Characterization of dominant giant rod-shaped magnetotactic bacteria from a low tide zone of the China Sea*
2018-07-11TENGZhaojie滕兆洁ZHANGWenyan张文燕CHENYiran陈一然PANHongmiao潘红苗XIAOTian肖天WULongFei吴龙飞
TENG Zhaojie (滕兆洁) 2 ZHANG Wenyan (张文燕) CHEN Yiran (陈一然) PAN Hongmiao (潘红苗) XIAO Tian (肖天) WU Long-Fei (吴龙飞)
1CAS Key Laboratory of Marine Ecology&Environmental Sciences,Institute of Oceanology,Chinese Academy of Sciences,Qingdao 266071,China
2University of Chinese Academy of Sciences,Beijing 100049,China
3Laboratory of Marine Ecology and Environmental Science,Qingdao National Laboratory for Marine Science and Technology,Qingdao 266000,China
4Laboratoire de Chimie Bactérienne,UMR7283,Institut de Microbiologie de la Méditerranée,Aix-Marseille Université,CNRS,Marseille,France
5Laboratoire International Associé de la Bio-Minéralisation et Nano-Structures,CNRS,Marseille,France
AbstractMagnetotactic bacteria are a group of Gram-negative bacteria that synthesize magnetic crystals,enabling them to navigate in relation to magnetic fi eld lines. Morphologies of magnetotactic bacteria include spirillum, coccoid, rod, vibrio, and multicellular morphotypes. The coccid shape is generally the most abundant morphotype among magnetotactic bacteria. Here we describe a species of giant rod-shaped magnetotactic bacteria (designated QR-1) collected from sediment in the low tide zone of Huiquan Bay(Yellow Sea, China). This morphotype accounted for 90% of the magnetotactic bacteria collected, and the only taxonomic group which was detected in the sampling site. Microscopy analysis revealed that QR-1 cells averaged (6.71±1.03)×(1.54±0.20) μ m in size, and contained in each cell 42-146 magnetosomes that are arranged in a bundle formed one to four chains along the long axis of the cell. The QR-1 cells displayed axial magnetotaxis with an average velocity of 70±28 μ m/s. Transmission electron microscopy based analysis showed that QR-1 cells had two tufts of flagella at each end. Phylogenetic analysis of the 16S rRNA genes revealed that QR-1 together with three other rod-shaped uncultivated magnetotactic bacteria are clustered into a deep branch ofAlphaproteobacteria.
Keyword: Alphaproteobacteria; flagella; motility; rod-shaped magnetotactic bacteria
1 INTRODUCTION
Magnetotactic bacteria (MTB) are a heterogeneous group of fastidious microorganisms that are capable of biosynthesizing nano-sized, membrane-bound,magnetic iron-rich mineral particles termed magnetosomes (Lefèvre and Wu, 2013). The magnetosomes enable MTB to orient and navigate along geomagnetic fi eld lines in a unique form of movement referred to as magnetotaxis. Magnetotaxis is thought to enable MTB to more rapidly locate optimal conditions in vertical chemical and/or redox gradients (Frankel et al., 1997; Schüler, 1999;Bazylinski and Frankel, 2004; Faivre and Schüler,2008). MTB are ubiquitous in sediments of freshwater,brackish, marine, and hypersaline environments, and in the overlying chemically stratified water columns.Morphological and phylogenetic characterization of MTB has shown that this group is highly diverse(Lefèvre and Bazylinski, 2013).
MTB morphologies include spirillum, coccoid,rod, vibrio, and multicellular morphotypes (Bazylinski and Frankel, 2004). The magnetotactic cocci are the most abundant morphotype of MTB and globally distributed in freshwater and marine sediments (Mann et al., 1990; Spring et al., 1992, 1995, 1998; Flies et al., 2005; Pan et al., 2008; Xing et al., 2008; Lin and Pan, 2009; Lin et al., 2009; Zhang et al., 2010, 2013;Bazylinski et al., 2013; Le Sage et al., 2013). Most MTB belong to theProteobacteriaphylum, including theAlphaproteobacteria,DeltaproteobacteriaandGammaproteobacteriaclasses; others belong to theNitrospira,Omnitrophica, andCandidatusLatescibacteria phyla (Lefèvre et al., 2010, 2012;Kolinko et al., 2012; Williams et al., 2012; Lin and Pan, 2015). MTB belonging to theAlphaproteobacteriaclass represent the dominant proportion of uncultured MTB in many freshwater and marine environments(Spring et al., 1992, 1995, 1998; DeLong et al., 1993;Flies et al., 2005). Recently, it has been reported that the genomes ofCandidatusMagnetococcus massalia MO-1 andMagnetococcusmarinusMC-1 have coding sequences (CDSs) with a similarly high proportion of origins fromAlphaproteobacteria,Betaproteobacteria,DeltaproteobacteriaandGammaproteobacteria, and represent a novel group ofEtaproteobacteria(Ji et al., 2017).
