Hepatoprotective Effect of Phenylethanoid Glycosides from Cistanche deserticola against Chronic Hepatic Injury Induced by Alcohol
2018-07-23GUOYuanhengCAOLiliZHAOBingZHAOQingshengHUANGYuanrongXIAOChuanming
GUO Yuanheng , CAO Lili, ZHAO Bing,, ZHAO Qingsheng,, HUANG Yuanrong, XIAO Chuanming
(1. Division of Biorefinery Engineering, State Key Laboratory of Biochemical Engineering, Institute of Process Engineering,Chinese Academy of Sciences, Beijing 100190, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Beijing Keepyoung Technology Co. Ltd., Beijing 101509, China)
Abstract: The aim of this paper is to explore the hepatoprotective effect of Herba Cistanchis against alcohol-induced chronic hepatic injury and its underlying mechanism. Phenylethanoid glycosides (PhGs) were extracted and purified from Cistanche deserticola. Then, the PhGs composition was analyzed and the antioxidant activity was determined. The hepatoprotective activity was studied both in vitro and in vivo. In vitro, the survival of HepG2 cells was promoted by PhGs.In vivo, PhGs restored the alterations induced by alcohol, including serological indexes (alanine aminotransaminase, aspartate aminotransferase, γ-glutamyltranspeptidase, acid phosphatase, triglyceride and low density lipoprotein-cholesterol) and hepatic indicators (superoxide dismutase, glutathione S-transferase, glutathione, glutathione peroxidase, malondialdehyde and triglyceride) levels. The prominent microvesicular steatosis and necrosis in hepatic histopathology of model animals were also notably attenuated by PhGs administration. These results indicated that PhGs possess hepatoprotective properties against chronic alcohol-induced liver injury, the mechanisms involve the modulation of related enzyme (including superoxide dismutase, glutathione S-transferase, glutathione peroxidase) activities and the reduction of lipid peroxidation products such as malondialdehyde.
Keywords: Herba Cistanchis; phenylethanoid glycosides; antioxidant activity; hepatoprotective effect; alcoholic liver disease
Alcohol is a worldwide consumed beverage and food additive. Published reports reveal that excessive alcohol abuse induced nearly 2.5 million deaths each year in the world[1-2]. In human body, the liver is a protective organ,which helps metabolization and removal of harmful toxin such as alcohol. For this reason, the liver is very vulnerable to alcoholic injury[3]. Pathological evidences reveal that liver injury induced by excessive drinking is one of leading risk factors for the hepatic disease and many other diseases[4-5].Alcohol induced chronic hepatic disease (AICH) is a complex multistep progression, normally develops from alcoholic steatosis to alcoholic hepatitis, and finally turns to alcoholic cirrhosis[6]. Despite the modern medical science has been highly developed, hepatopathy is still a worldwide healthy problem that has not been resolved. Thus, identifying active natural products for those alcohol consumers to prevent or slow down the progression of alcoholic liver injury in early stage seems to be a beneficial management strategy.
Herba Cistanchis is the stems of Cistanche deserticola Y. C. Ma, which parasitizes in the roots of the dicotyledonous plant Haloxylon ammodendrun. Cistanche Herba, first recorded in Shen Nong’s Chinese Materia Medica, has long been used as a traditional herbal medicine, for the treatment of liver and kidney deficiency[7-9], impotence, senile constipation, soreness and weakness of waist and knees,and blood deficiency[10-11]. Traditional Chinese medicine believed that the liver and kidney were consanguineous.Herba Cistanchis is beneficial for the liver and kidney, mild in nature, and is an invigorate medicine, no side effects for long term usage[7]. Thus Herba Cistanchis was the prominent component in a classical compound medicine (named Congrong Wan) used for nourishing liver and kidney[12-13].
Some papers have been published to elucidate the hepatoprotective effect of C. deserticola. But the aim was focused on acute liver injury induced by carbon tetrachloride(CCl4) or D-galactosamine and lipopolysaccharide[14-15],rather than the chronic liver disease related to alcohol abuse. For acute liver injury, especially CCl4induced model, the toxic mechanism was relatively clear and has been reported frequently. AICH involves more complex toxic mechanism, and it had become a serious social problem. But for the hepatoprotective effect of C. deserticola against AICH, relative reports are still rare[16]. This article researched the hepatoprotective effect of polysaccharide from C. deserticola, rather than ethanol soluble substance. Thus, study on hepatoprotective effect against AICH and its mechanism are meaningful both for large number of alcohol consumers and for the industry development of C. deserticola. Therefore, in this paper, our focus was on the composition and the hepatoprotective activity of phenylethanoid glycosides (PhGs) extracted from C. deserticola, as well as the underlying mechanism.
