How to overcome ATP-binding cassette drug efflux transporter-mediated drug resistance?
2018-07-27AdrianJaramilloFarahAlSaigJacquelineCloosGerritJansenGodefridusPeters
Adrian C. Jaramillo, Farah Al Saig, Jacqueline Cloos, Gerrit Jansen, Godefridus J. Peters
1Department of Hematology, VU University Medical Center, Cancer Center Amsterdam, Amsterdam 1007 MB, The Netherlands.
2Department of Medical Oncology, VU University Medical Center, Cancer Center Amsterdam, Amsterdam 1007 MB, The Netherlands.
3Amsterdam Rheumatology and Immunology Center, VU University Medical Center, Amsterdam 1007 MB, The Netherlands.
Abstract P-glycoprotein (ABCB1), multidrug resistance protein-1 (ABCC1) and breast cancer resistance protein (ABCG2) belong to the ATP-binding cassette (ABC) superfamily of proteins that play an important physiological role in protection of the body from toxic xenobiotics and endogenous metabolites. Beyond this, these transporters determine the toxicity profile of many drugs, and confer multidrug resistance (MDR) in cancer cells associated with a poor treatment outcome of cancer patients.It has long been hypothesized that inhibition of ABC drug efflux transporters will increase drug accumulation and thereby overcome MDR, but until now no approved inhibitor of these transporters is available in the clinic. In this review we present molecular strategies to overcome this type of drug resistance and discuss for each of these strategies their promising value or indicate underlying reasons for their limited success.
Keywords: Breast cancer resistance protein, ATP-binding cassette transporters, multidrug resistance, multidrug resistance protein
INTRODUCTION
One of the most challenging obstacles in cancer treatment is multidrug resistance (MDR). Despite having achieved lower mortality rates due to recent advances in cancer therapy, the long term survival rate remains poor, primarily due to chemotherapy resistance[1-3]. Resistance can be caused by numerous mechanisms in cancer cells, such as activation of drug-metabolizing enzymes (e.g., cytochrome P450), activation of DNA repair mechanisms, disruptions in apoptotic signaling pathways, reduced drug infux and increased activity of drug efflux pumps[4-6]. Specifically, several members of the ATP-binding cassette (ABC) superfamily mediate the efflux of multiple chemotherapeutic drugs and so contribute to MDR. The ABC superfamily represents one of the largest protein families in biology, including 49 ABC genes identified in the human genome[7,8]. This superfamily is subdivided into seven distinct groups (ABCA through ABCG) and currently at least 15 ABC transporters have been implicated to confer resistance to clinically active drugs, notably P-glycoprotein (P-gp, ABCB1), multidrug resistance protein-1 (MRP1, ABCC1) and breast cancer resistance protein (BCRP, ABCG2)[9].
Structurally, each ABC transporter is composed of at least one hydrophobic membrane-spanning domain(MSD) consisting of 6 α-helices and 1 hydrophilic nucleotide binding domain (NBD). All ABC proteins share a highly conserved region in the ATP binding domain, including the Walker A and Walker B sequences, the ABC signature motif, the H loop and the Q loop[8]. Depending on the clustering of the MSD and the NBD in the transporters, these are grouped into full transporters (e.g., ABCB1 and ABCC1, consisting of 2 MSDs and 2 NBDs), half transporters (e.g., ABCG2, containing only 1 MSD and 1 NBD) and non-transporters,which possess two NBDs, without the MSDs[10]. The subfamily ABCE belongs to the non-transporters, whose proteins play a role in the assembly of the pre-initiation complex and RNAse inhibition[11,12]. ABCF subfamily members also encode non-transporters, they are involved in ribosome assembly and protein translation[13,14].
ABC transporters are present in all living species, in prokaryotes they function as drug importers or exporters, in eukaryotes solely as drugs exporters[15]. By coupling ATP binding and hydrolysis these transporters are able to extrude substrates out of cells or into cellular compartments like cytoplasmic vesicles and lysosomes[8]. This superfamily transports a wide range of substrates, including lipids, bile salts, sugars,amino acids, steroids, peptides, nucleotides, endogenous metabolites, ions and toxins, including antibiotics and chemotherapeutic drugs. Collectively, ABC transporters fulfill both physiological and pharmacological functions impacting drug transport, tumor growth modulation and regulatory pathways (e.g., apoptosis and complement-mediated cytotoxicity[16-18]and immune cell-regulatory pathways[19]). This review focuses on current developments in drug discovery to circumvent MDR induced by P-gp, MRP1 and BCRP in cancer cells.
P-GLYCOPROTEIN
P-glycoprotein (or ABCB1 or MDR1) was originally identified as the first mammalian ABC multidrug transporter in 1976, when Juliano and Ling[20]observed that Chinese hamster ovary cells displayed pleiotropic cross-resistance to a wide range of amphiphilic drugs. This transporter was named P-gp and is also known as ABCB1 or MDR1.
P-gp is encoded by a single polypeptide chain with 2 homologous NBD and 2 homologous transmembrane domains (TMD)[21,22]. The 2 homologous TMD are joined by a linker region of about 60 amino acids[23]. Each TMD contains 6 transmembrane (TM) α-helices and 1 NBD [Figure 1].

Figure 1. Schematic representation of the domain structure of P-gp, MRP1 and BCRP. P-gp has 2 TMD consisting of 6 α-helices each and 2 ATP bindings sites. MRP1 also contains 2 ATP-binding regions and 2 TMD containing 6 α-helices and it is extended by 1 additional TMD consisting of 5 α-helices and 1 amino terminus loop (L0). The half transporter ABCG2 contains 1 TMD of 6 α-helices and 1 ATP-binding domain. The ATP-binding site of this transporter is found on the amino-terminal side (N) in contrast to P-gp and MRP1. P-gp:P-glycoprotein; MRP1: multidrug resistance protein-1; BCRP: breast cancer resistance protein; TMD: transmembrane domain
Aller et al.[24]reported the X-ray structure of mouse P-gp, which shares 87% sequence identity with the human P-gp. The structure revealed that the inward facing conformation is formed by 2 bundles of 6 TMs arranged as TMs 1-3, 6, 10, 11 and TMs 4, 5, 7-9, 12. This conformation results in a large internal cavity, which is open to the cytoplasm and the inner leafet. There are 2 portals (formed by TMs 4/6 and 10/12) that allow hydrophobic molecules to enter the cavity directly from the inner leafet. The drug binding pocket generally contains hydrophobic and aromatic residues and is large enough to accommodate at least two substrate molecules simultaneously[25]. In fact,this was supported by the X-ray structure reported by Aller et al.[24]. Expanding on these observations, Pajeva and Wiese[26]compared the inward facing homology model of human P-gp based on the earlier mentioned mouse structure with the outward facing homology model of human P-gp based on Sav1866 structure. It appeared that residues in TM4 and TM10 face the cavity in the inward facing conformation are completely buried in the outward facing form, while the ligands remain bound to the same residues of TM6 and TM12 in both conformations. This implies that each of the portals has different roles: TM4 and TM10 as “portal keepers”(preventing substrates that entered to escape back to the inner leafet) and TM6 and TM12 as “portal carriers”(these are mainly responsible for substrate interactions). Together, this model suggests that ligands remain bound to the same residues during the transition from the inward to the outward facing conformation.