Rod-shaped MTB have been affliated with theAlphaproteobacteria,DeltaproteobacteriaandGammaproteobacteriaclasses and the Nitrospira phylum. The cultured rod-shaped strains SS-2 and BW-1, which belong to theDeltaproteobacteria(Lefèvre et al., 2011), share similar morphological characteristics with strains BW-2 and SS-5, which belong to theGammaproteobacteria(Lefèvre et al.,2012). The rod-shaped MTBMagnetobacterium bavaricum(Spring et al., 1993), strain MHB-1 (Flies et al., 2005), and a rod-shaped MTB strain belonging to the ‘C.Magnetobacterium’ genus (Lin et al., 2014)(affliated with the Nitrospira phylum) contain multiple bullet-shaped magnetosome chains in their cells. Three other uncultured rod-shaped strains,clone 1-2-1 (named after its OTU), Cux-03, and CS-05, were proposed to be affliated with theAlphaproteobacteria. The uncultured rod-shaped magnetotactic bacterium clone 1-2-1 was reported from the intertidal zone in Qingdao, China (Zhang et al., 2013), and two other uncultured rod-shaped strains Cux-03 and CS-05 have been reported from a marine intertidal mudf l at sediment of the Wadden Sea(southeastern North Sea) and a freshwater sediment from Lake Chiemsee (Germany), respectively(Kolinko et al., 2013). It is notable that these three strains share an intriguing morphologically similarity.They are unusually large in size, are divided in two parts, have several refractive vesicles at the middle of the cell (visible by differential interference contrast microscopy), and all have elongated prismatic magnetosome crystals; both Cux-03 and CS-05 also have large electron dense inclusion particles at their poles.
In an intertidal zone in China we found a novel dominant giant rod-shaped MTB (designated strain QR-1: Qingdao Rod-shaped strain-1) that has a similar morphology to the threeAlphaproteobacteriastrains described above. In this study we used micromanipulation sorting combined with single cell whole genome amplifi cation and fluorescence in situ hybridization (FISH) to identify the 16S rRNA gene of QR-1 and analyze its taxonomic affliation.
2 MATERIAL AND METHOD
2.1 Site description
From 21 March to 7 July 2014, sediment samples were collected at low tide from a seawater pond located in the intertidal zone at Huiquan Bay (36° 02′ N,120° 20′ E; Qingdao, China). Samples were collected approximately once each week (no sampling occurred in May) on a total of nine occasions. The pond had previously been used for the culture of sea cucumbers,but had not been used for this purpose for at least for 6 years prior to the collection of samples for this study. The pond comprises a permanently submerged part and a low tide area (Pan et al., 2008); during rising tides the pond forms a lagoon as water flows in from Huiquan Bay via the low tide area. The sampling site was immediately in front of the low tide area in the middle of the permanently submerged part. During the sampling period the salinity was approximately 31, and the pH of samples ranged from 7.78 to 8.12.The temperature during the sampling period ranged from 16 to 18°C. The salinity was determined using a hand-held refractometer (YW100; Chenhua,Chengdu, China), and the temperature and pH were measured using a benchtop pH/temperature meter(JENCO 6173, Shanghai, China).
2.2 Sampling and magnet collection of MTB
Surface sediments and overlying seawater (1:1 ratio) from the permanently submerged part of the pond were collected in 500 mL plastic bottles. Back to the laboratory, some samples were processed immediately, while others were stored under dim light at ambient temperature (approximately 25° C) for subsequent analysis. Collection of MTB was performed as described previously (Moench and Konetzka, 1978). Cells were enriched by attaching the south poles of permanent magnets to the outside on two opposite sides of each plastic sampling bottle,adjacent to the water/sediment interface. After 30 min,approximately 1 mL of sample water was withdrawn using a Pasteur pipette from adjacent to the magnets and transferred to 1.5 mL centrifuge tubes for subsequent optical microscopy and micromanipulation sorting.