1 Materials and Methods
1.1 Materials, animals and reagents
The stems of C. deserticola were collected from Alashan League, Inner Mongolia of China, and identified by Prof. Wang Xiaodong (Division of Biorefinery Engineering,Institute of Process Engineering, Chinese Academy of Sciences).
HepG2 cells China Center for Type Culture Collection, Beijing, China.
Adult female ICR mice (22–25 g) purchased from the Beijing Vital River Laboratory Animal Technology Co. Ltd..
Assay kits including alanine aminotransaminase (ALT),aspartate aminotransferase (AST), glutathione (GSH),glutathione peroxidase (GSH-Px), glutathione S-transferase(GST), malondialdehyde (MDA), γ-glutamyltranspeptidase(γ-GTP), acid phosphatase (ACP) and superoxide dismutase (SOD) Nanjing Jiancheng Bioengineering Institute; low density lipoprotein cholesterol (LDL-C) and triglyceride (TG) BioSino Bio-Technology & Science Inc.; 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)(ABTS), 1,1-diphenyl-2-picryl-hydrazyl (DPPH), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Sigma Chemical Co. (USA); Dulbecco’s modified Eagle medium (DMEM)and fetal bovine serum (FBS) Invitrogen Inc. (USA); Er Guo-tou white spirit Beijing Red Star Co., Ltd.; bicyclol Beijing Union Pharmaceutical Factory; Aqueous solutions were prepared with ultra-pure water from a Milli-Q water purification system. All other reagents were of analytical grade.
1.2 Instruments
MultiskanTMFC 96-well plate reader Thermo Scientific (USA); DS-L1-5M Light microscope Nikon Corporation (Japan); SW-CJ-1D Super net worktable Shanghai Tiancheng Technology Co., Ltd.; PCS-100 CO2incubator Shenyang Shengda Bioelectrical Equipment Co., Ltd.; high performance liquid chromatography (HPLC)Shimadzu Corporation (Japan).
1.3 Preparation and chemical component analysis of PhGs
PhGs was prepared as the previous reported method of our group[17]. The total PhGs content was determined by spectrophotometry[18]. And its composition was analyzed by HPLC[19]. The chromatographic conditions were as follows:HPLC on LC-20AT system equipped with a SPD-M20A diode array detector and XTerra C18column (250 mm ×4.6 mm, 5 μm) was used. The mobile phase A was formic acid aqueous solution (0.2%), B was acetonitrile.Gradient elution program was as follows: 0–23 min, 14%of B; 23–24 min, 14%–17% of B; 24–35 min, 17% of B;35–36 min, 17%–20% of B; 36–60 min, 20% of B. The detection wavelength was 330 nm, flow rate was 0.8 mL/min,oven temperature was 30 ℃ and injection volume was 10 μL.Data were the means of three parallel experiments.
1.4 Hepatoprotective activity in vitro
1.4.1 Cell culture and treatment
HepG2 cells were cultured in DMEM containing heat inactivated FBS (10%), streptomycin (0.1 μg/mL)and penicillin (100 IU/mL), and non-essential amino acid. The cells were incubated in humidified atmosphere of 5% CO2at 37 ℃. The medium was changed every 48–72 h. Cells were harvested at the exponential growth phase, seeded into 96-well plates (4 × 104cells per well,100 μL) and incubated for 24 h. Naitive group (Nai) was treated with water. Test cells, including PhGs group,positive group (Pos) and negative group (Neg), were treated with alcohol (final concentration was 3.5%, V/V). After that PhGs group were added PhGs (final concentrations were 0.11, 0.33, 1.00, 3.00 mg/mL, respectively),Pos were added bicyclol (final concentration was 0.20 mg/mL), Neg was added water. Then cells were incubated in humidified atmosphere of 5% CO2at 37 ℃ for another 48 h.
