Molecular Structure and Electronic Spectra of CoS under the Radiation Fields
2018-07-12QijunWuLiminHanLingxuanWangandXunGong
QijunWu,LiminHan,LingxuanWangandXunGong
Abstract:We optimized the ground-state stable configuration of CoS molecule in different external radiation fields (0-0.04 atomic units (a.u.)) at the basis set level of 6-311G++ (d, p) using the B3LYP density functional theory. On this basis, the molecular structure, total energy, energy gap, and the intensities of infrared ray (IR) spectra, Raman spectra, and ultraviolet-visible (UV-Vis) absorption spectra of CoS molecule were computed using the same method. The results showed that the molecular structure changed greatly under the effect of the external radiation fields and had significant dependency on the radiation fields. The total energy of CoS molecule grew slightly at first and then significantly decreased in a monotonous manner. The bond length, dipole moment, and energy gap of the molecule all reduced at first and then increased, with the turning point all at F=0.025 a.u. of the radiation field. The absorption peak of IR spectra and Raman optical activity both had maximums at F=0.03 a.u. with significant red shift.In the external radiation field of F=0.030 a.u., the absorption wavelength of the UV-Vis absorption spectra showed large blue shift, and a strong absorption peak was observed.
Keywords:CoS, density functional theory, molecular structure, radiation field, spectra.
1 Introduction
The research on the effect of external radiation fields of molecules provides theoretical support for many fields, so it has potential application and guidance significance. For this reason, the research on the external radiation fields of molecules is paid great attention to by scholars across the world [Wang, Gu, Ma et al. (2016); Liu, Li, Wang et al. (2017);Yin, Liu, Lin et al. (2018)]. Under the effect of external radiation fields, molecules have many high-energy excited states which undergo a series of physical and chemical changes and thus cause occurrence of new phenomena. These phenomena include the changes in the bond length as well as the structure and properties of energy bands of molecules, the breakage of original chemical bonds, and the formation of new chemical bonds [Iwamae, Hishikawa and Yamanouchi (2000); Yin, Liu, Zhang et al. (2017)]. At present, numerous studies have been carried out on the properties of materials under theeffect of external radiation fields, for example, investigations on SiC, SiO, and MgO molecules [Yin, Liu, Zhang et al. (2018); Huang, Wang, Min et al. (2018); Xu, Lv and Liu (2009)].
Co is a kind of transition metal element. Cobaltous sulfide (CoS) is a typical representative sulfide of transition metals. CoS is a non-toxic and environmental semiconductor material,and its electrons are configured in a special structure on the 3d orbit. The special structure endows the material with favorable physical and chemical performance, such as magnetic,electrical, and catalytic performance [Sadjadi, Pourahmad and Sohrabnezhad (2007);Wang, Ng and Wang (2006); Wang, Anghel and marsan (2009)], so it attracts people’s great attention and has been used in various fields in material research. Considering the advantages of CoS in the field of semiconductor materials, it has also become one of the research focuses in recent years [Strbac, Mihajlovic and Zivkovic (2006); Justin and Rao(2010); Yu, Du and Guo (2002)]. However, to our knowledge, no research has been reported on the physical properties and spectra of CoS in the radiation fields, so studying its properties and application is of great significance.
The research investigated the changes of physical properties including bond length, bond angle, dipole moment, and energy and the change characteristics and oscillator strengths of IR spectra, Raman spectra, and UV-Vis spectra of CoS molecule using the density functional theory at the basis set level of B3LYP/6-311G++ (d, p). The research provided a significant theoretical basis for the research on the characteristics of the molecule under the effect of external radiation fields.
2 Theory and calculation methods
The HamiltonianHof a molecular system under the effect of external radiation fields is H=H0+Hint, where H0and Hintrepresent the Hamiltonian under conditions without external fields and under the interaction of external radiation fields and the molecular system. Under the dipole approximation, the interaction energy between the molecular system and the external radiation field F is Hint=-μ×F, where μ represents the electric dipole moment of the molecule. The external radiation field used in the research was in the range of (0-0.04 atomic units (a.u.)), where 1a.u.=5.14225×1011V/m.