The expression pattern of P-gp indicates that its main function is the protection of the body from toxic substances and xenobiotics by excreting them into bile, urine, feces and avoiding their entry into fetus,brain and testes. To this end, P-gp is expressed on the canalicular surface of hepatocytes in the liver, the apical surface of epithelial cells of proximal tubules in the kidneys, columnar epithelial cells of the intestine,epithelial cells of placenta, and the luminal surface of capillary endothelial cells in brain and testes[27,28][Table 1]. Next to this important physiological function, pharmacologically P-gp constitutes a considerable obstacle to the delivery of various clinically used drugs to their targets, such as anti-cancer drugs, anti-epileptics,immunosuppressive agents, cardiac glycosides, cholesterol-lowering statins, human immunodeficiency virus(HIV) protease inhibitors, anti-hypertensives, calcium channel blockers, anti-histamines and antibiotics[15].From knockout mice studies it became clear that P-gp plays a major role in determining the drug ADME(absorption, distribution, metabolism, excretion) profile[29,30]. These P-gp knock-out mice showed increased absorption and decreased elimination of drugs, which gave rise to severe toxicity. In addition, P-gp works synergistically with the major phase I drug metabolizing enzyme P450 (CYP3A4), as they share a remarkable similarity between their substrates and regulation, to decrease the oral drug bioavailability.High expression levels of P-gp have been identified in various solid tumor malignancies, e.g. kidney, colon,liver, ovarian, breast and sarcomas[4]. P-gp overexpression has been reported to be an independent negative prognostic factor in clinical outcome. In high grade osteosarcoma, increased levels of P-gp correlated with a > 3-fold increased risk of adverse events and shorter event-free survival[31]. P-gp RNA levels increased after lung perfusion with doxorubicin of patients with unresectable sarcoma pulmonary metastases[32].Furthermore, Gregorcyk et al.[33]reported that P-gp positive breast cancer patients are at significantly higher risk for relapse. In hematological malignancies, P-gp mRNA expression and protein function are increased after chemotherapy treatment. Expression of P-gp in myeloid blasts is correlated with treatment failure and shorter survival in adult acute myeloid leukemia (AML) patients[34,35]. However, childhood acute lymphoblastic leukemia (ALL) results have shown contradictory results, some found no association between high P-gp expression and prognosis[36,37], while other groups showed prognostic impact for P-gp in ALL[38,39].These differences can at least partially be attributed to different treatment strategies and methods used to detect P-gp[40].

Table 1. Characterization of P-gp, MRP1 and BCRP
MULTI-DRUG ASSOCIATED PROTEIN 1
A decade after the discovery of P-gp, the first member of the C subfamily, MRP1 (systematic name, ABCC1)was discovered[41]. MRP1 is widely expressed in normal tissues, with relatively high levels in the kidney,lung, testis, heart and placenta, while there is a low expression in colon, brain, and small intestine and peripheral blood mononuclear cells[42,43][Table 1]. Notably, in the above-mentioned tissues, MRP1 expression can vary between different cell types of the same tissue[44]. The highest expression is found in cells with specialized barrier function, e.g., the choroid cells in the blood cerebrospinal fuid barrier[45], or cells with a high proliferative status, e.g., reactive type II pneumocytes in the alveoli of the lung[46]. In contrast to P-gp and BCRP, MRP1 are mainly expressed at the basolateral membrane of polarized cells, with the exception of brain capillary endothelial cells. Consequently, it has been suggested that MRP1 functions to protect cell types from xeno- and endobiotics and pumping them into the interstitial space of the body, instead of their expulsion into bile, urine or gut[47]. In addition, studies with mrp1 (-/-) mice revealed other physiologic functions of MRP1 than transporters. For example, MRP1 plays a role in immunological responses by its involvement in leukotriene extrusion[48]. Furthermore, being a transporter of glutathione (GSH) and glutathione-conjugates, MRP1 plays a crucial role in the elimination of toxic substances formed during oxidative stress[49,50].
Despite the fact that both P-gp and MRP1 can confer resistance to a wide range of drugs, P-gp prefers neutral,or positively charged, hydrophobic compounds, while MRP1 binds and transports anionic substrates, mainly in the form of anionic glutathione, glucuronate or sulfate conjugates[51][Table 1]. Unmodified drugs can also be co-transported with free glutathione[50]. Some examples of MRP1 substrates are: various anticancer drugs,antibiotics, pesticides, HIV protease inhibitors, peptides, heavy metals, glutathione conjugates, glucuronide conjugates and sulfate conjugates[15].
The currently accepted MRP1 topology consists of the following domain arrangement: TMD0-L0-TMD1-NBD1-TMD2-NBD2 [Figure 1]. Compared to P-gp, this transporter is extended by an additional TMD0 containing 5 TM helices and 1 amino terminus loop (L0) consisting of 32 amino acids, which links TMD0 to TMD1. The exact function of the TMD0 still needs to be fully elucidated. It was previously thought that TMD0 had no role in trafficking to the plasma membrane or efflux function, but this view has been revisited by several mechanistic and cell biological reports[52]. It has been shown that TMD0 is important for the processing and trafficking of human MRP1[53]. Additionally, by mutation studies, Yang et al.[54]reported that the amino terminus is required for proper MRP1 function and structure. It has also been suggested by Chen et al.[55]that the amino terminus forms a U-shaped structure which might serve as a gate to regulate the substrate transport activity of MRP1. Finally, TMD0 and L0 seem to contribute to MRP1 homo-dimerization, but whether the functional form of MRP1 is a homodimer or a monomer needs to be determined[56].