1.4.2 Determination of cell survival rates
The cell survival rates were determined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay method mentioned by Yu et al[20]with slight modification. Briefly, MTT (5 mg/mL, 20 μL per well)were added into the cell-seeded 96-well plates described above, and the cells were incubated at 37 ℃ for 4 h. The solutions were removed and DMSO (150 μL per well) were added. The absorbance in each well was measured at 492 nm using a 96-well plate reader. The cell survival rates were calculated using the formula below. Five parallel experiments were repeated.

Where Asamplewas the absorbance of the experimental group; Acontrolwas the absorbance of the control group without sample; Ablankwas the absorbance of culture medium without sample and not seeded cell.
1.5 Hepatoprotective activity in vivo
1.5.1 Animals feeding conditions
Animals were housed in an environmentally-controlled room (relative humidity was 40%–60%, 22–26 ℃), air ventilation 12–18 times/h, and a 12 h light/dark cycle of 150–300 Lux, with feed and water ad libitum. The mice were allowed to acclimatize to the animal room conditions for 7 days prior to experiments. All procedures involving animals throughout the experiments were conducted in strict accordance with the rules for Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health.
1.5.2 Experimental design
ICR mice were randomly divided into the following 6 groups with 10 animals in each group. The naive group(Nai) was orally administered with distilled water. Negative control group (Neg) was orally administered with alcohol (Er Guo-tou white spirit, 56%, 6 mL/kg). Positive control group(Pos) was orally administered with alcohol and 5 hours later administered with bicyclol (300 mg/kg). Three PhGs groups(orally administered with PhGs at the doses of 200, 600,1 800 mg/kg and 5 h later with alcohol). All mice were administered for 31 consecutive days.
1.5.3 Determination of serological indexes
About 4 h after last alcoholic treatment, blood was collected from eye socket and centrifuged at 3 000 × g for 15 min. After the serum was separated, the enzyme activities of ALT, AST, γ-GCP and ACP were determined. The serum TG and LDL-C were also analyzed using diagnostic kits. Ten parallel experiments were repeated.
1.5.4 Determination of hepatic indicators
After the collection of blood, mouse was executed and its liver was immediately excised, washed with physiological saline. A piece of hepatic tissue was separated from left lobe of liver. Then the tissue was minced and homogenized in aqueous potassium chloride solution (1.15:100, m/V) in a glass homogenizer for 60 s to make a liver homogenate(1:10, m/V). The enzyme activities of SOD, GST, GSH and GSH-Px, the contents of MDA and TG were measured using diagnostic kits. Ten parallel experiments were repeated.
1.5.5 Histopathologic studies
Residual part of the liver was fixed in Bouin’s fixative for 24 h, after dehydrated using aqueous ethanol solution(50%–100%, V/V), the liver tissue was cleared in xylene and embedded in paraffin. Hepatic sections (5 mm) were stained with alum haematoxylin and eosin (HE). Images of liver sections and histopathological distortion was captured by a light microscope.
1.6 Statistical analysis
Data was expressed as means ± SE from five (cell experiment) or ten (animal experiment) independent experiments. Statistical significances of differences between groups were analyzed by One-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls’s multiple range test using SPSS 19.0 software. In all analyses, P < 0.05, P < 0.01 or P < 0.001 indicated statistical significance.
2 Results and Analysis
2.1 Chemical components of PhGs
The content of the PhGs was analyzed. The structures and chromatograms of different component are shown in Fig. 1. PhGs content was 79.6%. Contents of echinacoside and acteoside (the two key marker compounds recorded in Chinese Pharmacopoeia) of PhGs were 27.27% and 9.61%,respectively.