All theoretical calculations in the research were conducted by using the Gaussian 09 quantum chemical calculation software and the basis set B3LYP/6-311G++ (d, p) [Frisch,Trucks, Schlegel et al. (2009)]. The calculated CoS molecular was optimized in the same calculation method. The change characteristics of the geometrical configuration, energy gap, IR spectra, and Raman spectra under the effect of different external radiation fields(0 a.u.-0.04 a.u.) were obtained. The absorption wavelengths and resonance lengths corresponding to 30 lowest excited electronic states were calculated using the TDB3LYP method. In this way, the change characteristics of ultraviolet-visible (UV-Vis)spectra under external radiation fields were obtained.
3 Results and discussion
3.1 Stable configurations of the molecule without the radiation fields
The change characteristics of physical properties and spectra of CoS under the effect ofexternal radiation fields were calculated using the density functional theory at the basis set level of B3LYP/6-311G++ (d, p). The calculated and optimized stable configurations are shown in Fig. 1, where x and y axes are Cartesian coordinate axes and z axis extends along the direction of the connecting line of Co-S bond.
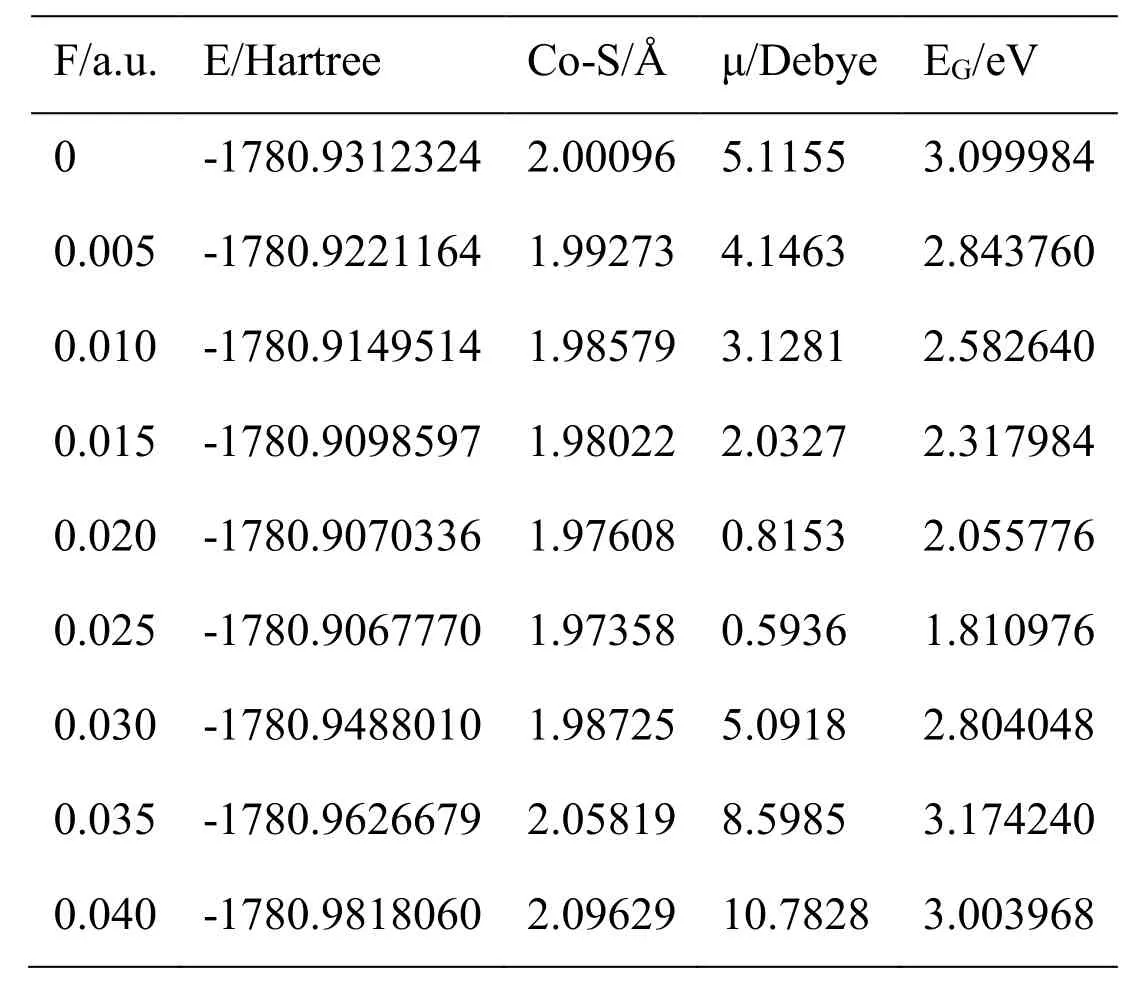
Table 1: The calculated physical characteristic parameters of the optimized structure of CoS at different external electric fields
3.2 Influences of external radiation fields on the physical properties of CoS
When different radiation fields (0 a.u.-0.04 a.u.) were applied along thezaxis (the connecting line of Co-S bond), the structure of CoS molecule was optimized at the basisset level of B3LYP/6-311G++ (d, p). In this way, the stable molecular structures of CoS under different radiation fields were obtained. The calculation results provided various physical parameters change including the Co-S bond length, single-point energy E,transition dipole moment μ, lowest unoccupied molecular orbital (LUMO) energy EL,highest occupied molecular orbital (HOMO) energy EH, and energy gap EGin different radiation fields (0a.u.-0.04 a.u.). Among them, EGis calculated using Formula EG=(ELEH)×27.2 eV.