Depending on expression levels, MRP1 can confer resistance to a variety of antineoplastic drugs,including vinca alkaloids, anthracyclines, epipodophyllotoxins, saquinavir, methotrexate, mitoxantrone,camptothecins, paclitaxel, glucuronide, doxorubicin, epirubicin, and tyrosine kinase inhibitors such as imatinib[57,58]. High MRP1 expression levels have been identified in different cancer types, e.g., lung cancer, breast cancer, prostate cancer, gastrointestinal carcinoma, melanoma, neuroblastoma, ovarian and hematological malignancies[44,59](AML, ALL and chronic lymphoblastic leukemia). In these results, it has been difficult to define statistically significant correlations between high MRP1 expression in the indicated tumors and acquired resistance or prognosis. This was largely due to the fact that tumor samples contained a variable number of MRP1 expressing normal cells, cells, while other drug efflux transporters might contribute as well[44]. However, a prospective study demonstrated that MRP1 is an independent prognostic indicator of outcome in neuroblastoma patients[60]. In addition, a high expression of MRP1 was associated with shorter tumor-free survival (TFS) and overall survival (OS) in non-small cell lung cancer (NSCLC)[61].Similarly, in breast cancer MRP1 expression was related to shorter relapse-free survival (RFS) and OS[62].
BREAST CANCER RESISTANCE PROTEIN
ABCG2, also known as BCRP, ABCP or MXR, is expressed in a variety of normal tissues, including epithelial cells of the gastrointestinal tract and liver, placental syncytiotrophoblast cells, prostate epithelium, kidney cortical tubules, endothelial cells of the blood-brain barrier (BBB) and hematopoietic stem cells[10,63][Table 1].Like P-gp, ABCG2 is expressed on the apical surface of epithelial cells and since it is expressed in tissues with secretory and barrier function, this suggests that its function is similar to P-gp, which is protecting the body from toxic compounds. ABCG2 mRNA expression was higher compared to other efflux transporter in the human small intestine, which is a rate-limiting barrier to oral drug absorption[64]. The important impact of ABCG2 on the intestinal absorption was further supported by several knockout mice research, which have indicated that ABCG2 affects the pharmacological and toxicological behavior of many drugs[65-67]. Also,polymorphisms might infuence the role of ABCG2 in intestinal absorption. For example, ABCG2 c.421C>polymorphism renders BCRP more than 75% less active than its wild-type variant. Since ABCG2 c.421C>A is a common polymorphism, present in more than 30% of the Asian and 15% of European population,decreased drug absorption due to BCRP polymorphisms deserves clinical attention[68]. Additionally,Kruijtzer et al.[69]reported that ABCG2 affects the bioavailability and systemic concentration of topotecan in patients. Inhibition of ABCG2 by elacridar (GF120918), a dual inhibitor of P-gp and ABCG2, increased the bioavailability of topotecan from 40% to 97% after oral administration and reduced the biliary and renal excretion of topotecan after intravenous administration. It has also been noted that the polymorphic variant 421C>A of ABCG2 can alter the pharmacokinetics of several drugs, including difomotecan, terifunomide,sulfasalazine, gefitinib, imatinib, and many statins[63]. Furthermore, it has been suggested that ABCG2 plays a role in the maintenance of human pluripotent stem cells in an undifferentiated state and their protection from toxic xenobiotics and hypoxia[10].BCRP exhibits broad substrate specificity and can transport either positively or negatively charged drugs,hydrophobic or hydrophilic and conjugated or unconjugated substrates[70]. Examples are a wide range of anticancer drugs, sulfate and glucuronide conjugates of xenobiotics, tyrosine kinase inhibitors (TKIs),statins, fuorescent dyes and favonoids[10,63]. In vitro assays showed that ABCG2 might have more than two binding sites, since ABCG2 inhibitors displayed different inhibition profiles, depending on the tested substrate[71-73]. For instance, the inhibitor Ko143 inhibited the transport of all the substrates tested by Giri et al.[72], which suggested its binding to a region that allosterically inhibits the transport of these substrates.However, the ABCG2 inhibitors elacridar, nelfinavir and Pluronic P85 seemed to bind to a different region,which inhibited the transport of the nucleoside analogs abacavir and zidovudine, but showed no or a partial effect on the transport of prazosin and imatinib. Muenster et al.[73]showed that two different substrates, such as topotecan and albendazole sulfoxide, could simultaneously be transported without hindering each other’s transport, which also suggest multiple binding sites in ABCG2. Furthermore, using homology modeling a complete homodimer ABCG2 model was created based on the crystal structure of the bacterial multidrug exporter Sav1866, which also suggested a multiple binding site[74]. ABCG2 is a half transporter consisting of 6 putative transmembrane segments and 1 NBD [Figure 1]. The simplest functional form of ABCG2 is a homodimer, which is bridged by disulfide bridge bonds[75]. Other research has suggested higher order of homo-oligomer on plasma membranes, which could also regulate ABCG2 function by dynamic association and dissociation of ABCG2 monomers[76]. Accordingly, it is currently unknown whether a homodimer or a homo-oligomer is the dominant functional unit of ABCG2 in the plasma membrane[77,78].
Clinically, high expression levels of ABCG2 have been found in many solid tumors, especially adenocarcinomas of the digestive tract, endometrium, lung and melanoma[77]. In addition, Damiani et al.[79]found that ABCG2 was overexpressed in 33% of AML patients and that this feature was significantly associated with shorter diseasefree survival and higher risk of relapse. ABCG2 expression is also higher in B-lineage than in T-lineage ALL,while adult ALL patients have a higher expression than infant ALL patients[80,81]. Contradictory results have been published on the correlation between ABCG2 expression and prognosis of solid tumors and on the correlation between ABCG2 expression and prognosis of hematological malignancies[10,39,82]. Overall, even though BCRP activity contributes to MDR and drug pharmacokinetics in cancer, the exact mechanisms of action and interaction remain to be elucidated.
ABC TRANSPORTER INHIBITION
Since overexpression of some of the ABC transporters was correlated with a poor chemotherapeutic response and prognosis of patients with specific cancer types, inhibition of ABC transporters appears a logical approach to circumvent MDR and improve patient’s outcome[82,83]. However, other than in a preclinical setting where ABC transporters could reverse MDR, this promise has still not been fulfilled in clinical practice. Inhibition of ABC transporters has been evaluated with rationally designed or natural inhibitors, including competitive and non-competitive inhibitors. Competitive inhibitors exert their function by tightly binding and blocking the substrate binding pockets. Non-competitive inhibitors exert their function by binding to a non-substrate binding site thereby inhibiting the ATPase activity or modulating transporters’ function allosterically[15,83-85].Another strategy to circumvent MDR that will be discussed further is the application of small interfering RNA (siRNA)[86-88]and microRNA (miRNA)[89,90]inoculation to down-regulate ABC transporter expression.Currently, approximately 30 siRNA candidates are being studied in clinical trial, yet unfortunately their high cost, poor stability in vivo, delivery challenges and off-target effects still remain a challenge for treatment[89].A third approach relates to the rational design of new chemotherapeutics which are non-substrates for ABC transporters. TKIs are small molecules developed to inhibit the uncontrolled activity of various tyrosine kinases(TK) involved in cancer. Many of them can also act as substrates or non-substrates to alter efflux mechanisms mediated by ABC transporters[91]. TKIs have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) since 2001 (such as imatinib, gefitinib and erlotinib and more recently ceritinib, lenvatinib) while several others are under investigation in clinical trials and can potentially be used to inhibit transporter-mediated efflux, as discussed below[92-96]. The main focus of this section of the review will be to discuss modulators which are lead compounds for clinical application.