Fig. 1 Molecular structure (A and B) and HPLC (C) of PhGs
2.2 Effects of PhGs on HepG2 cell survival rates
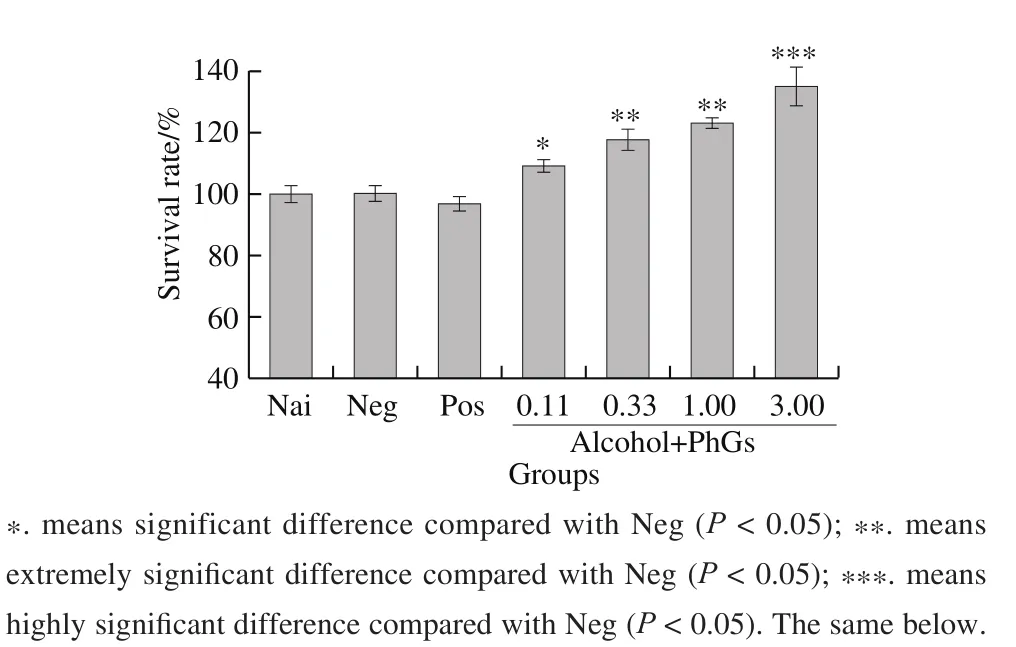
Fig. 2 Effects of PhGs on survival rate of alcohol-treated HepG2 cells
In vitro studies of the hepatoprotective activity of PhGs were performed by assess survival rates of HepG2 cells. Fig. 2 shows that PhGs can notably improve the HepG2 cells viability in a dose dependent manner.
2.3 The effects of PhGs on serological index
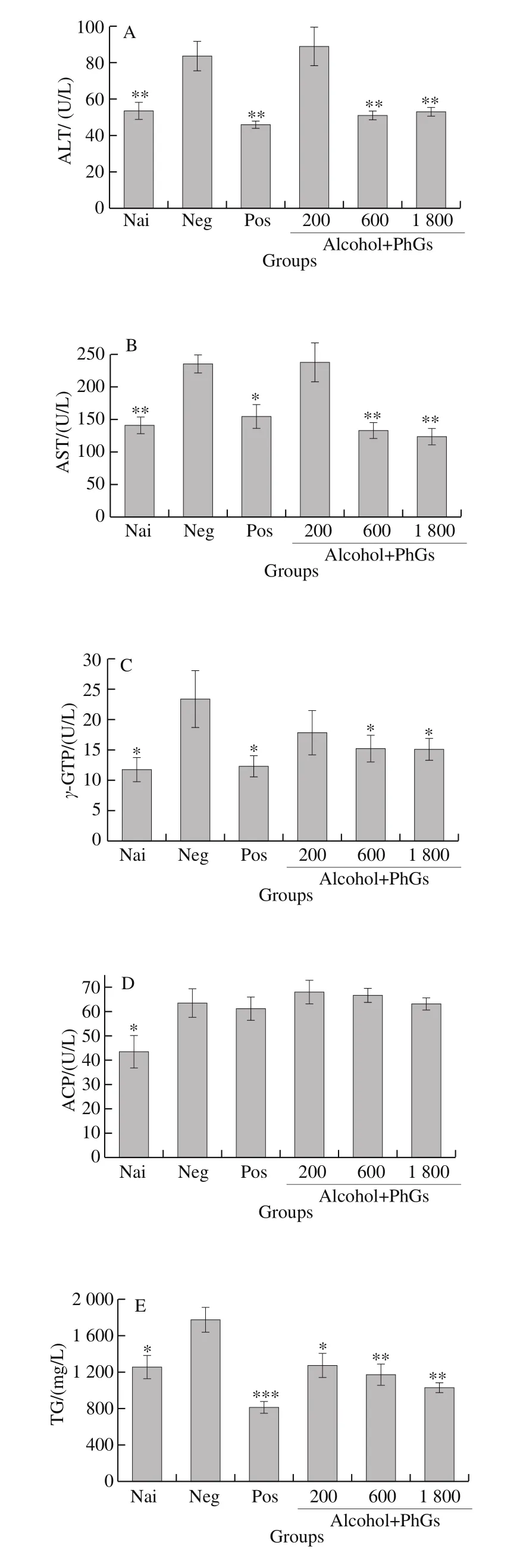

Fig. 3 Effects of PhGs on serum biochemical parameters of alcohol-treated ICR mice, including ALT (A), AST (B), γ-GTP (C), ACP (D), TG (E) and LDL-C (F) levels
The results of cell experiment indicated that PhGs have a potential hepatoprotective activity. To certified this property and research its mechanism. In vivo experiments were carried out. Fig. 3A–F exhibit the effects of PhGs on ALT, AST,γ-GGT, ACP, serum TG and LDL-C levels, respectively. As clearly shown in these pictures, six serum indicators drastically promoted by alcohol treatment in negative group. PhGs(especially in its medial and high concentration) can significantly attenuate the other five indicators except ACP, which also attenuated by PhGs but not exhibited statistical significance.