Figure 1: The optimized geometry of CoS on the level of B3LYP/ 6-311G++ (d, p)
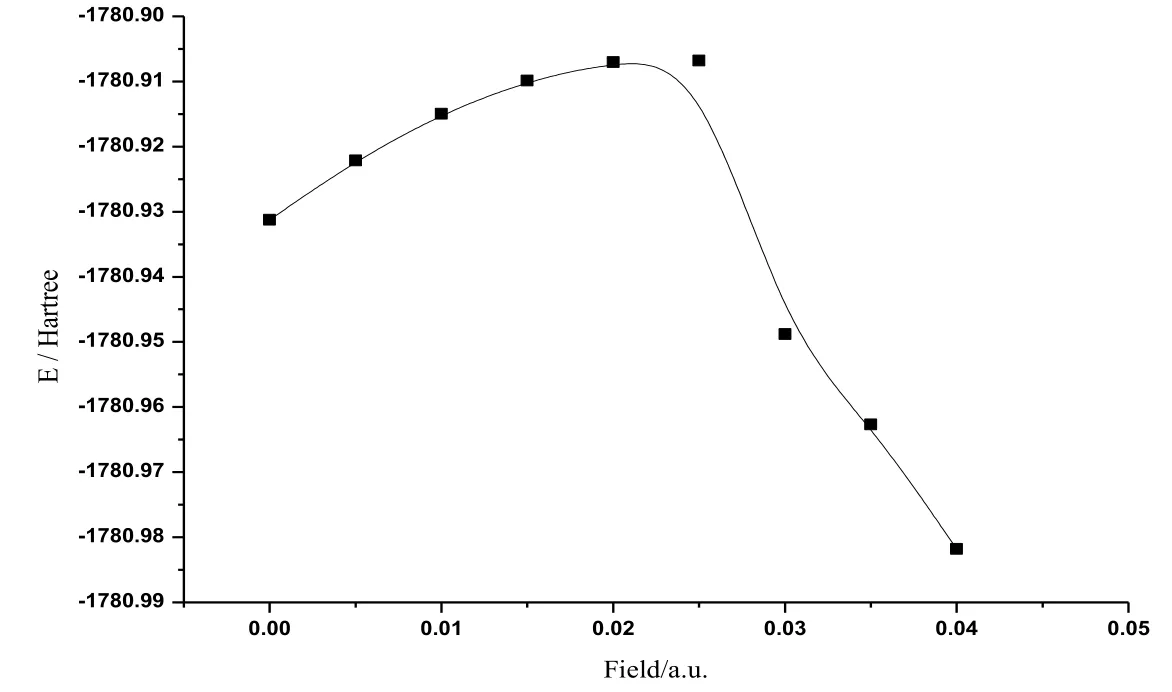
Figure 2: The variations of total energy of CoS in radiation field
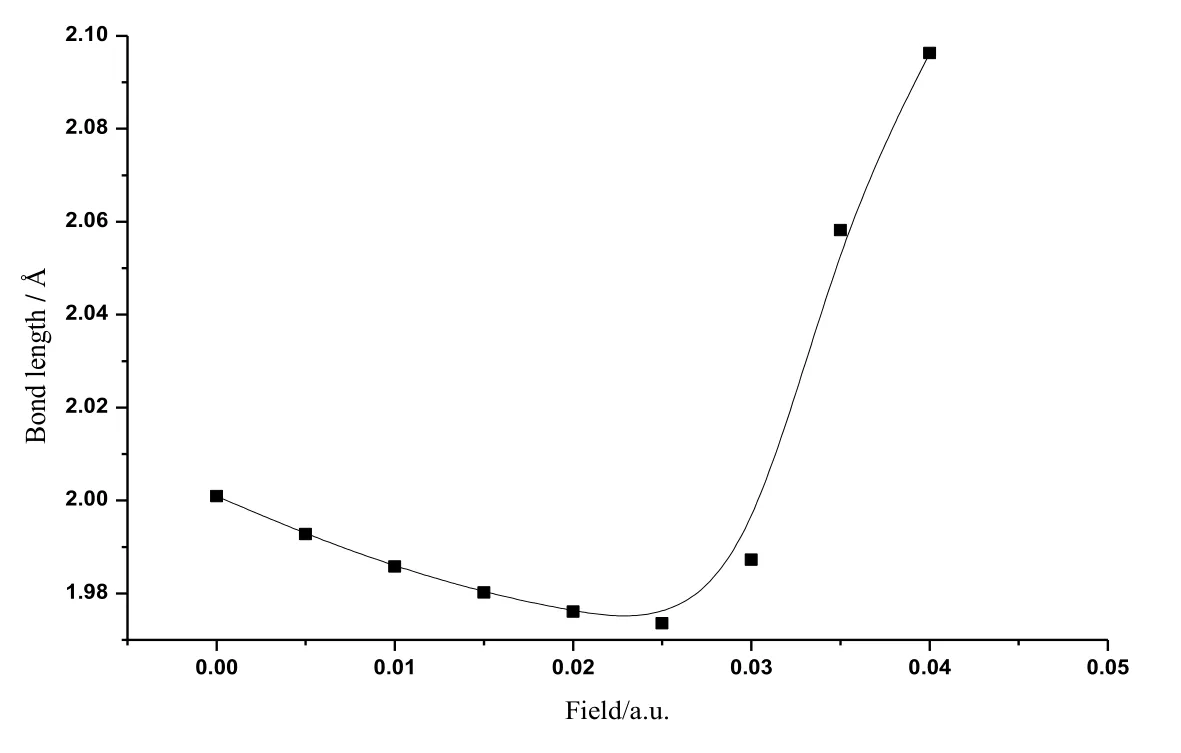
Figure 3: The variations of bond distances of CoS in radiation field
The calculated data about CoS molecule are listed in Tab. 1. Fig. 2 shows the change curve of the total energy of CoS molecule in different radiation fields. As shown in the figure, the total energy increased at first with the enlarging radiation field (0a.u.-0.04 a.u.)and reached the peak value at about 0.025 a.u.. Then the total energy reduced substantially in a monotonous manner with the increasing radiation field. Fig. 3 illustratesthe change curve of the bond length of CoS molecule in different radiation fields. It can be seen from the figure that as the radiation field increased (0a.u.-0.04 a.u.), the bond length reduced at first and had a minimum at about 0.025 a.u..Afterwards, with the further increase of the radiation field, the bond length greatly increased in a monotonous manner. The change curves of dipole moment of CoS molecule in different radiation fields are demonstrated in Fig. 4. The figure shows that with the increase of the radiation field (0a.u.-0.04 a.u.), the dipole moment decreased at first and then reached the minimum at about 0.025 a.u. This was followed that the monotonous increase of the dipole moment with the further enlargement of the radiation field. Fig. 5 shows the change curve of the energy gap of CoS molecule in different radiation fields. As displayed in the figure, the energy gap decreased to the minimum at about 0.025 a.u. with the increasing radiation field (0a.u.-0.04 a.u.). As the radiation field further grew, the energy gap increased and stabilized at about 0.035 a.u..Data in Tab. 1 and curves in Figs.2, 3, 4, and 5 all indicate that physical parameters including single-point energy E, bond length Å, transition dipole moment μ, and energy gap EGall had a turning point at 0.025 a.u.. The reason is that radiation field (0 a.u.-0.025 a.u.), the internal stress of the molecule is greater than the external field force, and the internal stress of the molecule is less than the external field force in radiation field (0.025 a.u.-0.04 a.u.). When radiation field 0.025 a.u., the internal stress of the molecule equals the external field force, at this time the internuclear distance reaches the minimum value, the molecular polarity is at the minimum, the molecular total energy reaches the maximum and the molecular gap reaches the minimum value, indicating that the molecule is most likely to have chemical reaction.