OVERCOMING P-GP RESISTANCE
P-gp specific inhibition is particularly challenging due to its large binding pocket with low substrate specificity which enables P-gp to interact with over 200 different known substrates[9,97]. Nevertheless, the discovery of new agents to inhibit P-gp is still ongoing, building on encouraging in vitro studies and individual clinical trials such as the Southwest Oncology Group (SWOG 9126) study that showed a strong benefit of the use of the P-gp inhibitor cyclosporine A (CSA) in patients with relapsed or refractory AML[98]. To date, three generations of P-gp inhibitors can be distinguished. Both, the first-generation agents (including verapamil,tamoxifen, cyclosporine A, quinidine and dexverapamil), and the second-generation agents [including VX-710 (biricodar), GF120918 (elacridar) and PSC833 (valspodar)] ultimately failed to show improvement in overall drug efficacy in multiple randomized clinical trials due to poor potency and increased toxicity,respectively[99]. Consequently, the third-generation agents were developed featuring a high transporter affinity and a low pharmacokinetic interaction, including XR-9576 (Tariquidar), LY335979 (Zosuquidar) and R101933 (Laniquidar), which will be discussed below.
Inhibition of P-gp
Agents from all the three generations have been tested in clinical trials. Even though some of the early trials showed benefit from P-gp inhibition, results of most large randomized trials have been disappointing[100,101].As an illustration, a phase I study of tariquidar in combination with vinorelbine showed that tariquidar is a potent P-gp inhibitor, with no noteworthy side effects and pharmacokinetic interactions[102]. Similar findings were observed for zosuquidar. Phase I research with zosuquidar showed that the drug could be administered safely to patients with AML or solid tumors[103,104]. However, a randomized placebo-controlled doubleblind trial in 449 patients with newly diagnosed AML or high-risk myelodysplastic syndrome, showed no improvement in OS, or complete remission (CR) rates of drug treatment combined with zosuquidar[105].
The main reason for the initial failures in early clinical trials which started within 10 years after the discovery of P-gp-mediated MDR, was the lack of full understanding of P-gp properties and lack of knowledge on other ABC transporters. A clear example of this is tariquidar, which initially was considered as a specific P-gp inhibitor, but later results showed inhibitory effects on BCRP and MRP1 leading to the synthesis of more potent tariquidar derivatives[106,107]. Mechanistic studies also showed that tariquidar can act, in a dosedependent manner, both as a substrate or as a transport inhibitor[106,108,109]. Furthermore, recent 3-dimensional quantitative structure-activity relationship (QSAR) studies of anthranilamide derivatives of tariquidar suggest that a hydrophobic domain is needed for increased P-gp specific inhibitory function[26]. Some tariquidar analogs with sulfonamide groups are P-gp specific, nevertheless tariquidar was toxic to healthy cells[107]. Since its discovery, tariquidar was described as non-competitive and non-transported inhibitor of P-gp[110], while Bankstahl et al.[111]have shown that tariquidar is concentration-dependently transported by P-gp. Recent transepithelial drug transport assays using radioactive tariquidar in human and mouse cell lines indicate that tariquidar functions as a high-affinity P-gp substrate rather than a non-competitive inhibitor[112].
The insufficient knowledge of the P-gp transporter led to the design of clinical trials with inadequate inhibitors and patient selection. Agents such as verapamil belonging to the first-generation inhibitors were low in potency, and since dose escalation was limited due to toxicity, ineffective dosages were used[113,114].Other clinical trials failed due to their choice of compounds that interfered with the physiological role of P-gp and sister proteins. For instance, the dose of the anticancer agents used in combination with the second-generation P-gp inhibitor PSC 833 had to be reduced to prevent toxicities of the anticancer agent because PSC 833 caused a decreased drug clearance via inhibition of its metabolism by cytochrome P450[112].Regarding patient selection, initial clinical trials enrolled patients irrespective of their P-gp status, whereas rationally patient selection should be based on adequate transporter(s) expression and/or function in tumor specimen and whether the targeted transporter is the dominant mechanism of drug resistance[91]. Moreover,early clinical trial designs did not take into account the role of P-gp polymorphisms. Polymorphic variants of the transporters infuence their expression, function and localization and thereby might affect the pharmacokinetics of their substrates[115]. Inclusion of patients with such polymorphic variants might confuse treatment outcome assessments, since these patients might not develop significant MDR via efflux pumps,but face bone marrow toxicity in combination therapy with the inhibitor.
Novel molecules that can successfully interact with P-gp-specific NBDs have been identified through in silico and computational studies (compound 19, 29, 45)[116,117]. The aim of such approach is to find molecules that can interfere with the energy harvesting system of a transporter, avoiding its non-specific drug binding domains. Nanayakkara et al.[118]identified through computational studies (compound 29, 34, 45) which,when co-administrated with vinblastine and paclitaxel decreased viability, survival and motility of prostate cancer and other resistant cell lines. Furthermore compound 34 and 45 were found to be P-gp specific while inhibitor 45 also had affinity for ABCG2[117]. These techniques may be a promising approach in the search for P-gp specific NBD inhibitors[119].
Evading P-gp (nanoparticle drug delivery systems)
Focus has now shifted towards downregulating the transcription of P-gp with siRNA[87,120,121]or microRNA[122,123]using nanoparticle (NP) drug delivery systems[124]. Hyaluronic acid based nanoparticles can effectively target CD44+ ovarian cancer cells and downregulate P-gp and increase intracellular concentration of paclitaxel. High CD44+ expression in ovarian cancer cells is related to metastasis, therefore this cell-specific approach has the potential to improve the cell sensitivity in ovarian cancer patients with poor prognosis[120,121]. Encapsulation drugs in liposomal nanoparticles show promising results in the clinical setting. Doxorubicin HCl liposomal injection (Doxil) was approved for the treatment of AIDS-related Kaposi’s sarcoma (USA in 1995 and Japan in 2007), and for the treatment of ovarian cancer (USA in 1999 and Japan in 2009) after failure of prior systemic chemotherapy or intolerance or after failure of platinumbased chemotherapy[125]. In addition, in 2007 the FDA approved Doxil for the treatment of multiple myeloma in combination with bortezomib. Another candidate is the nanoparticle albumin-bound paclitaxel (nabpaclitaxel, Abraxane), which in 2011 has been approved in 42 countries for the treatment of metastatic breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months after adjuvant chemotherapy[126]. Additionally, encapsulation of doxorubicin in hydrophobic polymeric micelles cause indirect inhibition of P-gp efflux and enhance intracellular drug concentration without affecting P-gp expression[127]. The BBB is also another target of investigation with NP drug delivery. A recent study analyzed the permeability kinetics of siRNA NPs in a BBB monolayer model using human brain endothelial cells. It was concluded that NP delivery can inhibit P-gp expression by half and increase rhodamine 123 permeability by a third[87]. siRNA-loaded NP carriers could be a promising approach to circumvent the human BBB in order to facilitate drug penetration in the central nervous system. Unfortunately, side effects such as skin and hypersensitivity reactions and neurotoxicity remain common limiting factor[128,129].
