Modulation of microglial functions by methyl jasmonate
2018-07-21JordanMcKenzieAndisKlegeris
Jordan A. McKenzie, Andis Klegeris
Biology Department, University of British Columbia Okanagan Campus, Kelowna, BC, Canada
Abstract Neuroin fl ammation contributes to the neurodegenerative processes in Alzheimer’s disease (AD); therefore,characterization of novel drug candidates aimed at combatting in fl ammation in the central nervous system is one of the potential avenues for the development of effective AD treatment and prevention strategies.Non-neuronal microglial cells orchestrate neuroin fl ammatory reactions, and their adverse activation has been linked to AD pathogenesis. Methyl jasmonate (MJ) has anti-cancer properties and has also been shown to reduce peripheral in fl ammation in pre-clinical models. Recently, anti-neuroin fl ammatory activity of MJ was demonstrated in mice, but the exact cellular and molecular mechanisms responsible for this bene fi cial effect are unknown. We hypothesized that MJ can regulate select microglial functions, and used two different in vitro models of microglia to test this hypothesis. MJ inhibited the production of damaging reactive oxygen species by differentiated human HL-60 promyelocytic leukemia cells without reducing their viability. MJ also selectively upregulated phagocytic activity of murine BV-2 microglia, but had no effect on nitric oxide secretion by these cells. Since microglial phagocytosis can be bene fi cial for clearance of amyloid β aggregates in AD, the observed upregulation of phagocytic activity by MJ, combined with its inhibitory effect on reactive oxygen species production, supports continued studies of MJ as a candidate drug for managing adverse neuroin fl ammation in AD.
Key Words: Alzheimer’s disease; anti-inflammatory; glia; neuroinflammation; neurodegeneration;neuroprotection; nitric oxide; phagocytosis; reactive nitrogen species; reactive oxygen species
Introduction
Methyl jasmonate (MJ), a cyclopentanoic compound,was fi rst isolated from the jasmine plant and is the most active anti-cancer derivative among natural jasmonates(Umukoro et al., 2017). Recent studies have shown that in lipopolysaccharide (LPS)-treated mice MJ reduces brain levels of pro-inflammatory mediators, in addition to lowering amyloid β (Aβ) levels, and that MJ treatment enhances cognitive performance in mice (Eduviere et al., 2015; Umukoro et al., 2017), even thoughin vitroMJ does not inhibit LPS-induced microglial expression of pro-inflammatory cytokines (Taki-Nakano et al., 2016).It has been demonstrated that MJ possesses free radical-scavenging properties, which could be the basis of its neuroprotective activity (Eduviere et al., 2015). These studies provide indirect evidence that MJ could have bene fi cial effects on neuroin fl ammation, which is a contributing factor to a broad range of neurodegenerative disorders including Alzheimer’s disease (AD). MJ has been shown to selectively kill cancer cells without being toxic to normal cells (Eduviere et al., 2015). MJ was not toxic when administered to mice at 10–40 mg/kg doses(Umukoro et al., 2017).
Neuroinflammatory responses in the brain results from adverse activation of microglial cells, which release pro-inflammatory mediators such as cytokines, chemokines, as well as reactive oxygen and nitrogen species(ROS and RNS) (Lee et al., 2010). Microglia represents the mononuclear phagocyte system in the brain and they express proteins that are characteristic of professional phagocytes such as major histocompatibility complex(MHC) glycoproteins and scavenger receptors. Microglial phagocytosis and release of RNS and ROS are directed at removing pathogens and pathological structures such as AD Aβ aggregates (Lee et al., 2010). It has been suggested that microglial phagocytosis exerts a beneficial effect in repair and regeneration; however, there is also evidence that phagocytic activity facilitates transition of microglia to a neurocytopathic state. In this study, we assessed the potential of MJ to regulate microglial phagocytosis and their release of ROS and RNS.
Materials and Methods
Cell culture
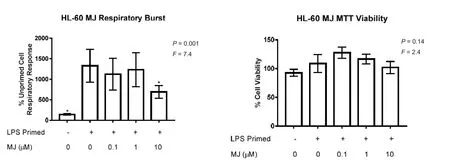
Figure 1 Methyl jasmonate (MJ) inhibits priming of the respiratory burst of HL-60 cells at non-toxic concentrations.