2.3 Optical and electron microscopy
The swimming behavior and cell morphology of the numerically dominant QR-1 strain were investigated on-site using differential interference contrast (DIC) microscopy (Olympus BX51 equipped with a DP71 camera system; Olympus, Tokyo, Japan)of cells enriched in a magnetic fi eld applied to hanging drop preparations of the bacterium (Schüler, 2002).For transmission electron microscopy (TEM), cells collected by microsorting were adsorbed onto 200 mesh carbon-coated copper grids (Beijing Zhong Jing Ke Yi Technology Co., Ltd.) and examined (Hitachi H8100 TEM) without staining. The arrangement and morphology of the magnetosomes and the morphology ofinclusions were assessed at 100 kV. The composition of magnetosomes and inclusions was investigated using energy-dispersive X-ray spectroscopy (EDXS)and high-resolution TEM (HRTEM; Jeol, JEM2100).To determine the surface morphology, cells were examined using scanning electron microscopy (SEM;KYKY-2800B, Beijing, China,) at 25 kV.
2.4 Microsorting and whole genome amplifi cation
The procedures for microsorting and whole genome amplifi cation (WGA) of the cells of strain QR-1 have been described previously (Chen et al.,2015). To phylogenetically identify the strains in this study, we combined microsorting with WGA,followed by polymerase chain reaction (PCR) analysis of the 16S rRNA genes.
2.5 Sequence analysis of 16S rRNA gene
The 16S rRNA gene was amplified from WGA products of the rod-shaped MTB. PCR using the bacteria-Specific primers 27f and 1492r (Sangon Biotech, Shanghai, China) was performed using a Mastercycler (Eppendorf, German). The resulting PCR products were cloned into the pMD18-T vector(TaKaRa, Dalian, China) and transformed into competentEscherichiacoliTOP10 cells. The clones were randomly selected for sequencing, which was carried out by Nanjing Genscript Biotechnology(Nanjing, China). The 16S rRNA gene sequences obtained were analyzed using the BLAST search program (http://www.ncbi.nlm.nih.gov/BLAST/).The 16S rRNA gene sequences of other strains were obtained from GenBank data; all of the 16S rRNA gene sequences were aligned using CLUSTAL W multiple alignment software; sequence identities were calculated using the BIOEDIT software.
A phylogenetic tree was constructed via the neighbor-joining method using MEGA 6, and bootstrap values were calculated from 1 000 replicates.
The 16S rRNA gene sequence has been submitted to the GenBank database under accession number KY317945.
2.6 Probe design
The 16S rRNA gene sequences of QR-1 were aligned with those of Cux-03, CS-05, and clone 1-2-1, and completely conserved regions were used to check for Specificity by screening the bacterial DNA and structural RNA database sections of the GenBank database.
A Specific probe QR197 (position 197-215; 5′-GAC GCA GGA CCC TCT CAA G-3′) was designed.The general probe EUB338 (5′-GCT GCC TCC CGT AGG AGT-3′), which anneals to the 16S rRNA genes of most bacteria, was also used as a control in Specificity and permeability tests (Amann et al., 1990).The Specific oligonucleotide DNA probes were labeled at the 5′ end with Cy5 dye, and the general probe was labeled at the 5′ end with 6-FAM (FITC). All probes were synthesized at Shanghai Sangon Biotech.
2.7 Fluorescence in situ hybridization
The tiptube-purified samples collected from the sampling site were fi xed with 4% paraformaldehyde in artifi cial seawater (pH 7.2) for 12 h at 4°C, and then washed in artifi cial seawater. Other bacteria in the sample were used as positive controls for general probes, and as negative controls for Specific probes.For each fi xed sample, a 4- μ L drop was placed onto an adhesion microscopy slide and dried for approximately 10 min at room temperature. The slide was then immersed in 50%, 80% and 100% ethanol for 3 min each, and then air-dried until the ethanol had evaporated. Hybridization buffer (13.5 μ L) and 1.5 μ L each of Specific and general probe (50 ng/ μ L)were dropped onto the sample and incubated for 3 h at 46°C. A subsequent wash was performed at 48°C for 25 min using elution buffer. The slide was washed three times in dd H2O, then dried. Fluorescence quenching agent (5 μ L; Solarbio) was dropped onto the sample, and the slide was examined using fluorescence microscopy. The hybridization buffer contained 35% formamide, 1 mol/L Tris-HCl (pH 8.0), 5 mol/L NaCl, dd H2O, and 10% SDS; the elution buffer contained 0.5 mol/L EDTA, 1 mol/L Tris-HCl(pH 8.0), 5 mol/L NaCl, dd H2O, and 10% SDS.

Fig.1 Optical microscopy morphology of the rod-shaped magnetotactic bacterium QR-1 from Huiquan Baya. differential interference contrast images of QR-1 showing that the strain is the dominant morphotype among the collected MTB; b. detailed morphological characteristics of QR-1, The black arrow indicates the direction of the applied magnetic fi eld lines. The black, white, and yellow triangles indicate magnetosome chains, inclusion bodies, and refractive vesicles, respectively; c. fluorescence image showing that the cells emit strong blue fluorescence when exposed to UV light (wavelength: 330-385 nm) without staining. The yellow arrows indicate blue fluorescence at the ends and the middle of the cell; d. cell stained with Nile red, show lipid granules evident under blue light (wavelength: 450-480 nm). The yellow arrows indicate orange fluorescence at the ends and the middle of the cell. Scale bars=20 μ m in panel a, 2 μ m in panel b, and 5 μ m in panels c and d.
3 RESULT
3.1 Occurrence and morphology of the giant rodshaped strain QR-1
Freshly collected MTB comprised various cell shapes, including coccoid, vibrio, spirilla, and dominant rod-shaped morphotype. On six of the nine sampling occasions the rod-shaped magnetotactic bacterium QR-1 accounted for >90% of the MTB at the sampling site, with cells abundances up to 104ind./cm3in samples that had not been incubated under laboratory conditions. It is noteworthy that strain QR-1 was the only morphotype of MTB present on 50% of the sampling occasions (Fig.1a). The cells of strain QR-1 averaged (6.71±1.03)×(1.54±0.20) μ m in size (n=226), which is larger than most of the rodshaped MTB previously reported, except for clone 1-2-1 (Zhang et al., 2013). The DIC microscopy revealed that the QR-1 cells were usually separated into two parts. Each part contained one inclusion particle and several refractive vesicles at the middle of the cell (Fig.1b). These morphological characteristic are very similar to those of clone 1-2-1 (Zhang et al.,2013), and strains Cux-03 and CS-05 (Kolinko et al.,2013), which are affliated to theAlphaproteobacteria.
Fluorescence microscopy observation of cells without staining revealed blue fl orescent spots in the middle and at the poles of the cell (Fig.1c). Nile red staining showed that these spots may be lipid granules(Fig.1d).
3.2 Magnetosomes and inclusion bodiesgiant rodshaped
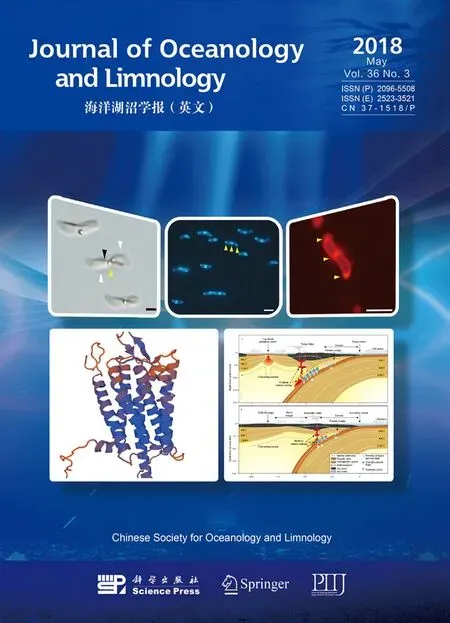
Fig.2 Ultrastructural analysis of QR-1a. TEM images showing the characteristics of QR-1 cells; b. magnetosomes. The white arrows indicate the electron-dense inclusion bodies; c. HRTEM analysis of magnetite magnetosomes; d. a selected area at higher magnifi cation; e. EDXS analysis of magnetite; f. polyphosphate. The black arrows identify peaks ofiron and oxygen in panel e, and peaks of phosphorus and oxygen in panel f. g. SEM image showing the surface structure of QR-1 cells. The yellow arrows indicate the projecting imprint of lipid granules, coinciding with the morphological characteristics evident by TEM. Scale bars=1 μ m in panels a and g, 50 nm in panel b, 5 nm in panel c, and 1 nm in panel d.
Magnetosomes are membrane-enveloped nanocrystals that impart a magnetic moment and enable geomagnetic navigation of MTB (Schüler, 2008).TEM-based observations revealed that the giant rodshaped MTB strain QR-1 contained 42 to 146 magnetosomes (n=15) per cell, arranged in a bundle formed by 1-4 parallel chains along the long axis of the cell; approximately 53.3% of observed cells had two chains (Fig.2a). Each elongated prismatic magnetosome was 106±18 nm in length and 93±22 nm in width (n=271), and this produced a shape factor of approximately 0.876±0.1 (n=271)(Fig.2b). EDXS analysis indicated that the magnetosome crystals were composed ofiron and oxygen, and analysis by HRTEM identified that the crystals were magnetite (Fig.2c, d, e). Multiple magnetosome chain bundles have previously been observed in strain clone 1-2-1 (Zhang et al., 2013),manyNitrospirastrains from freshwater (Flies et al.,2005; Lin et al., 2012), and Multicellular Magnetotactic Prokaryotes (MMPs; Abreu et al., 2013; Zhou et al.,2013; Chen et al., 2015, 2016). EDXS analysis revealed that the two polar electron-dense structures contained large quantities of phosphorus and oxygen(Fig.2f), and were found to be composed of polyphosphate. SEM analysis of cellular morphology revealed a projecting imprint at the position of the lipid granules (Fig.2g), while TEM distinguished an electron-dense structure at each end of the cell(Fig.2a), as has been observed in Cux-03 and CS-05(Kolinko et al., 2013). Each of the two elongated oval-shaped polyphosphate particles was approximately 2 μ m long, and is much larger than similar structures in other strains (Silva et al., 2007;Lefèvre et al., 2009, 2012; Kolinko et al., 2013).Similar polyphosphate particles also occur in Cux-03 and CS-05 (Kolinko et al., 2013). Polyphosphate is a common type ofinclusion body in bacteria, which use it as an energy source, phosphorus reserve material,or active metabolic regulator (Kulaev and Vagabov,1983; Achbergerová and Nahálka, 2011). The use of polyphosphate by these MTB strains, and its possible role in regulation of magnetosome formation, is unclear.
3.3 Phylogenetic analysis
Identifi cation of the taxonomic position of QR-1 in this study was performed by the micromanipulation sorting technique in combination with whole genome amplifi cation and 16S rRNA gene amplifi cation and sequencing, which has been shown to be a highly effcient method to identify MTB and MMPs in phylogenetic studies (Jogler et al., 2011; Kolinko et al., 2012, 2013; Zhang et al., 2014; Chen et al., 2015,2016). Micromanipulation sorting enabled QR-1 with the giant rod morphotype to be separated from other bacteria (Chen et al., 2015) (see Materials and Methods). A total of 39 random clones were sequenced, and 27 of these (>69%) provided sequences most similar to Cux-03 (97% identity)(Schüler, 2002). All the 27 sequences belonged to the same OTU (QR-1), and among these 24 of the sequences differed by less than 0.4%. To verify the 16S rRNA gene sequence a Specific oligonucleotide DNA probe was designed, based on the 16S rRNA gene sequence for QR-1. QR-1 cells were collected from the sampling site, along with other MTB as controls. The general probe recognized both the QR-1 and other marine bacteria, while the Specific probe only recognized QR-1 cells (Fig.3a, b). The three rodshaped QR-1 cells were easy to tell apart by their shape based on fluorescence microscope, and the cells had strong fluorescence area in round shape may owing to high concentration of ribosomes and high ratios of hybridiztion (Frickmann et al., 2017). This result corroborates the authenticity of the 16S rRNA gene sequence belonging to QR-1 and shows the effciency of micromanipulation-WGA-sequencing approach in taxonomic identifi cation of MTB.

Fig.3 FISH analysis of QR-1The same microscopic fi elds are shown following: a hybridization with the 5′-FAM labeled bacterial universal probe, EUB338; and b hybridization with the 5′-Cy5 labeled Specific probe, QR197. White and red arrows indicate QR-1 cells and other MTB, respectively. Scale bars=5 μ m.
Phylogenetic analysis of the 16S rRNA gene showed that QR-1 is closely related to three other rodshaped MTB (Cux-03, CS-05, clone 1-2-1) (Fig.4).Although QR-1 showed a high level ofidentity (97%)with Cux-03 (Kolinko et al., 2013), the QR-1 cells are approximately double the size of Cux-03 (length:6.7 μ m versus 3.5 μ m), and it has one to multiple magnetosome chains; this suggests that QR-1 may be a novel species. In addition, QR-1 has 92% identity with clone 1-2-1 (Zhang et al., 2013), and 89.4%identity with freshwater strain CS-05 (Kolinko et al.,2013). Other two uncultured clones MRT-120 (Xing et al., 2008) and QOCT164 which were also collected from Huiquan Bay and identified by mass collective method, clustered into the same clade with QR-1,suggesting that those two strains may be rod-shaped MTB. Interestingly, the uncultivated bacterium clone 1-2-1, Cux-03 and CS-05 were considered to be affliated withAlphaproteobacteriabecause that is the most closely related Class (Kolinko et al., 2013;Zhang et al., 2013). However, these three strains together with QR-1 and most uncultivated magnetococcus bacterium were fi rst clustered into a branch withCand. Magnetococcus massalia MO-1 andMagnetococcusmarinusMC-1 that are representatives of the novelEtaproteobacteria(Ji et al., 2017), and the branch clearly distinguish from representatives of magnetospillum and magnetovibrio.Alphaproteobacteria magnetotactic bacteria were cluttered with MO-1 and MC-1 that are representatives of the novelEtaproteobacteria, and genomic comparison is required to clarify the taxonomic classifi cation of the QR-1 and its relatives.
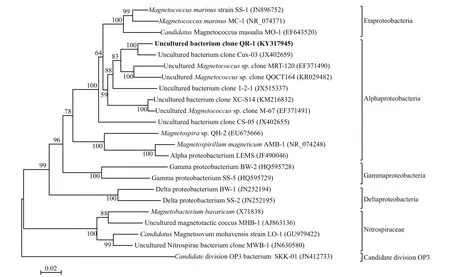
Fig.4 Phylogenetic tree of QR-1 and other MTBThe tree was constructed based on neighbor-joining analysis using full length 16S rRNA gene sequences, and the bootstrap values were calculated from 1 000 replicates. The sequence determined in this study is shown in bold. The GenBank accession numbers of the utilized sequences are indicated in parentheses.Rod-shaped MTB in the Alphaproteobacteria are circled in the dotted red box. Scale bar=0.02 substitutions per nucleotide position.

Fig.5 Motility of QR-1a. composite images showing uniform velocity of QR-1; b. composite images showing excursion; c. transient cessation of motility; d. return swimming; e-g.composite images showing collective magneto-aerotaxis behavior of QR-1 cells. The black and white arrows indicate the direction of the applied magnetic fi eld lines and the direction of movement of QR-1 cells, respectively. The white circle indicates non-moving cells. Scale bars=20 μ m.
3.4 Motility and flagella
For many bacteria, motility is essential for survival,growth, virulence, biofi lm formation, and intra/interspecies interactions (Nan and Zusman, 2016).Motility associated with magnetotaxis typically involves the rotation of flagella, which are complex molecular machines that, like propellers, generate force to push cells forward or backward at differing speeds (Kearns, 2010).
The QR-1 cells swam at 20 to 132 μ m/s (Fig.5a),with an average of 70±28 μ m/s (n=85), which is slower than most ovoid-shaped MTB (Lefèvre et al.,2009, 2011; Zhang et al., 2012), but faster than that of the magnetotactic spirillum QH-2 (20-50 μ m/s) (Zhu et al., 2010),M.bavaricum(average speed 40 μ m/s)(Spring et al., 1993), and the slow moving MHB-1(Flies et al., 2005). As with some magnetotactic spirilla including AMB-1, MS-1, andMagnetospirillummagnetotacticum(Balkwill et al., 1980;Spormann and Wolfe, 1984; Matsunaga et al., 1991),when exposed to an applied magnetic fi eld QR-1 displayed axial magnetotaxis (Fig.5b, c, d), which involves cells being equally likely to swim parallel or antiparllel to the magnetic fi eld, with random abrupt changes in direction (Faivre and Schüler, 2008). For these cells the magnetic fi eld only represents an axis of swimming, but does not determine direction of movement (Frankel et al., 1997). When the direction of the applied magnetic fi eld was reversed, QR-1 cells rotated 180°, but continued to swim back and forth;this response was similar to that ofM.magnetotacticum(Frankel et al., 1997).

Fig.6 Flagella characteristics of QR-1a. overall TEM image showing lophotrichous flagella; b. magnifi cation of the area of the white rectangle in panel a, showing the flagella bundle at one end of the cell. The black arrows and parallel lines indicate 7 flagella in a bundle; c. magnifi cation of the area of the yellow rectangle in panel a, showing the flagella bundle (black arrow and parallel lines) and numerous flagellar fi laments (yellow arrows) at the other end of the cell; d. magnifi cation of the black rectangle area in panel a, showing the root of the flagella bundle (indicated by white arrows and parallel lines). Scale bars=1 μm in panel a and 200 nm in panels b-d.
Bacteria have traditionally been viewed as unicellular organisms that grow as dispersed individual cells (Henrichsen, 1972). Remarkably,laboratory DIC microscopy observations of the edge of the hanging drop revealed a collective magnetoaerotaxis behavior by QR-1 cells apparently aggregating as multicellular groups rather than as individuals, when a magnetic fi eld (500 Gs) was applied (Fig.5e, f, g). The composite image of the trajectories of groups of QR-1 cells revealed that this phenomenon was through the combined axial magnetotaxis ofindividual cells, which could be divided into 3 phases: (1) excursion swim from the droplet edge towards the center, at right angles to the magnetic fi eld lines; (2) transient cessation of motility for approximately 0.2 s; and (3) return swimming back to the droplet edge parallel to the magnetic fi eld lines.
TEM-based analysis showed that QR-1 cells have two tufts of flagella at each end of the cell (Fig.6a).Each tuft of flagella was composed of 7 flagellar fi laments and many fi brils (Fig.6b, c). A similar flagella apparatus has been reported in the bilophotrichous flagella strains MC-1 (Frankel et al.,1997), MO-1 (Zhang et al., 2014), and QH-3 (Zhou et al., 2010). All the three coccid strains have flagellar sheaths, but no sheath was observed in QR-1. The diameters of the roots of the flagella bundles (Fig.6d),the fi laments, and the fi brils was approximately 115 nm (n=21), 17.9±0.4 nm (n=123), and 12 nm(n=15), respectively.
4 DISCUSSION
In this study, a dominant rod-shaped MTB strain QR-1 was found from the seawater pond in intertidal zone. Compared with the low tide area, where the magnetotactic coccus QHL dominates (Pan et al.,2008), the permanently submerged part of the pond used as the sampling site in the present study was generally more stable and subject to less tidal disturbance. It had been reported that both environmental heterogeneity and geographic distance play significant roles in shaping the dominant populations in MTB communities (Lin et al., 2013).The geographic distributions of QR-1 and QHL are in very close proximity, suggesting that environmental heterogeneity between the ecological niches for these two bacteria may be the major factor determining the dominant species within the MTB communities at each site (Lin et al., 2013). At the population level,microbial growth followed established patterns known as the growth law (Schaechter et al., 1958).The individual cells’ width did not change significantly between birth and division, and the average instantaneous elongation rate was identical to the average nutrient-imposed growth rate of the population (Taheri-Araghi et al., 2015). Thus, the long rod-shaped QR-1 surpassed the nutrient-imposed growth rate of other MTB among MTB community,and then turned into the dominant population in biotope.
Our study shows that the giant rod-shaped QR-1 cells are larger than most of the rod-shaped MTB previously reported. So, how do QR-1 cells win in the competition for nutrients to maintain size homeostasis?Actually, bacteria are strictly osmotrophic, thus, their size is limited by surface area to volume (SA/V) ratios(Laf l amme et al., 2009). Small cells, like bacteria,have large SA/V ratios. Typically, the larger SA/V is,the more effciently the cell can exchange materials with the environment. The large SA/V precludes the need for complicated internal transport systems(Zhang, 2016). For bacteria, small variations in the width ofindividual cells had a strong negative effect on the SA/V values, whereas SA/V ratios are relatively constant when changing cell lengths (Steinberger et al., 2002; Laf l amme et al., 2009). The QR-1 cells are longer than most of the MTB reported, but have no significant difference with them in width. Therefore,the SA/V rations of QR-1 cells are not significantly affected by cell widths, and their lengths inf l uence the surface area and volume more significantly than the SA/V. Hence, other growth strategies to increase SA/V allowing for rapid and effective transport of nutrients directly through the integument into QR-1 cells are needed.Thiomargaritaare largest bacteria with sizes up to 750 μm in diameter (Schulz and Schulz, 2005). However, the tremendous dimensions greatly reduce the feasibility osmotrophy which is restricted to bacteria with sizes 100 μm (Schulz and Jørgensen, 2001). To reduce the SA/V threshold, theThiomargaritacell usually contain a large central vacuole and numerous sulfur particles which strategically reducing the overall active organic volume to increase SA/V. In fact, this growth strategy is also used by QR-1 cells because they possessed two large oval-shaped polyphosphate particles. The incorporation ofinactive matter within the volume significantly restricts the effective volume used by the organism, thereby allowing QR-1 cells to maintain high SA/V ratios and also consistent with morphological expectations for osmotrophs.
Recently, microsorting and WGA are widely used as effective techniques for MTB strain isolation and identifi cation (Jogler et al., 2011; Kolinko et al., 2012,2013; Zhou et al., 2012; Chen et al., 2015, 2016).These techniques easily linked phylo- to morphotypes and led to the discovery of novel low-abundant MTB(Kolinko et al., 2013), and facilitated the amplifi cation and d e novo assembly of whole genomes even from an individual bacterial cell (Rodrigue et al., 2009;Woyke et al., 2010). Here, microsorting and WGA were applied for the phylogenetic and ultrastructural analysis of giant rod-shaped QR-1. This strain is phylogenetically affliated closer with the marine strains clone 1-2-1 and Cux-03 than to the freshwater strain CS-05, indicating that phylotype is related to habitat conditions (Zhang et al., 2010). Despite their different geographic origins and ecology, these three marine strains share considerable morphological similarity, and clustered into one clade in the phylogenetic tree, apparently separate from other morphotypes; this indicates that these morphologically distinct strains may have originated from a common ancestor.
It should be noted that the flagella apparatus of QR-1 cells was fi rst reported in rod-shaped MTB,which have mostly been reported to be monotrichous(Spring et al., 1993; Lefèvre et al., 2010, 2011;Kolinko et al., 2013). It has been reported that, during swimming motility, the flagella of a cell coalesce into a bundle and rotate to propel the bacterium forward,while tumbling can occur when only a single fl agellum changes its direction of rotation (Kearns, 2010). The bundling of flagella effectively increases flagellar stiffness during rotation, and makes force generation more effcient in viscous liquids (Atsumi et al., 1996;Zhang et al., 2014). Because of this powerful flagella apparatus, strain QR-1 exhibited distinct magnetotactic motility and demonstrated higher speeds than other reported rod-shaped strains (Spring et al., 1993; Flies et al., 2005).
With the powerful flagella apparatus of QR-1, the cells group exhibited a special collective magnetoaerotaxis behavior which distinguished them from all rod-shaped MTB reported previously (Spring et al.,1993; Flies et al., 2005; Lefèvre et al., 2011; 2012;Kolinko et al., 2013). It was inferred that this behavior occurred in conjunction with aerotaxis, with cells exhibiting a positive response to the marked change in oxygen concentration ([O2]) when they were transferred from sediment to the hanging drop (Mao and Liu, 2015). QR-1 cells probably have an oxygen sensory system (Taylor, 1983; Spormann and Wolfe,1984) that can determine the change in [O2], enabling them to regulate the direction of flagellar rotation(Wadhams and Armitage, 2004; Turner et al., 2010).One interpretation for the observed movement is that the accumulation of cells in high densities may result in depletion of oxygen (Frankel et al., 1997) to a concentration lower than optimum, resulting in clockwise flagellar rotation and swimming at right angles to the magnetic fi eld until a higher [O2] is encountered. At this point the cells pause and reverse flagellar direction to counterclockwise, then swim parallel to the magnetic fi eld until they reach a low[O2] threshold. Whether this behavior occurs in situ is unknown.
5 CONCLUSION
The giant rod-shaped MTB QR-1 described here were dominant morphotype in sampling environment,contrary to previous reports that magnetotactic cocci are the most abundant morphotype of MTB. The strain QR-1 was separated into two parts with one polyphosphate particle and several lipid granules at the middle of the cell. Each cell usually possessed 1-4 parallel magnetosome chains arranged along the long axis of the cell. These morphology characteristics were similar to those reported rod-shaped MTB belonged toAlphaproteobacteria. To identity the taxonomic position of QR-1, micromanipulation sorting technique in combination with WGA and FISH were performed, and the analysis showed that QR-1 affliated within the deep branch ofAlphaproteobacteria. Besides, the QR-1 cells swam faster than other rod-shaped MTB and displayed a unique collective magneto-aerotaxis behavior which was a combined axial magnetotaxis ofindividual cells. All of these motility characteristics were due to the powerful flagella apparatus. QR-1 cells have two tufts of flagella with 7 flagellar fi laments and many fi brils in each tuft, and this kind of flagella apparatus was fi rst reported in rod-shaped MTB.
6 DATA AVAILABILITY STATEMENT
Sequence data that support the fi ndings of this study have been deposited in the GenBank database with accession number KY317945.
7 ACKNOWLEDGEMENT
We thank XU Jianhong for his assistance with biological sampling, JIANG Ming, LIU Jing, and MA Xicheng for their assistance with the TEM analysis,and LIU Wei for supporting our SEM observations.
猜你喜欢
——邢伟中檀香扇作品欣赏
杂志排行
Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- The post-larval and juvenile fi sh assemblage in the Sukhothai Floodplain, Thailand*
- Effects of probiotic on microf l oral structure of live feed used in larval breeding of turbotScophthalmus maximus*
- Comparison ofintestinal microbiota and activities of digestive and immune-related enzymes of sea cucumberApostichopus japonicusin two habitats*
- Otolith shape analysis for stock discrimination of twoCollichthysgenus croaker (Pieces: Sciaenidae,) from the northern Chinese coast*
- The impact of spatial autocorrelation on CPUE standardization between two different fi sheries*