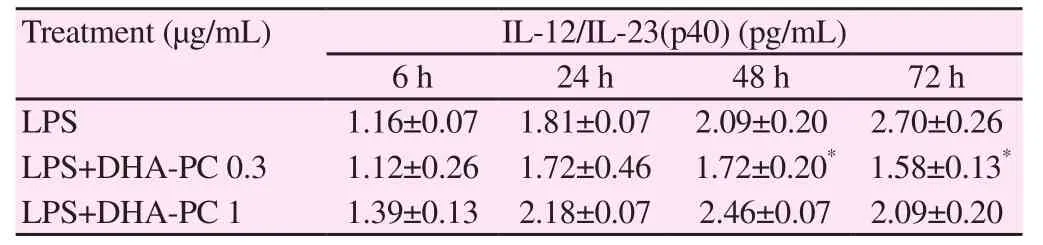
Table 6 Effect of DHA-PC on production of LPS induced IL-12/IL-23(p40) at different times in mouse spleen cells.
4. Discussion
N-3 highly unsaturates, such as DHA and EPA, have been known to protect against immune disease and inflammatory side effects in rodents[19] and humans[20]. Meydaniet al.[21] indicated that feeding with n-3 fatty acid deficiency for 3 mon inhibits production of IL-1β, TNF,IL-6 and IL-2 induced by LPS. Liuet al.[22] found that fish oil and its functional components including EPA and DHA conducted an antiinflammatory effect by decreasing cytokine production (IL-1β and IL-2) in a weaning pigs, which might be associated with the modulation of intracellular signaling, especially that of protein kinase C. However,there have been few studies on the effects of DHA-PC on cytokine production. Huanget al.[23] suggested that the anti-inflammatory action of lysophosphatidylcholine containing DHA was partially linked to the decreased formation of leukotriene C4, TNF-α and IL-6. IL-2 is a soluble, locally released inflammatory mediator with a multitude of regulatory roles, which therefore is a possible marker that can be applied to measure the functional effects of antiinflammatory intervention[24]. We found that DHA-PC decreased the expression of pro-inflammatory cytokine, IL-2.
There is big significant evidence that these n-3 fatty acids are capable to inhibit development of inflammatory cells and generation of eicosanoids from n-6 fatty acids[25,26]. According bothin vitroand clinical studies, administration with EPA, DHA and fish oil could reduce production of pro-inflammatory cytokines[27]. Wanget al.[28] demonstrated that macrophages supplemented with EPA and DHA resulted in decreased levels of TNF-α and IL-6 compared to controls, suggesting the ability of EPA, DHA and EPA+DHA to decrease pro-inflammatory markers. For maintaining health, it is important to balance pro-inflammatory and anti-inflammatory cytokines. Excessive chronic production of pro-inflammatory cytokines contributes to inflammatory diseases[29-31]. Here we evaluated the effects of synthesized DHA-PC on the production of cytokines in murine splenocytes induced by LPS. There was few studies on relationship between DHA-PC and cytokine expression.Our results suggest that DHA-PC inhibits expression of proinflammatory cytokines [IL-2, IL-6 and IL-12/IL-23(p40)] on induction of inflammation. Further studies are warranted including dose response trials and gene expression studies.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1A2B4005915).
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Role of toll like-receptor 2 in inflammatory activity of macrophage infected with a recombinant BCG expressing the C-terminus of merozoite surface protein-1 of Plasmodium falciparum
- Characterization of Cnidoscolus quercifolius Pohl bark root extract and evaluation of cytotoxic effect on human tumor cell lines
- Anti-epileptic effect of morin against experimental pentylenetetrazol-induced seizures via modulating brain monoamines and oxidative stress
- Expression of fluorescent tagged recombinant erythroferrone protein
- Identification of a toxin coding fragment in pBSSB1, a linear plasmid from Salmonella enterica serovar Typhi that can stabilize a multicopy plasmid
- Influence of different cultivars of Phoenix dactylifera L-date fruits on blood clotting and wound healing