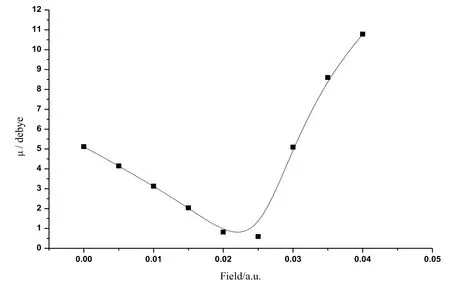
Figure 4: The variations of dipole moment of CoS in radiation field
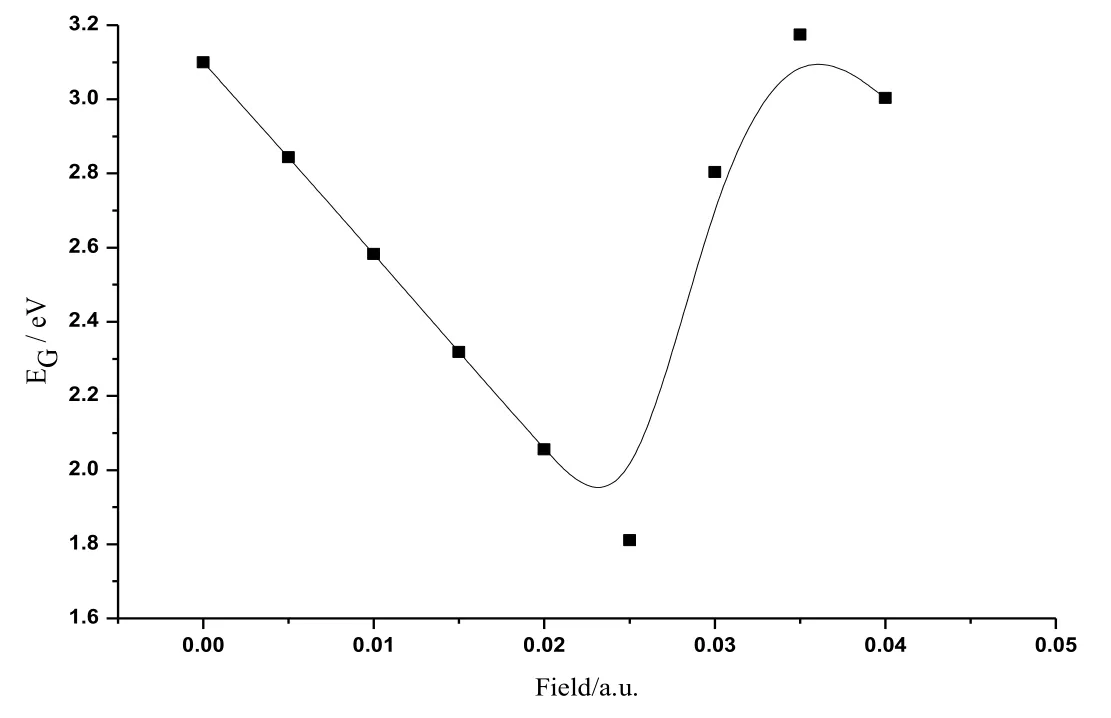
Figure 5: The variations of energy gap of CoS in radiation field
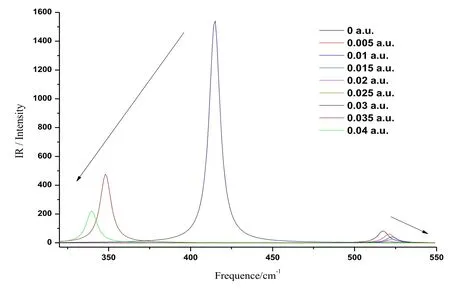
Figure 6: The variations of IR spectra of CoS in radiation field
3.3 Influences of radiation fields on the IR spectrum
Likewise, different radiation fields (0a.u.-0.04 a.u.) were applied in the z axis to compute the IR spectrum of CoS molecule at the basis set level of B3LYP/6-311G++ (d, p). By doing so, the IR spectrum of CoS molecule under the effects of different radiation fields(0 a.u.-0.04 a.u.) was obtained, as shown in Fig. 6. It can be seen that the radiation field significantly influenced the vibration frequency and infrared intensity of CoS molecule.When the radiation field in the z axis increased from 0 a.u. to 0.025 a.u., the vibration frequency slightly increased in the range of 510-530 cm-1, the vibration intensity mildly reduced, and slight blue shift occurred to the IR spectra, as indicated by the little arrow in the right side of Fig. 6. As the external radiation field increased to 0.03 a.u., a strongvibration peak appeared abruptly at the low frequency (410 cm-1), the intensity of which was about 200 times of that when no radiation field was applied (0 a.u.). It indicates that the ability of electron transition decreases and the peak intensity increases under external radiation field. Afterwards, both the vibration frequency and the vibration intensity reduced greatly and the IR spectrum showed significant red shift in the radiation field of 0.03 a.u.-0.04 a.u.. The change trend was indicated by the large arrow in the left side of Fig. 6.
3.4 Influences of the radiation field on the Raman spectrum
Similarly, different radiation fields (0 a.u.-0.04 a.u.) were applied in the z axis to compute the Raman spectrum of CoS molecule at the basis set level of B3LYP/6-311G++ (d, p).In this way, the Raman spectrum under the effect of different radiation fields (0 a.u.-0.04 a.u.) was obtained, as illustrated in Fig. 7. The changes in the vibration frequency of the Raman spectrum of CoS molecule in different radiation fields were basically similar to those of IR spectrum. Under the effect of different radiation fields (0 a.u.-0.01 a.u.), the vibration frequency increased while the vibration intensity basically remained unchanged.At 0.015 a.u., the vibration frequency was the same with that at 0.01 a.u. while the vibration intensity increased slightly. When the radiation field was in the range of 0.015 a.u.-0.04 a.u., the vibration frequency shifted greatly to the low frequency and the vibration intensity grew substantially. At 0.03 a.u., the amplitude of the vibration intensity reached to the peak value. Afterwards, the vibration intensity reduced with the increasing radiation field and red shift was observed in the Raman spectrum. The arrow in the left side of the figure indicates the change trend of the spectrum in the range of 0.03 a.u.-0.04 a.u.. The right part of Fig. 7 shows the locally enlarged change curve of Raman spectrum in the range of 0 a.u.-0.025 a.u.. The arrow with a fold line indicates the change trend of the spectrum.
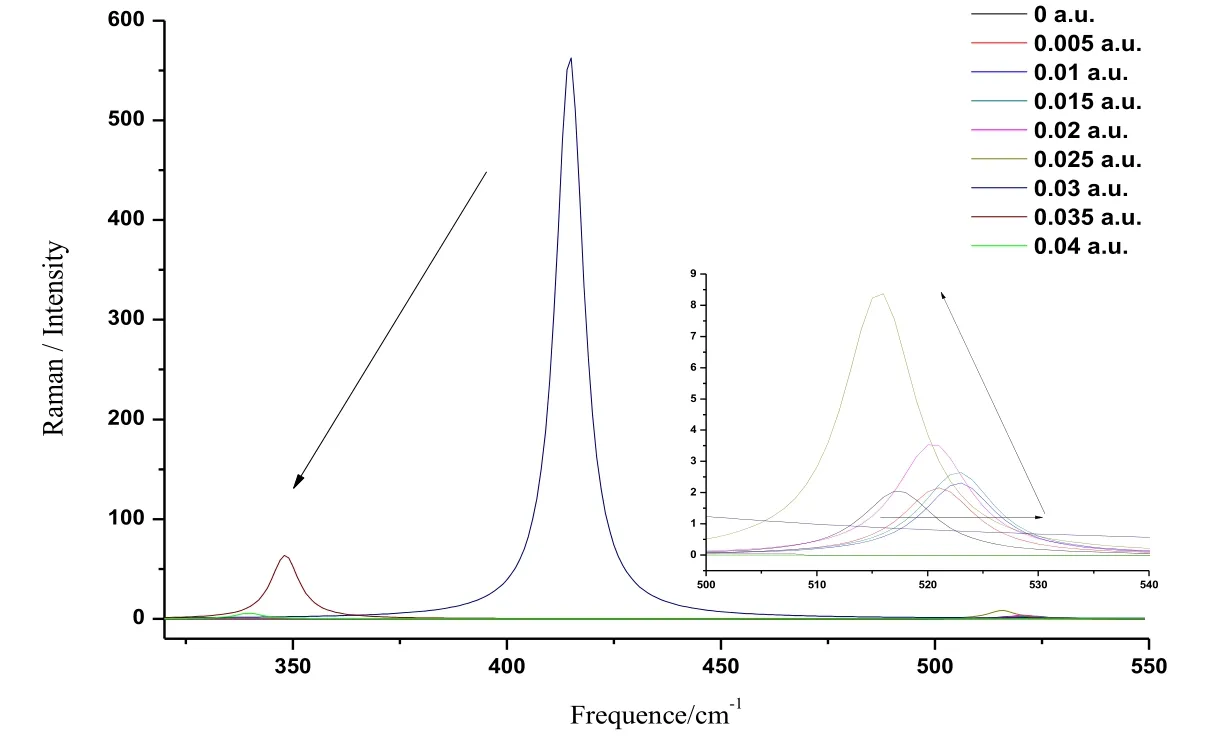
Figure 7: The variations of Raman spectra of CoS in external field
Fig. 8 shows the change curve of the Raman optical activity of CoS molecule. It can be seen from the figure that when the radiation field was in the range of 0 a.u.-0.02 a.u., the Raman optical activity increased slightly. When the radiation field was in the range of0.02 a.u.-0.04 a.u., the Raman optical activity grew substantially at first, then reached to the maximum at 0.03 a.u., and finally decreased remarkable in a monotonous manner before reaching low activity.
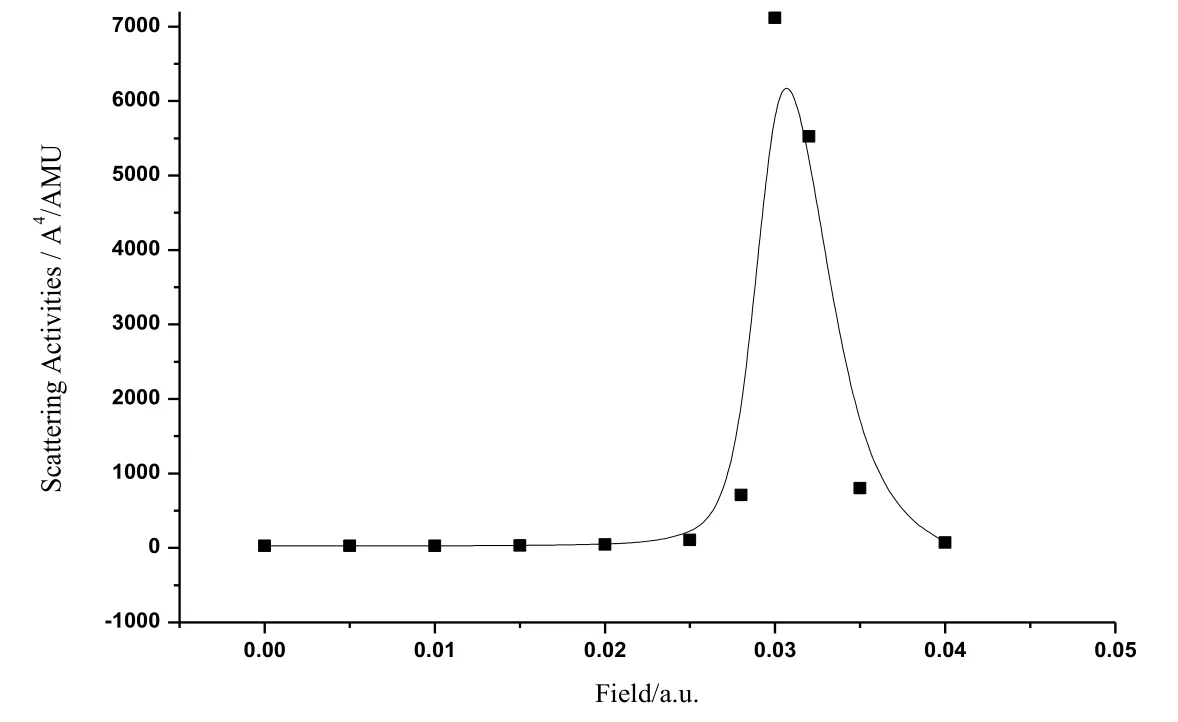
Figure 8: The activity variations of Raman spectra of CoS in radiation field
3.5 Influences of radiation fields on the UV-Vis absorption spectrum
The UV-Vis absorption spectrum of CoS molecule was computed by applying different radiation fields (0 a.u.-0.04 a.u.) in the z axis at the basis set level of B3LYP/6-311G++(d, p). In this way, we obtained the UV-Vis absorption spectrum of CoS molecule under effects of different radiation fields (0 a.u.-0.04 a.u.), as shown in Fig. 9.