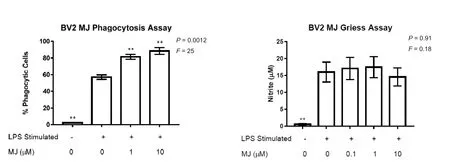
Figure 2 Methyl jasmonate (MJ) upregulates phagocytic activity of LPS-stimulated BV-2 microglia without affecting their nitric oxide (NO)secretion.
The human HL-60 promyelocytic and the murine BV-2 microglia cells were used as microglia models. The HL-60 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The BV-2 cells were donated by Dr. G. Garden (University of Washington, Seattle, WA, USA). Cells were grown in Dulbecco’s modi fi ed Eagle medium (DMEM)/F12 supplemented with 10% calf bovine serum (CBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (250 ng/mL)(F10 media).
Respiratory burst assay
The ability of MJ to reduce ROS by inhibiting microglial respiratory burst was assessed as described by Muranaka et al. (2005). Dimethyl sulfoxide (DMSO)-differentiated and LPS-primed HL-60 cells were utilized since they express higher levels of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase components compared to BV-2 cells allowing detection of ROS in response to N-formylmethionyl-leucyl-phenylalanine (fMLP) stimulation. HL-60 cells were fi rst differentiated in the presence of 1.3% DMSO for 5 days. HL-60 cells were then washed and plated at 1 × 106cells/mL in 250 μL clear DMEM medium without phenol red, supplemented with 2% CBS. Cells were treated with MJ (0.1, 1, or 10 μM), or its vehicle solution (0.2% DMSO, 0 μM) for 15 minutes.Subsequently, cells were primed with LPS (0.5 μg/mL) or left unprimed for 24 hours. Real-time luminol-dependent chemiluminescent response of cells to their stimulation with the bacterial peptide fMLP (20 μM), was measured for 30 minutes as described by Madeira et al. (2012).
Phagocytosis assay
Murine BV-2 cells were used to study the effect of MJ on LPS-induced phagocytic activity of microglia as described by He et al. (2002). 500 μL of 1 × 105BV-2 cells/mL in F5 media were added onto 18 mm glass cover slips placed in a 12-well plate and allowed to adhere for 24 hours, followed by their incubation with MJ (1 μM or 10 μM), or its vehicle solution (0.2% DMSO), for 15 minutes before stimulation with LPS (0.5 μg/mL) to induce phagocytic activity. After 24 hours of incubation, 0.8 μm diameter polystyrene beads (Sigma Aldrich, Oakville, ON, Canada)were added to each well at a concentration of 1 μL/mL for 2.5 hours. Cells were washed with phosphate-buffered saline (PBS) to remove beads and then fixed with 4%paraformaldehyde solution in PBS for 20 minutes. Zeiss AxioObserver.Z1 wide- fi eld microscope (Carl Zeiss Canada, Toronto, ON, Canada) was used to visualize cells and count beads phagocytosed by the BV-2 cells. Confocal microscopy (Carl Zeiss Canada) was used to con fi rm that the beads were in fact internalized by BV-2 cells (data not shown).
Nitric oxide (NO) and cell viability measurements
The Griess assay was performed as described previously to assess the effect of MJ on the secretion of NO by LPS-stimulated BV-2 cells (Spielman et al., 2017). The viability of cells was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)assay as described by Madeira et al. (2012). For all cell types used, the non-toxic MJ concentration range and incubation times were selected in preliminary experiments.
Statistical analysis
GraphPad Prism software (version 7.0, GraphPad Software Inc., La Jolla, CA, USA) was used for the statistical analysis. Data are presented as means ± standard error of the mean (SEM); randomized block one-way analysis of variance (ANOVA), followed by the Fisher’s least signi ficant differencepost-hoctest was used to assess the statistical signi fi cance of the effects observed. Signi fi cance was established atP< 0.05.
Results
Effect of MJ on reactive oxygen species production by primed HL-60 cells
Since increased release of neurotoxic molecules, including NADPH oxidase-generated ROS, has been shown to cause neuronal damage, we studied the effects of MJ on the phagocyte respiratory burst response. HL-60 cells responded to fMLP stimulation by ROS production, which was 13-fold higher in LPS-primed compared to unprimed cells (Figure 1A). Treatment of primed HL-60 cells with 10 μM MJ led to a statistically signi fi cant 48% reduction of their respiratory burst. Since MJ was added to cells 15 minutes before their priming with LPS and stimulation with fMLP, the inhibitory effect of MJ could have been due to inhibiting the speci fi c priming events of the differentiated HL-60 cells, or through direct interaction with the NADPH oxidase complex involved in the production of ROS. Therefore, an additional experiment was performed, where MJ was added to already LPS-primed cells 15 minutes before their stimulation with fMLP. Under these experimental conditions, MJ (0.1–10 μM) did not signi fi cantly reduce the respiratory burst of fMLP-stimulated cells (data not shown) indicating that MJ interfered with the respiratory burst priming mechanisms in HL-60 cells.