The role of microRNAs in P-gp modulation
MicroRNAs are post-transcriptional regulators composed of noncoding RNAs, which can regulate gene expression and have been implicated in drug resistance in many cancers[122,123]. MicroRNA can bind to the 3’ untranslated region (UTR) of RNA with high precision, causing RNA degradation and inhibition of gene expression[130]. Several studies report that miRNAs can regulate MDR by modulating the expression of P-gp.For example, it has been shown that miR-451 and miR-27a upregulation was P-gp dependent in cell lines representing ovarian cancer, leukemia and hepatocellular carcinoma[112,114,115]. In contrast, deregulation ratherthan upregulation of certain miRNAs, such as miR27a and miR 331-5p, can also cause drug resistance reversal and decreased P-gp expression, as seen in doxorubicin resistant leukemia cell lines K562 and HL60[131-133].Furthermore one study found a possible prognostic value for miR-296, whose expression can distinguish long-term survivors from short-term survivors, while its downregulation sensitized cells to known P-gp and BCRP substrates[134].

Table 2. Novel approaches to P-gp inhibition
MiRNA mediated P-gp inhibition and drug resistance reversal seem to be dose-dependent, as seen with colorectal adenocarcinoma and breast cancer cell lines[135,136]. For example, Bao et al.[136]reported that P-gp expression could be reduced with miR-298 and reversed doxorubicin resistance in breast cancer cells in a dosedependent fashion. In vivo studies also report that miR-873 elicits a dose-dependent response, measured by tumor growth inhibition and cisplatin sensitization in previously resistant cell lines[122]. Other miRNAs with BCRP-modulating capacity include miR-122[137], miR-381, miR-495[138], miR-223[139], miR-9[140], and miR-873[122][Table 2].
Unfortunately, full understanding of miRNA post-transcriptional modulation is still insufficient and concerns with off target effects on top of which RNA degradation still remains an unressolved issue[89].
Bypassing P-gp: none substrates
Several approaches have been investigated to evade P-gp mediated MDR with novel drugs: (1) decreasing their molecular affinity for P-gp and (2) increasing the affinity to drug targets. This has been challenged for microtubule stabilizing agents (e.g., epothilones, second- and third-generation taxanes), microtubule destabilizing agents (e.g., cryptophycins, halichondrins, hemiasterlins and STX140), inhibitors of topoisomerase I (e.g., lipophilic camptothecins, homocamptothecins and dibenzonaphthyridinones) and inhibitors of topoisomerase II (e.g., lipophilic anthracyclines). Many of these agents have already undergone wide preclinical and clinical investigation, and are comprehensively reviewed[141].
The epothilones including epothilone B (patupilone) and its analogs BMS-310705, sagopilone and ixabepilone,and epothilone D and its derivative KOS-1584, are poor substrates of P-gp. They show a diverse susceptibility profile to P-gp mediated resistance, with ixabepilone the most and sagopilone the less affected by P-gp overexpression[142,143]. Currently, ixabepilone is FDA approved for the treatment of metastatic breast cancer and has been tested in phase III clinical trials in combination with capecitabine[144-146].
TPI 287, a novel microtubule destabilizing drug of the taxane family designed to circumvent MDR, was recently tested in a small group phase I study of children with neuroblastoma and medulloblastoma. TPI 287 was well tolerated in half of patients with neuroblastoma who completed one cycle of treatment with TPI 287 alone showed a stable disease although the study was not designed to evaluate treatment response[147]. In the case of epothilones, there are a number of active clinical trials to test its efficacy in a range of advanced solid tumors such as breast cancer, ovary, head and neck, esophagus, lung, gastrointestinal tract cancers and brain tumors (clinical trial reference: NCT00030173, NCT0035516 and NCT00450866). Being a P-gp substrate, ixabepilone might exert its antitumor activity preferentially in tumors where the main resistance mechanism is βIII-tubulin or/and BCRP dependent[148,149]. The exact working mechanism of these novel agents is still unclear. However, bypassing a resistance mechanism such as P-gp-mediated MDR does not imply that patients are not at risk of developing other mechanisms of drug resistance which will lead to intrinsic or acquired tumor refractoriness. For epothilones, expression of some MRPs, such as MRP7, and β-tubulin mutations has been related to the development of tumor drug resistance to these agents[150,151].
Using P-gp to kill cancer cells
Instead of inhibition of the P-gp function or evading its efflux to overcome P-gp-mediated MDR, an alternative approach is to actually exploit P-gp expression to specifically kill MDR cells. This strategy makes use of the phenomenon of collateral sensitivity (CS). which refers to the fact that some agents show preferential activity toward MDR cancer cells relative to their non-MDR parental cells, as was previously noticed for verapamil, cytosine arabinoside and gemcitabine in MDR small cell lung cancer cells lines[152,153]. Different CS agents have been synthesized from lead compounds such as NSC73306 (1,1-Isatin-4-(4′-methoxyphenyl)-3-thiosemicarbazone) and the favonoid desmosdumotin B[154,155]. It has been hypothesized that CS agents exert their effect by the generation of reactive oxygen species. This strategy is still experimental. Of further interest,within a class of isatins drugs, particularly N-alkylisatins were found not to be susceptible to P-gp mediated efflux, destabilize microtubule formation and induce apoptosis in P-gp overexpressing tumor cells[156].
Another approach to bypass P-gp is to use drugs that show a higher sensitivity to P-gp expressing cells compared to P-gp negative cells. Bergman et al.[153,157]showed that both small cell lung cancer and NSCLC cells with an overexpression of either P-gp or MRP1 were more sensitive to the deoxynucleoside analogs cytarabine and gemcitabine. This was related to an increased activity of the activating enzyme deoxycytidine kinase (dCK) for these nucleoside analogs, leading to an increased accumulation of the active metabolites,ara-CTP and dFdCTP, respectively. It was argued that in P-gp cells steroids are effluxed efficiently decreasing their intracellular concentration. Since steroids regulate dCK, this may lead to an increased enzyme activity[158]. The increased sensitivity was observed in cells with acquired resistance to P-gp substrates, such as etoposide, doxorubicin, colchicine, but also in cells transfected with the gene for P-gp or MRP1[153,157].
Increase oral bioavailability by increasing gut epithelial uptake
A strategy that can benefit of P-gp inhibition is the increase of the oral bioavailability of drugs, particularly when a drug is a P-gp substrate and is efficiently being effluxed back to the gut leading to a low oral bioavailability. The oral administration of drugs has several advantages over intravenous formulation[159].First of all, patients prefer oral chemotherapy over intravenous due to the ease of administration, avoiding the discomfort of injection and the related risk of infection[159,160]. Secondly, oral medication is cost saving for hospital and health insurance, since there is no need for hospitalization. Finally, oral administration of drugs enables metronomic therapy with the advantages of chronic exposure of the drugs to its targets and reducing dose-related toxicities, such as the optimizing of anti-angiogenic efficacy of chemotherapeutic agents including paclitaxel and docetaxel[161,162]. Wild-type mice showed limited oral bioavailability of paclitaxel compared P-gp knockout mice[163]. Following this, multiple preclinical mice results have shown that inhibition of P-gp using inhibitors such as cyclosporine, PSC 833 or elacridar improved the oral treatment of paclitaxel in mice[164-166]. Therefore, clinical trials were started to explore the effect of the oral administration of paclitaxel in combination with cyclosporine. Three phase ІІ trials have tested this combination in patients with NSCLC, advanced gastric cancer and breast cancer. These studies have shown promising results, but long-term oral use of cyclosporine might be complicated by immunosuppressive effects. Therefore, other inhibitors might replace cyclosporine such as elacridar (GF120918) or HM30181, respectively[167,168]. Being a P-gp substrate in addition to the encouraging results of oral paclitaxel, preclinical studies were also initiated with another taxane, namely docetaxel[169]. Different inhibitors were used such as OC144-093 (a P-gpinhibitor), cyclosporine (a dual inhibitor of P-gp and CYP3A4) and ritonavir (CYP3A4 inhibitor), which have shown increased oral bioavailability of docetaxel[170]. In line with this observation, which indicates that CYP3A4 is the major determinant of the low oral bioavailability of docetaxel, it seems rational that future trials will continue with oral docetaxel in combination with ritonavir[167,170].
Other studies have shown that P-gp restricts the oral bioavailability of the topoisomerase II inhibitor,etoposide, and that P-gp inhibition enhances its oral bioavailability[171]. The appropriate inhibitor and associated dosages (of both drug and inhibitor) still need to be determined to improve the therapeutic outcome with minimal toxicities, since P-gp infuences the excretion of drugs in addition to its role in protecting of vital organs such as the brain, the fetus and the testes. Furthermore, attention needs to be paid to specific polymorphisms of P-gp that might impact the drug oral bioavailability.
Monoclonal antibodies against P-gp
Already in the 1986 Hamada and Tsuruo[172]developed two monoclonal antibodies against human P-gp in adriamycin-resistant myelogenous leukemia cells (MRK-16 and MRK-17). Initially, these antibodies were intended to study the membrane changes in MDR cells, but later research demonstrated that MRK-16 was able to inhibit P-gp mediated efflux of vincristine and actinomycin D in vivo[172,173]. The addition of a P-gp inhibitor such as cyclosporine A to MRK-16 treatment, further sensitized MDR myelogenous leukemia cells to vincristine and doxorubicin[174,175]. This innovative approach with MRK-16 is currently being investigated again in phase I clinical trials[9].
OVERCOMING MRP1 RESISTANCE
MRP1 was identified as a drug transporter 15 years after P-gp, and therefore its role in MDR in human tumors has not been fully understood and fewer MRP1-specific inhibitors with sufficient potency and efficacy have been identified[176]. Several novel strategies that have been applied to P-gp are being tried on MRP1 overexpressing cells are listed on Table 3. Nanoparticle drug delivery systems have been also applied successfully to inhibit MRP1 mediated resistance[177,178]. For example, Wang et al.[177]used redox-responsive polymeric micelles with a disulfide bond that serves as a linker for delivery of paclitaxel as chemotherapeutic,and indomethacin, as chemosensitizer. Also, siRNA delivery in porous silicon nanoparticles has shown to effectively downregulate MRP1 mRNA[178]yet more studies are needed, especially in vivo.
As with P-gp, numerous TKIs have been also identified to modulate the efflux of MRP1. Cediranib (AZD217)is an orally administered potent small-molecule TKI that inhibits the vascular endothelial growth factor(VEGF) family of receptors (VEGFR 1-3), platelet-derived growth factor receptor (PDGFR) and a stem cell factor receptor, c-Kit[179]. A study by Tao et al.[180]showed that cediranib inhibited the ATPase activity of P-gp in a dose - dependent manner and it also reversed both P-gp and MRP1 mediated MDR, suggesting that cediranib acts as dual inhibitor. Another TKI, vandetanib (ZD6474, Zactima), an inhibitor of VEGFR,epidermal growth factor receptor (EGFR) and rearranged during transfection (RET) tyrosine kinases[181],stimulated P-gp ATPase activity in a dose-dependent manner and reversed MDR in cancer cells by directly inhibiting the function of P-gp, MRP1 and ABCG2[181,182]. Other TKIs such as AG-1393 and EKI-785, have also been found to interact and inhibit P-gp activity[183].