While studying the influences of the radiation field on the UV-Vis absorption spectrum,the maximum absorption wavelength λmaxcorresponding to the absorption peak is an important physical parameter and plays a significant role. Existing research indicates that λmaxis indispensable in the qualitative spectrometric analysis of a material. Fig. 9 clearly shows the concrete influences of the different radiation fields (0 a.u.-0.04 a.u.) on the absorption wavelength λmaxof UV-Vis absorption spectrum of CoS. It can be seen that the radiation field exerted great influences on the UV-Vis absorption spectrum of CoS molecule. When the radiation field in the direction of the z axis increased from 0 a.u.-0.025 a.u., the absorption wavelength grew constantly while the peak intensity changed slightly, as indicated by the arrow in the lower part of Fig. 9. On conditions that the radiation field was in the range of 0.03 a.u.-0.04 a.u., the absorption wavelength showed substantial blue shift abruptly and the peak intensity was 7-10 times of that in the radiation field of 0 a.u.-0.025 a.u.. The research on the maximum absorption wavelength λmaxof UV-Vis absorption spectra of CoS molecule under the effect of different radiation fields (0 a.u.-0.04 a.u.) provides a theoretical basis for the further research.
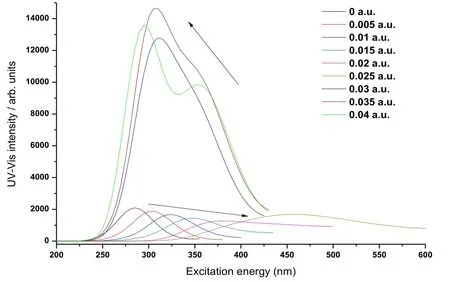
Figure 9: The λmaxvariations of UV-Vis spectra of CoS in radiation field
4 Conclusions
The research calculated the change characteristics of the geometrical configuration, bond length, energy gap, IR spectra, Raman spectra, and UV-Vis absorption spectra of CoS molecule under the effect of different radiation fields (0 a.u.-0.04 a.u.) using the firstprinciples method. The results showed that in a certain range, the total energy of the molecule slightly increased at first (reached the maximum of -1780.90678 Hartree at F=0.025 a.u.) and then significantly reduced in a monotonous manner. The bond length and dipole moment of the molecule both decreased at first (at F=0.025 a.u. of the radiation field, both the bond length and the dipole moment had the minimums of 1.97358 Å and 0.5936 Debye, respectively), followed by an increase. As to the energy gap, it constantly decreased at first, then increased, and finally stabilized after F=0.035 a.u., with the minimum appeared when F=0.025a.u.. In addition, the external radiation field also greatly influenced the excited energy, oscillator strength, and the vibration frequencies and vibration intensities of IR spectra, Raman spectra, and UV-Vis absorption spectra of CoS molecule. Under the effect of the external radiation field (0 a.u.-0.04 a.u.),the vibration frequencies and vibration intensities of IR and Raman spectra both changed slightly at first. When F=0.03 a.u., the vibration frequency shifted remarkably to the low frequency by about 200 cm-1, and the intensity of the vibration peak and the Raman optical activity grew to 100 times of those without applying the external radiation field,followed by the monotonous decrease. The absorption wavelength of the UV-Vis absorption spectrum grew constantly at first while the peak intensity changed mildly. When the radiation field was F=0.03a.u., the absorption wavelength presented substantial blue shift and a strong absorption peak. Afterwards, the absorption wavelength and peak intensity basically remained unchanged.
Acknowledgement:The authors are pleased to acknowledge the financial support of this research by the National Natural Science Foundation of China (Grant No. 21667010) and support from this startup project for high-level talents of Guizhou Institute of Technology(XJGC20150404).
杂志排行
Computers Materials&Continua的其它文章
- Biodegradation of Medicinal Plants Waste in an Anaerobic Digestion Reactor for Biogas Production
- Feature Selection Method Based on Class Discriminative Degree for Intelligent Medical Diagnosis
- A Spark Scheduling Strategy for Heterogeneous Cluster
- Rare Bird Sparse Recognition via Part-Based Gist Feature Fusion and Regularized Intraclass Dictionary Learning
- Event-Based Anomaly Detection for Non-Public Industrial Communication Protocols in SDN-Based Control Systems
- Inverted XML Access Control Model Based on Ontology Semantic Dependency