2.4 The effects of PhGs on hepatic indicators

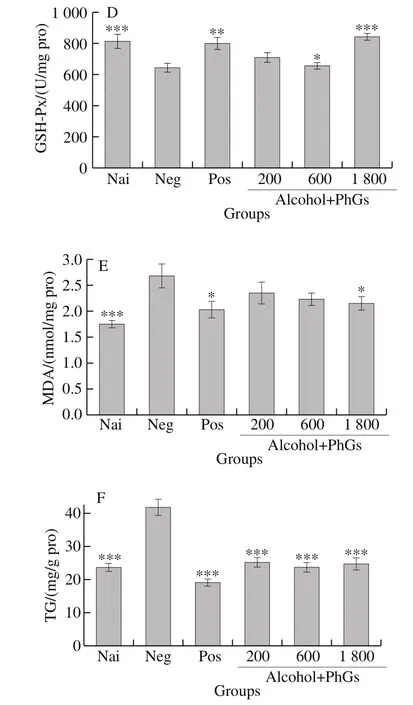
Fig. 4 Effects of PhGs on hepatic indicators of alcohol-treated ICR mice, including SOD (A), GST (B), GSH (C), GSH-Px (D), MDA (E) and TG (F) levels
The effects of PhGs on liver function are shown in Fig. 4.Fig. 4A–E and F exhibit the effects of PhGs on SOD, GST,GSH, GSH-Px, MDA and TG levels, respectively. PhGs not only can evidently improve the SOD, GST, GSH and GSH-Px levels, but also can reduce the MDA and TG levels.
2.5 Histopathologic effects of on alcohol induced mice
Based on the histopathologic observations on the liver sections, the naive group exhibits normal cellular architecture with distinct hepatic cells and no histologic abnormalities(Fig. 5A). In comparison, the administration of alcohol caused serious liver damage. The liver sections show hepatocyte fat deposition, hepatocyte necrosis and swelling, vacuole formation in the cells, and the cellular boundaries also disappeared (Fig. 5B). Administration of bicyclol (Fig. 5C),or varying dose of PhGs (Fig. 6D–F) alleviate these alterations appeared in negative control group. These results validate that the alcohol induced hepatocyte experienced a certain extent of normal distortion in negative control group,but PhGs alleviated this distortion.
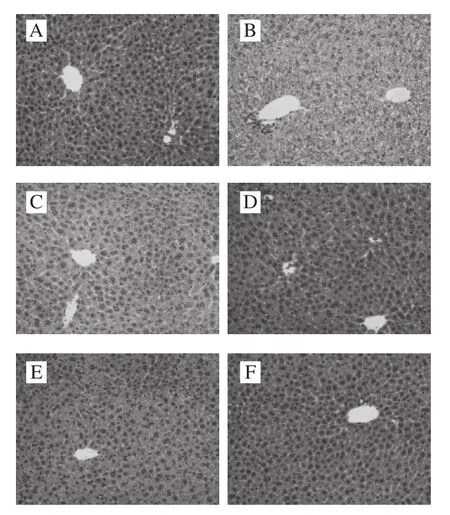
Fig. 5 Histological photos of liver tissues of naive group (A), negative group (B), positive group (C), and three PhGs + alcohol treatment groups(D, E and F, orally administered with PhGs at a dose of 200, 600,1 800 mg/kg and 5 hours later with alcohol)
3 Discussion
Alcohol abuse attributes to nearly 4% of all death rates,which has become a serious social problem in the world.In human body, the liver is one of the protective organs which helps metabolize and remove toxins such as alcohol.Alcohol is metabolized in the liver after it is absorbed through the gastric and small intestinal mucosa[21]. Alcoholic liver diseases usually occur after years of excessive alcohol ingestion[22]. Commonly pathological conditions such as fatty liver, hepatitis, fibrosis and cirrhosis[23], or even cancerous diseases such as hepato cellular carcinoma and colon cancer[24], are observed in alcohol linked liver disorders. For these reasons, alcohol is a typical hepatoxin and is widely used as an inducer of liver injury in scientific researches[25].Considering alcohol induced liver diseases were often caused by alcohol beverages and food additive but not industrial ethanol, thus Er Guo-tou white spirit was used to establish liver injury model in ICR mice in the present study. Bicylol is a commonly used agent used to treat the chronic liver injury,so it was used to set positive control model.
Measuring cell viability is a common way to assess the efficacy of natural drugs[26]. In present study, survival rates of HepG2 cells were used to evaluate the protective effects of PhGs on alcohol damaged model. PhGs exerts extensive activity in improve cell survival rates in a dose dependent manner.
Hepatic cells contain higher concentrations of ALT and AST in the cytoplasm and mitochondria. Because of various hepatic cell damages, the leakage of cytosol will cause increment of ALT and AST levels in serum. Thus the promotion of ALT and AST levels are indicators of cellular leakage and functional disorders of the liver[27]. Therefore, the serum AST and ALT levels were usually set as the key marker enzymes for the indirect assessment of the liver health[28]. For similar reasons, γ-GGT, ACP, TG and LDL-C levels were also used as the indicators to assess the healthy condition of liver[29-30].In the present study, the hepatoprotective activity of PhGs were assessed by determined the levels of ALT, AST, γ-GGT, ACP,TG and LDL-C. Comparing negative control group to naive group, these serum indicators were changed notably, that means alcoholic management made significantly injury in liver of mice.Comparing experimental group with negative control group,the results showed that treatments with PhGs attenuated these biochemical alterations induced by alcohol, implying that the PhGs possesses potential to protect the hepatic parenchyma and hepatocytes against alcoholic damage.
Organisms subject to oxidative stress usually evolved an antioxidant defense mechanism. SOD and GSH were two of typical enzyme based antioxidant systems[31-32]. The SOD and GSH play an important role in eliminating peroxides and regulate oxygen and hydrogen peroxide at the physiological levels. SOD catalyzes the dismutation of superoxide anion into O2and H2O2[33]. GSH is a tripeptide and plays a major protective role as a scavenger of toxic metabolites[34]and free radicals. Free radical toxicity normally combine with non-proteinthiols of the GSH reactive center and finally be cleared[35]. Studies have shown that both acute and chronic alcohol exposure induced the decreases of the hepatic GSH content[36-37]. GSH-Px is one of essential enzyme in regulation of GSH and oxidized glutathione (GSSG). GST, a soluble protein located in the cytosol, also plays an important role in the detoxification of liver. Besides, the GST functionally associated with GSH[38]. For the reasons mentioned above,SOD, GST, GSH and GSH-Px become the indicators in the alcohol induced hepatotoxicity[39]. In the present study, we found that alcohol management significantly depleted GSH content and activities of SOD, GST and GSH-Px. However,PhGs treatment restored these four indexes.
Oxidative injury always causing cell death or organ damage. Alcohol induced oxidative injury can be monitored by detecting MDA[40]. In our study, 31 days of alcoholic treatment notably increased the contents of TG and MDA compared with the naive group. PhGs administration decreased the MDA and TG contents in liver compared with the negative group.
The results showed that PhGs regulated relative enzyme activities, reduced oxidant substance like MDA. Chemical composition analysis exhibited that PhGs content is about 79.6%. Considering quantity of phenolic hydroxyl groups existing in PhGs, which have antioxidant activity. Accorded with its reducing MDA activity, which may be related with its phenolic hydroxyl structure. But how and why these PhGs regulate the relative enzyme activities and reduce relative oxidants substances, whether synergistic effects between the components exist or not need to be further studied.
The present study findings concluded that the PhGs possesses hepatoprotective property against AICH, which provide new insights in utilizing field of C. deserticola in the prevention of alcoholic liver disease. This is meaningful not only for majority of alcohol consumers, but also for promotion of C. deserticola industry.