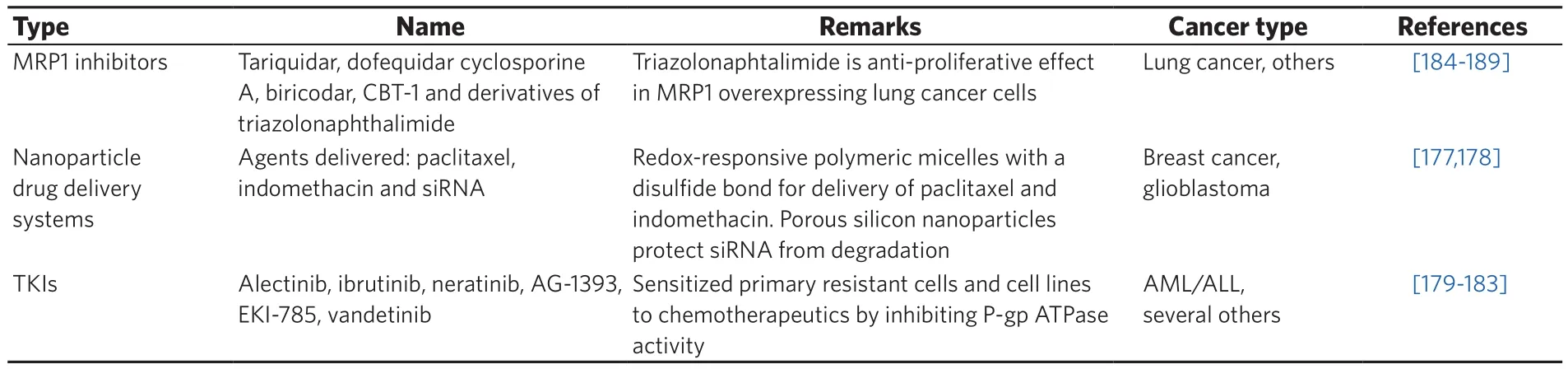
Table 3. Novel approaches to MRP1 inhibition
Among the three generations of P-gp inhibitors, there are inhibitors that also inhibit MRP1 and/or ABCG2.Examples of such inhibitors are cyclosporine A[26,184-187], biricodar (VX-710), dofequidar (MS-209), tariquidar and CBT-1. The novel triazolonaphtalimide derivatives have been reported to achieve an anti-proliferative effect in chemoresistant lung cancer cell lines via MRP1 downregulation without affecting P-gp function[188].Some of these preclinical studies reached phase III clinical trials, for instance for dofequidar[189]and CBT-1 (NCT00437749). However, inadequate trial design and poor drug specificity might hamper proper assessments of the full potential of these agents. Further investigation is warranted to assess the effect of these inhibitors on each targeted ABC transporter in clinical trials, to guide selection of the right population of patients to enhance the efficacy and minimize the toxicity.
The scarcity of specific MRP1 inhibitors refects the fact that there is still no clarity about the benefit of P-gp inhibition in the clinical practice, which retards research efforts to develop MRP1 inhibitors for clinical use[190]. However, the calcium channel blocker verapamil and its derivative NMeOHI2, in addition to the several non-steroidal anti-infammatory drugs (e.g., indomethacin), have shown collateral sensitivity toward MRP1 overexpressing cells[191,192]. These agents were proposed to trigger apoptosis in cells overexpressing MRP1 via intracellular GSH depletion. Furthermore, through high-throughput screening and chemical modification studies, the tricyclic isoxazole derivatives were identified to exhibit high potency and specificity to MRP1 inhibition. Nevertheless, further studies are needed to address MRP1 inhibition specificity, toxicity and effectivity both in vivo and in vitro.
OVERCOMING ABCG2 RESISTANCE
Ahmed-Belkacem et al.[193]divided ABCG2 inhibitors in four groups: (1) ABCG2-specific inhibitors; (2)broad-spectrum inhibitors, including inhibitors that inhibit P-gp and/or MRP1; (3) TKIs; and (4) naturally occurring favonoids and derivatives.
ABCG2 inhibitors
The mycotoxin fumitremorgin C (FTC) was identified as the first ABCG2 specific inhibitor, but its neurotoxic effect hampered its further clinical development[194,195]. This lead compound was the basis of a new non-toxic tetracyclic analogue of FTC, Ko143, which is a highly potent and specific inhibitor of ABCG2[195]. Ko143increased topotecan plasma concentration 4-6-fold in Mdr1a/1b-/- mice. Pick et al.[196]identified a new class of specific ABCG2 inhibitors which were structurally related to tariquidar, but did not show potency toward P-gp and MRP1. In their research they presented evidence for 3 different binding sites on ABCG2, one occupied by the ABCG2 substrate pheophorbide A (Pheo A), another by the TKI imatinib and a third by the two compounds that specifically inhibit ABCG2. Imatinib showed features of an allosteric modulator by increasing the inhibitory effect of the inhibitor compounds. Another ABCG2 inhibitor lead compound, PZ-39, was reported by Peng et al.[197]. Interestingly, this compound had a dual mode of action; it could effectively inhibit ABCG2 function and markedly reduced the half-life of ABCG2 protein from approximately 54 to 5 h by accelerating its protein degradation via endocytosis and trafficking to lysosomes. The ABCG2 inhibitor specificity of these compounds was confirmed with respect to P-gp and MRP1, but not for other ABC transporters.

Table 4. Novel approaches to ABCG2 inhibition
Transcriptional down regulation using siRNA or miRNA are also areas of interest in ABCG2 inhibition.One study shows that combination of siRNA and PI3K/Akt signaling inhibition can reverse chemoresistance in mitoxantrone-resistant BCRP-overexpressing cells lines[198][Table 4]. Similarly, nanoparticle drug delivery using polyethyleneimine as a carrier for siRNA has been reported to down-regulate ABCG2 and decrease the mitoxantrone IC(50) values by approximately 14-fold[86]. Other efforts to deliver ABCG2 and P-gp inhibitors with nanoparticles show complete reversal of resistance in ABCG2 positive MDR cells[199].
There are several miRNA that have been identified in a wide range of cancer cells, as potential inhibitors of ABCG2 expression (such as miRNA-328, -hsa-miR-328, -519, -520h, -212, -181a, 487a and miR-302[200-205]. The use of miRNA to interfere with transporter transcription confirmed the role of ABCG2 in drug resistance related signaling pathways. For example, it was seen that miR-132 enhanced cisplatin resistance in gastric cancer cells by targeting SIRT1 which regulates ABCG2 expression by promoting the de-acetylation of the transcription factor CREB (SIRT1/CREB/ABCG2 pathway). Moreover, Kaplan-Meier survival analysis showed that low miR-132 is related to longer survival in chemo resistant gastric cancer[206]. Another example is Wnt/β-catenin-ABCG2 pathway, which is modulated by miR-199a/b in colorectal cancer stem cells, and whose upregulation is related to cisplatin resistance[207].
Dual inhibitors
Some of the broad-spectrum inhibitors of P-gp, including elacridar, reserpine, cyclosporine A, tariquidar and PSC 833 also display ABCG2 inhibitory activity[10]. Such dual inhibitors will be effective in cases where both transporters participate in MDR and the cytotoxic drug is a substrate of both transporters. Conceivably,their inhibition will also increase the oral bioavailability of the drug, in addition to its penetration to the tumor site. However, since these two transporters are also expressed in sanctuary sites, caution for potential toxic side effects should be considered. Moreover, this awareness would also hold for cytotoxic drugs with a narrow therapeutic window, as minor elevations of drug exposure might lead to adverse reactions.
TKIs as ABCG2 inhibitor or substrate
Various TKIs are either a substrate, an inhibitor or both, for P-gp, MRP1 and ABCG2[168]. TKIs such as the BCR-ABL kinase inhibitors (imatinib, nilotinib and dasatinib), EGFRs kinase inhibitors (gefitinib, erlotinib,lapatinib) and others, show substrate-like properties at lower concentration and inhibitor-like properties at higher concentration via competitive inhibition of the transporters function[208]. In addition to the concentrationdependent type of interaction, it has been proposed that exposure time to TKIs also plays a role[209]. Short exposure (≤ 24 h) to either gefitinib or vandetanib demonstrated a synergic interaction with SN-38, whereas prolonged exposure (5 days) showed a strong antagonism between gefitinib or vandetanib and SN-38. Gefitinib and EK-785 at a low concentration (0.1 to 1 µmol/L) significantly stimulated the ATPase activity, suggesting that these TKIs are transported substrates of ABCG2 whereas higher concentrations induced strong inhibition of ABCG2[210]. Different types of interactions and corresponding possible effects have been suggested by different research groups, which are demonstrated in a hypothetical model [Figure 2].
Many combination therapies of TKIs with cytotoxic drugs have been performed (e.g., the combination of gefitinib with 5-fuorouracil, leucovorin, and irinotecan in patients with colorectal cancer, the combination of imatinib and cytarabine in newly diagnosed patients with chronic myeloid leukemia, and the combination of gemcitabine/cisplatin with gefitinib in NSCLC[95,211-214]. TKIs also interact with ABCG2 that can modify ADME-Tox profile of ABCG2 substrates. Gefitinib enhanced the oral bioavailability of irinotecan and topotecan, and increased their apparent bioavailability and decreased systemic clearance in mice[215,216].Therefore, studies are needed that investigate the exact type of interactions between TKIs and cytotoxic drugs[95,208,209].
Inhibitors from natural sources
ABCG2 inhibitors originating from natural products have been referred to as the fourth-generation inhibitors. Originally, these compounds showed effects in cancer prevention[217], but later on they were found to exert sensitizing effects to chemotherapeutics. Among them are the favonoids which are polyphenolic compounds found in foods and herbal products. Different favonoids are potent modulators of ABC transporters: kaempferol, apigenin and myricetin are inhibitors of MRP1[218], kaempferide,quercetin, diosmin and glabridin are inhibitors of P-gp[219]and silymarin, hesperetin and daidzein are inhibitors of ABCG2[220]. The biofavonoids neochamaejasmin B (NCB)[221]and chamaechromone from the root of Stellera chamaejasme L. are able to inhibit the expression and activity of MRP1. Interestingly,chamaechromone is MRP1 specific, while NCB is a substrate for MRP1 and P-gp. Furthermore NCB and chamaechromone co-administration increased chamaechromone bioavailability substantially[166]. However,further investigation is needed to define optimal dose schedules of favonoids capable of inducing ABC transporter inhibition[219]. Future studies would also have to take into account favonoid pharmacokinetics,bioavailability and activity of favonoid metabolites after ingestion, which would reduce active favonoid plasma concentrations[222,223].
CONCLUSION
Efflux transporters of the ABC superfamily including P-gp, MRP1 and ABCG2 can confer MDR in cancer cells. Additionally, overexpression of these transporters in tumor tissues has been associated with poor therapy outcome of cancer patients. Conceptually, inhibition of these ABC transporters will increase drug accumulation and thereby overcome MDR. However, despite accumulating knowledge of ABC transporters and their complex interplay, clinical trials evaluating the effect of ABC transporter inhibitors did not fulfill their promises. In part, this has also been accounted for by poor patient selection criteria.

Figure 2. Hypothetical model demonstrating the different possible interactions of TKIs with ABC transporters. (A) The competitive binding of TKI at the drug binding site and hereby preventing the binding of the drug and consequently its efflux; (B) the situation when TKI is not a substrate of ABC transporter, but it binds at the NBD or other site inducing allosteric modulation of the transporter which leads to its inhibition; (C) the case when the TKI behaves as a substrate at low concentration, where the “vacuum cleaner” model is applicable[30].However, when the concentration increases at a given moment saturation takes place and TKIs penetrate into the cell and bind at the NBD and thereby inhibiting the transporter. ABC: ATP-binding cassette; TKI: tyrosine kinase inhibitor; NBD: nucleotide binding domain
The design of ABC transporter inhibition research would require several considerations. The choice for a specific inhibitor will increase the likelihood of undesirable side effects when the inhibitor targets multiple ABC transporters, although due to redundancy of ABC transporters, sanctuary sites might be spared from the toxic insults of the cytotoxic drug. When multiple ABC transporters are involved in provoking the MDR phenotype, broad-spectrum inhibitors should be preferred, but as a double-edged sword will also impact physiological functions of these ABC transporters. The evading strategy may serve an alternative for the ABC transporter inhibition dilemma. At this stage, however, the magic bullet that escapes each of the 15 ABC transporters implicated in cancer drug resistance has not been identified and current studies aim to identify those compounds evading the major ABC transporters P-gp, MRP1 and BCRP. Also, MDR evading strategies using encapsulation of drugs have potential downsides such as immune reactions. Lastly,concerning the exploiting strategy, those drugs which cause collateral sensitivity would promote selective targeting of MDR cells, but this concept has not yet been sufficiently explored in animal and clinical studies.
Collectively, each of the MDR reversal strategies has its merits but did not translate in successful clinical application. Future directions should therefore be aimed at combining our current knowledge of clinically relevant ABC transporters in the field of molecular biology (including polymorphic variants), biochemistry,computational biology and sensitive detection and imaging techniques, which in conjunction with state of the art medicinal chemistry can generate new generation inhibitors for future MDR reversal studies. Such an integrated approach may also help to guide personalized therapy in cancer patients to achieve the most optimal treatment outcome.
DECLARATIONS
Authors’ contributions
All authors contributed equally to the paper.
Financial support and sponsorship
None.
Conflicts of interest
There are no conficts of interest.
Patient consent
Not applicable.
Ethics approval
Not applicable.
Copyright
© The Author(s) 2018.
杂志排行
Cancer Drug Resistance的其它文章
- Resistance mechanism to cisplatin in NCl-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage
- Reduction of mitomycin C resistance in human bladder cancer T24 cells by knocking-down ras oncogene
- lmproved potency of F10 relative to 5-fluorouracil in colorectal cancer cells with p53 mutations
- ldentification and targeting of CD22ΔE12 as a molecular RNAi target to overcome drug resistance in high-risk B-lineage leukemias and lymphomas
- Cancer drug resistance: a new perspective