To rule out MJ cytotoxicity at the concentrations studied, the MTT cell viability assay was performed, which showed no signi fi cant decrease of MJ-treated HL-60 cell viability, when compared to cells exposed to the MJ vehicle (0.2% DMSO, Figure 1B); therefore, the inhibitory effect of MJ on the respiratory burst activity of HL-60 cells was not due to its cytotoxicity at the concentrations studied.
Effect of MJ on the phagocytic activity of microglia
Figure 2A shows that LPS on its own induced a 24-fold increase in percent of phagocytic BV-2 cells. Adding MJ at 1 μM or 10 μM further increases numbers of phagocytic cells by 43% and 55% respectively compared to cells treated with LPS alone (MJ, 0 μM). Preliminary experiments showed that MJ (0.1–10 μM) did not induce phagocytosis in the absence of LPS stimulation (data not shown).
Figure 2B demonstrates that, unlike its activity on the respiratory burst response and phagocytosis, MJ at 0.1-10 μM did not affect the release of NO by LPS-stimulated BV-2 cells. Even though NO secretion was not inhibited by MJ indicating absence of toxicity, we confirmed the lack of toxic effects of MJ at the concentration studied on LPS-stimulated BV-2 cells by the MTT assay (data not shown).
Discussion
This study shows that MJ selectively modulates in fl ammatory responses of microglia. Two different cell lines were used to model microglia, and LPS was used as the in fl am-matory stimulus. We demonstrated that at low micromolar concentrations MJ inhibited phagocyte respiratory burst priming by 48%, while upregulating LPS-stimulated phagocytic activity by up to 55%. At the concentrations studied, MJ did not affect NO production by stimulated BV-2 cells and was not toxic to the cells studied. Inhibiting excess production of harmful ROS by microglia could be beneficial in the treatment of various neurodegenerative diseases, such as AD, which are accompanied by neuroin fl ammation. It has been previously suggested that the beneficial regulation of adverse microglia activation could involve inhibiting pro-inflammatory secretions while preserving their phagocytic activity (Lee et al.,2010). Our data showed upregulated phagocytic activity of immune-stimulated BV-2 microglia in the presence of MJ, which represents a novel observation. This result is especially exciting since upregulated phagocytosis could facilitate Aβ clearance by microglia in AD brains. The molecular mechanism mediating the observed bene fi cial effects of MJ will require further studies. MJ is structurally similar to prostaglandins and has been reported to inhibit aldo-keto reductase (AKR) 1C (Davies et al., 2009),which is involved in regulation of cellular oxidative and in fl ammatory responses. The molecular pathway engaged by MJ to increase the phagocytic activity of BV-2 cells is unknown, but could involve upregulation of vitronectin surface receptors similar to previous observations showing induction of phagocytosis by LPS (Brown et al.,2015).
Overall, our data demonstrate that MJ, at low micromolar concentrations, can selectively regulate microglial inflammatory responses by reducing excessive production of ROS and enhancing their phagocytic activity.Such modulation of microglial functions could lead to mitigation of adverse neuroinflammatory activation of these cells as well as increased clearance of pathological structures, such as Aβ aggregates in AD. We conclude that further studies assessing effectiveness of MJ in animal models of AD are justi fi ed.
Author contributions:JAM and AK conceived the study and wrote the manuscript. JAM conducted experiments and analyzed the data.Both authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Financial support:Supported by Grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Jack Brown and Family Alzheimer’s Disease Research Foundation.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Olga Chechneva, University of California Davis, USA.
Additional fi le:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Stem cells: a promising candidate to treat neurological disorders
- Injury of the superior longitudinal fasciculus by ventriculoperitoneal shunt: a diffusion tensor tractography study
- Degree of dopaminergic degeneration measured by 99mTc-TRODAT-1 SPECT/CT imaging
- Differences in brain pathological changes between rotenone and 6-hydroxydopamine Parkinson’s disease models
- Zishenpingchan granules for the treatment of Parkinson’s disease: a randomized, double-blind,placebo-controlled clinical trial
- Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury