lnsecticide resistance of the field populations of oriental armyworm,Mythimna separata (Walker) in Shaanxi and Shanxi provinces of China
2018-07-09ZHAOYuyuSULiLlShuaiLlYipingXUXiangliCHENGWeiningWANGYiWUJunxiang
ZHAO Yu-yu, SU Li, Ll Shuai, Ll Yi-ping, XU Xiang-li, CHENG Wei-ning, WANG Yi, WU Jun-xiang
1 State Key Laboratory of Crop Stress Biology in Arid Areas/College of Plant Protection, Northwest A&F University, Yangling 712100, P.R.China
2 Department of Plant Protection, Agricultural College, Guangxi University, Nanning 530005, P.R.China
3 College of Agriculture, Shanxi Agricultural University, Taigu 030800, P.R.China
1. lntroduction
The oriental armyworm, Mythimna separata (Walker)(Lepidoptera: Noctuidae), a typical migratory insect, is a polyphagous and gluttonous pest of grain crops in the Eastern Asia and some parts of Oceania. It has been reported in China except Tibet and Xinjiang. Larvae prefer to hosts in Gramineae, especially some primary cereal crops such as rice (Oryza sativa L.), maize (Zea mays L.) and wheat (Triticum aestivum L.), causing serious economic losses (Zhang et al. 2012; Jiang Y Y et al. 2014). In 2012,the third-generation armyworm seriously occurred for the acreage of more than 200 thousand ha in Shaanxi and Shanxi provinces, China (Zeng et al. 2013). In 2016, due to the impact of climatic conditions, the third-generation oriental armyworm outbroke again with more than 60 thousand ha in these two provinces, causing serious losses to the autumn growing crops (Zhao and Cheng 2016).
The control of M. separata has been heavily relying on the application of various insecticides, mainly organophosphates and pyrethroids. Previous studies showed that the oriental armyworm had developed certain levels of resistance to pyrethroid insecticides as early as since late 1980s and early 1990s (Yu 1987; Yang and Gong 1992; Yang et al. 1995). In recent years, there were also similar studies on the insecticide resistance of oriental armyworm (Dong et al. 2014). However, the strong migratory capability of M. separata, with an annual multigenerations round trip migrations between southern and northern China, can dilute the resistance and slow down its developmental rate (Yang et al. 1995). For this reason, no considerable attention has been made on the insecticide resistance of the pest. In recent years, with the significant increase in occurrence and damage caused by oriental armyworm in China, people have realized that in addition to climate conditions and planting structure, insecticide resistance should also be taken into account in analyzing its population dynamics and underlying mechanisms (Jiang X F et al. 2014). We hypothesized that the long-term and heavy application of chemical insecticides might have inevitably led to the development of insect resistance towards commonly applied chemicals. In this study,five field populations of M. separata were collected from Shaanxi and Shanxi provinces, the resistance status to six commonly used insecticides was tested using a standard leaf dipping bioassay. The goal of the study was to provide information about the development of insecticide resistance and to give suggestions to the design of appropriate resistance management strategies of M. separata.
2. Materials and methods
2.1. lnsects
Field populations of M. separata were collected respectively from five different regions of China. About 500 fifth- to sixth-instar larvae were collected from each sampling site(Fig. 1 and Table 1). Larvae were reared on maize leaves in climatically controlled chambers maintained at (25±1)°C,relative humidity 70–80%, with a photoperiod of 14 h L:10 h D(Wang et al. 2016). Pupae were distinguished with sex and then each pair was transferred to disposable plastic cup.The adults were fed with cotton ball soaked with 5% honey solution and folded paper was supplied for oviposition. New third-instar larvae from the first filial generation were used for bioassays.
The relatively susceptible strain (Lab-SS) was collected from Xingping City, Shaanxi Province, China in July 2014,which has been maintained in the laboratory for over 18 generations without exposure to any insecticides.
2.2. lnsecticides
Six formulated insecticides including four conventional insecticides and two new-type chemical insecticides were used in all bioassays. Conventional insecticides included organophosphates (phoxim, 92.0% purity; chlorpyrifos,97.0% purity) and pyrethroids (beta-cypermethrin, 95.9%purity; lambda-cyhalothrin, 97.4% purity). New-type chemical insecticides included emamectin benzoate (74.2%purity) and chlorantraniliprole (200 g L–1SC). They were all provided by Nanjing Agricultural University, China. All these insecticides except chloorantraniliprole were dissolved in acetone, constant volume in a 50-mL volumetric flask,respectively. Chlorantraniliprole was diluted directly and serially diluted seven times with distilled water containing 0.1% Triton X-100 for experiment.
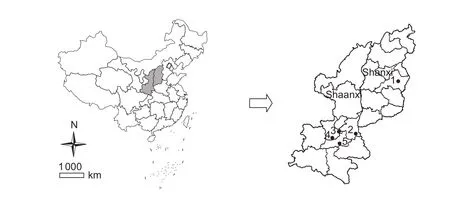
Fig. 1 Sampling sites of Mythimna separata field populations from Shaanxi and Shanxi provinces in China.
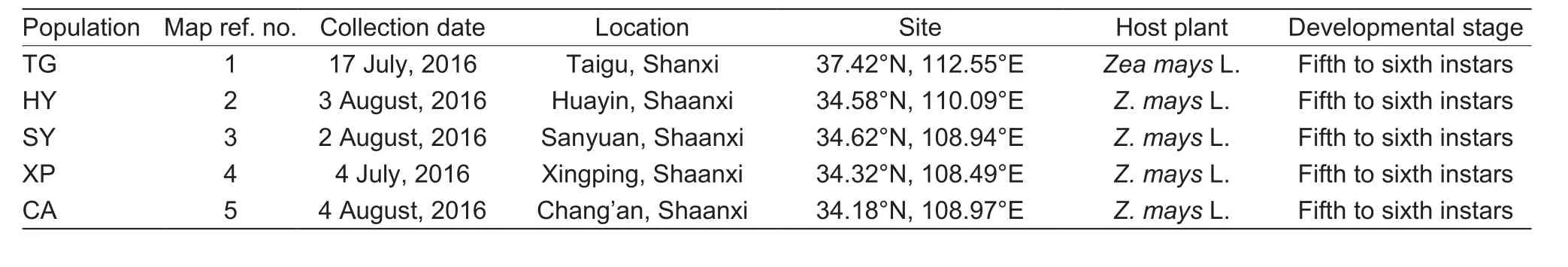
Table 1 Locations, sampling sites, dates, host plants and developmental stages of Mythimna seperata from fields in China
2.3. Bioassays
Bioassays were conducted using a standard leaf dipping bioassay method (Ahmad et al. 2007). Leaf discs of maize with 5 cm in length were dipped in an insecticide solution for 30 s. Then the leaf discs were dried at room temperature for 2 h and placed in a plastic Petri dish with filter paper to feed larvae that had been deprived of food for 2 h. Control discs were treated with 0.1% Triton X-100 solution in water only. The bioassay was repeated three times with 20 larvae per replicate, and assays were conducted under similar environmental conditions as described above. The mortality was assessed after 48 h for all insecticides except for the chlorantraniliprole which was accessed after 72 h (Dong et al. 2014). The oriental armyworms failing to exhibit movement after a gentle probe were considered dead.
2.4. Data analysis
Probit analysis was conducted using PoloPlus Software to estimate the median lethal concentration (LC50) and the slopes of the regression lines. LC50s were not considered different if their 95% confidence limits (95% CL) overlapped.Resistance ratios (RR) were determined as the LC50of field populations divided by LC50of the susceptible strain (Abbott 1925). Resistance level was considered as none (RR<3),very low (RR=3–5), low (RR=5–10), moderate (RR=10–40),high (RR=41–160) and very high (RR>160) (Shen and Wu 1995). Pairwise correlation coefficients of logLC50values of the five populations for each insecticide were calculated by the Pearson correlation formula using SPSS Software.
3. Results
3.1. Toxicity of insecticides against laboratory susceptible strain
The susceptible strain had the highest susceptibility to emamectin benzoate (LC50=0.024 mg L–1), while chlorpyrifos and phoxim were equally toxic with the LC50values of 0.030 and 0.066 mg L–1, respectively. The pyrethroids,lambda-cyhalothrin and beta-cypermethrin, were the least toxic among the tested insecticides against the Lab-SS of M. separata (Table 2).
3.2. Toxicity of conventional insecticides against field populations
The field populations of oriental armyworm have developed various levels of resistance to organophosphates, especially phoxim. All field populations showed moderate to high levels of resistance, ranging from 19.367 to 70.100 folds(Table 3), except for the Sanyuan (SY) population. But for the chlorpyrifos, the resistance was much lower. A moderate level of resistance was found in Taigu (TG) (32.045-fold)and Xingping (XP) (11.879-fold) populations, while no or very low resistances were observed in SY, Huayin (HY) and Chang’an (CA) populations.
Of the pyrethroids, compared with the Lab-SS population,lambda-cyhalothrin expressed moderate levels of resistance in all field populations, ranging from 12.683 to 32.827 folds(LC50=8.054–20.845 mg L–1), except for the TG population(LC50=0.081 mg L–1). The resistance ratios of field populations to beta-cypermethrin were less than 10-fold.SY and TG populations displayed low level of resistance with resistance ratio of 5.052 and 6.217, respectively. The resistance ratios of the other three populations were less than 1, indicating that they were highly sensitive to betacypermethrin (Table 3).
3.3. Toxicity of new-type chemical insecticides against field populations
Resistance levels to chlorantraniliprole (1.169–5.576 folds)were generally low for all field populations. However, low levels of resistance to chlorantraniliprole were found in SY and XP populations (5.152- and 5.576-fold) respectively.All armyworm populations were relatively sensitive to emamectin benzoate (0.583–1.583 folds) (Table 4).
3.4. Correlations between lethal concentration (LC)values of insecticides
A pairwise comparisons of the logLC50of insecticides showed no significant correlation between beta-cypermethrin and emamectin benzoate or chlorpyrifos, while betacypermethrin showed no correlation with chlorantraniliprole,lambda-cyhalothrin or phoxim (Table 5), suggesting that there was a lack of cross-resistance between theseinsecticides. Chlorpyrifos showed significant correlation only with emamectin benzoate. Correlation coefficients between lambda-cyhalothrin, chlorantraniliprole and emamectin benzoate, chlorpyrifos or phoxim respectively is negative, indicating that there was no significant cross-resistance.
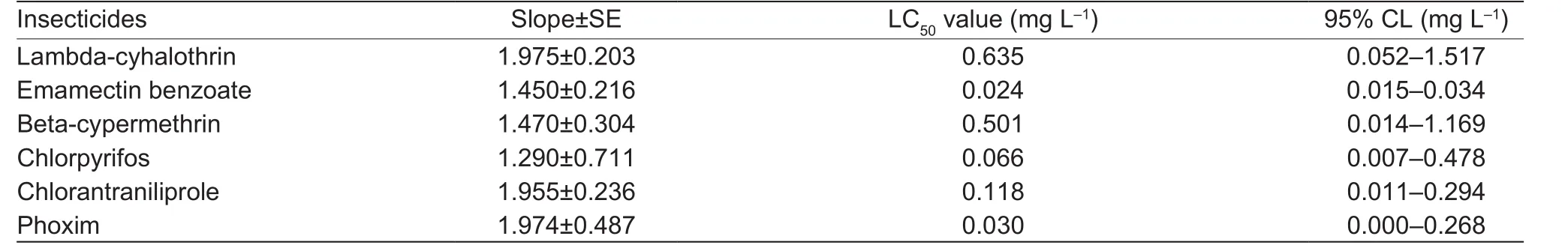
Table 2 Toxicity of six insecticides to third instar larvae of susceptible strain
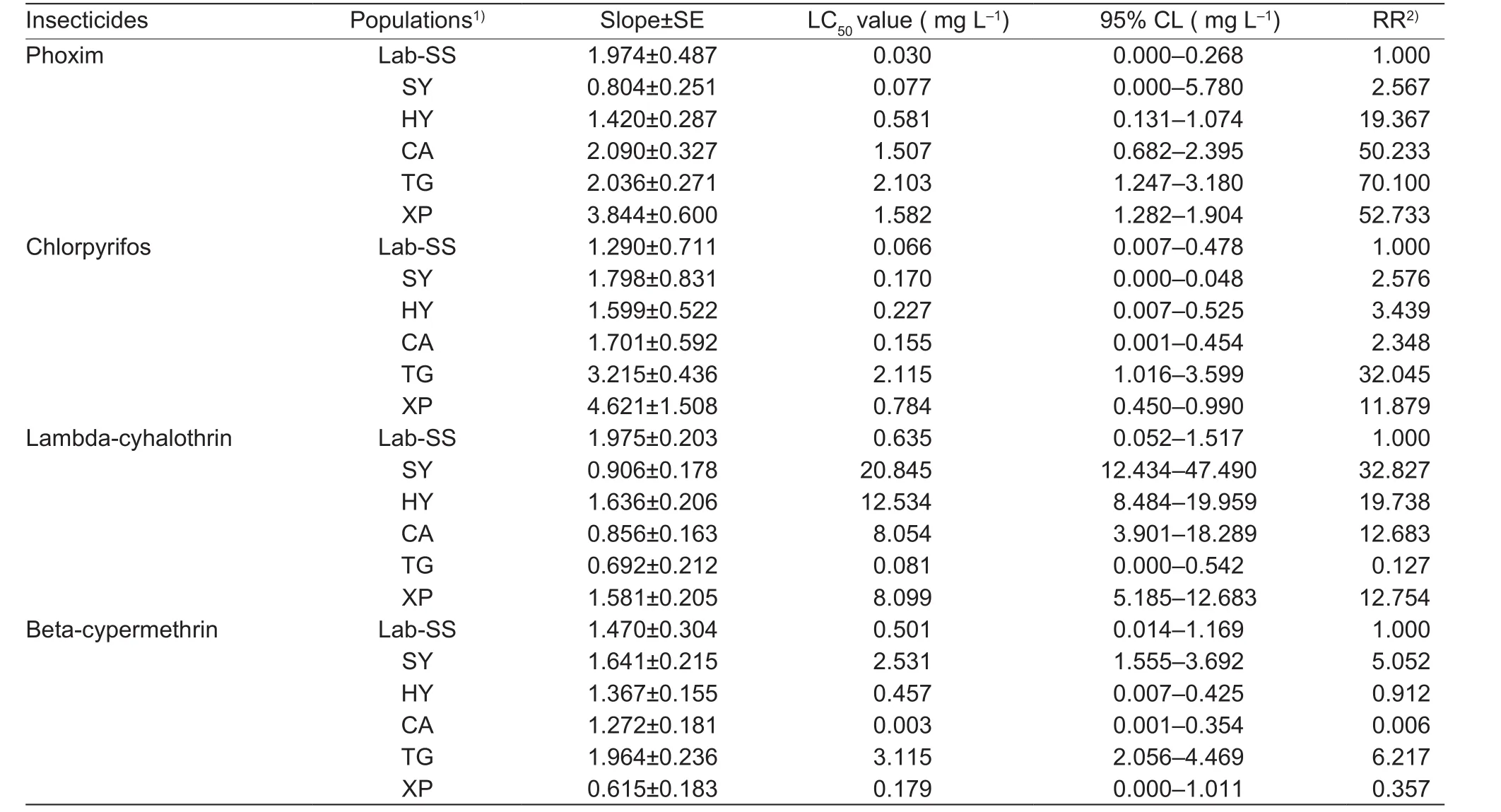
Table 3 Resistance status of third instar larvae of field populations to conventional insecticides
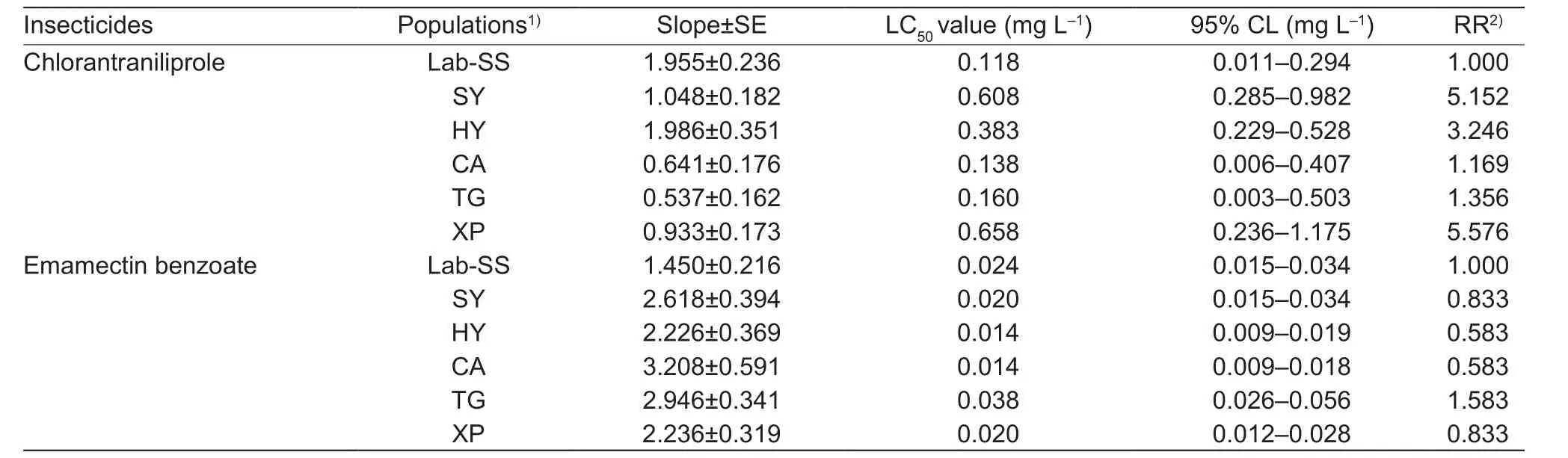
Table 4 Resistance status of third instar larvae of field populations to new-type insecticides
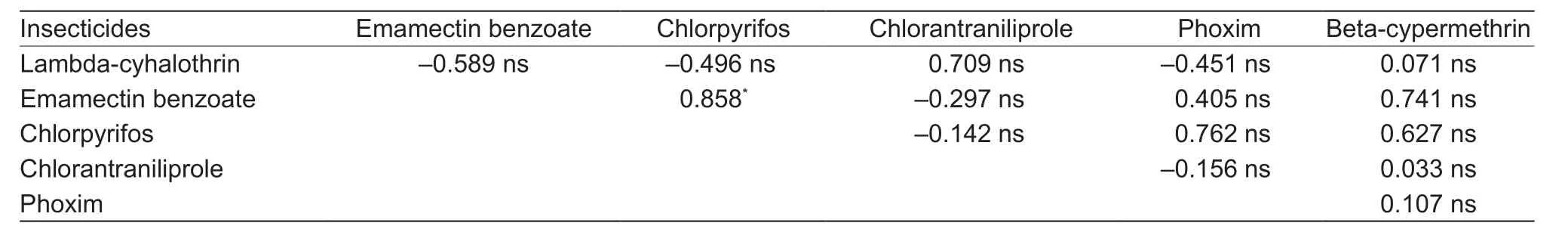
Table 5 Pairwise correlation coefficient comparisons between LC50 concentration values of the insecticides tested on the field populations of Mythimna separata
4. Discussion
Insecticide resistance of insects is a phenomenon of stress evolution, which is accompanied by the selection pressure exerted on sprayed populations that increases the frequency of resistant individuals (Metcalf 1989). Due to the difference in the frequency and intensity of insecticides used in different geographical regions, the sensitivity and the development rate of insecticide resistance were different (Chen et al.2014). The present study revealed that populations from different geographical regions displayed various resistance levels to conventional insecticides such as pyrethroids and organophosphates. This could be related to the long-term and unreasonable usage of chemicals in these areas.As early as 1987, it was reported that the resistance of M. separata significantly increased against pyrethroids especially deltamethrin (Yu 1987). This study showed that pyrethroids exhibited very low to medium resistance levels out of five populations of M. separata tested, which was also observed in other parts of China (Yang et al. 1995;Dong et al. 2014). This may be related with migration of M. separata, which is a well-known long-distance migratory insect and can overwinter south of 33°N in China where the isotherm in January is about 0°C. There are at least four distinct large-scale migration events of M. separata annually in China (Feng et al. 2008; Jiang et al. 2011), and in the south the host plants of M. separata are mainly rice where the use of pyrethroids is limited. Therefore, migration may dilute resistance through interbreeding with susceptible individuals and is responsible for the among-years cyclical variation of resistance frequencies (Yang et al. 1995).Similarly, in Australia, migration process was considered a major behavioral factor affecting the evolution of insecticide resistance in the cotton bollworm, Helicoverpa armigera(Daly 1993). Resistance ratios to chlorpyrifos in populations collected from Shanxi and Shaanxi provinces were 32.045-fold and <12-fold, respectively, which is accordant with the fact that chlorpyrifos is a preferred chemical to control oriental armyworm in these areas. While four populations of M. separata presented medium to high level resistance to phoxim. It suggests that phoxim should be withdrawn from the field application to prevent development of resistance and other alternative insecticides may be chosen to control M. separata.
Emamectin benzoate, a macrocyclic lactone with high potency against nematodes, mites and insects,is the most effective against lepidopteran insects in all insecticides, which has the advantages of ultra-efficient,non-toxic and pollution-free (Clark et al. 1995; Bi and Zhao 2003). Different levels of resistance were observed in field populations of Plutella xylotella, Spodoptera exigua and Spodoptera litura (Pu et al. 2010; Che et al.2013; Saleem et al. 2015), which could be attributed to the intensive and irrational use of emamectin benzoate.Two populations of the beet armyworm, S. exigua from Yanliang, Shaanxi Province in 2009 and 2010 were demonstrated to have very high resistance levels (461.8-and 203.0-fold, respectively) (Shi 2011). But it exhibited very low to low levels of resistance to S. exigua in nine and medium in three out of 15 populations tested in Pakistan(Ishtiaq et al. 2012). Our study also illustrated that M. separata were sensitive to emamectin benzoate in different regions of China and suggested that emamectin benzoate is still effective against M. separata and can be used for the control of this pest.
Chlorantraniliprole, the first broad-spectrum pesticide from a new class of chemistry, the anthranilic diamides,has exceptional insecticidal activity across a range of lepidopteran species. In addition, it serves as an effective alternative insecticide for control of pest resistance to other insecticides, and it has not been found to have crossresistance with existing insecticides (Sattelle et al. 2008;Lahm et al. 2009; Wang et al. 2010). However, in recent years, a medium to very high resistance to chlorantraniliprole has been reported in some Lepidopteran insects in various parts of China. Wang et al. (2012) tested 20 P. xylostella populations from 12 different regions in China, and very high levels of resistance were detected in Zengcheng(2 000-fold) and Zhuhai (140-fold), which may be resulted from variation in insecticide use intensity. The resistance of 18 field populations of S. exigua collected from China ranged from 2.8- to 17.1-fold for chlorantraniliprole when compared with the laboratory susceptible strain (Lai et al.2011). Narrow variations in chlorantraniliprole susceptibility were also observed in field populations of Choristoneura rosaceana (5.3-fold) (Sial et al. 2010), Cnaphalocrocis medinalis (5.8-fold) (Zheng et al. 2011), Chilo suppressalis(9.4-fold) (Huang et al. 2011) and Bemisia tabaci (5-fold) (Li et al. 2012). Selection experiment showed that S. exigua developed 11.8-fold resistance to chlorantraniliprole under continuous exposures (Lai and Su 2011). Among the five populations from Shaanxi and Shanxi provinces, low levels of resistance were detected in Xingping (XP) and Sanyuan(SY) in the current study. It suggests us that proactive resistance management strategies should be implemented to postpone the evolution of resistance.
Correlation analysis demonstrated that a highly positive correlation between emamectin benzoate and chlorpyrifos in M. separata. Similar cross-resistance between these two chemicals was reported in Frankliniella occidentals. Owing to different modes of action,cross-resistance between emamectin benzoate and chlorpyrifos may be mediated by one or more common mechanisms of metabolic detoxification (Wang et al. 2012).Simultaneously, chlorantraniliprole showed no crossresistance with any insecticide except lambda-cyhalothrin,which implies that chlorantraniliprole can be used for the management of insecticide resistance in M. separata.Sang et al. (2016) also did not find significant correlation between chlorantraniliprole and emamectin benzoate or chlorpyrifos in different regions of S. litura. To some extent, the correlation analysis could be used to explain the cross-resistant relationship of insecticides. However,due to the complexity of insecticide use in fields, pests of field population can develop multiple resistances to various insecticides, thus it would be more practical to rely more on insecticide application history in a field when determining cross-resistance (Shen and Wu 1995).Insecticides of dissimilar modes of action may have the same resistance mechanisms, i.e., decreased cuticular penetration, enhanced activity of mixed-function oxidases,esterases or cytochrome P450.
As M. separata has a strong mobility, migration can only slow but not completely prevent the development of resistance. It is important to timely monitor the changes in resistance of the pest and rotate the proposed chemicals in a scientific preventive manner. We propose emamectin benzoate, chlorantraniliprole, and beta-cypermethrin as effective insecticides. In order to postpone the development of resistance, a more reasonable integrated pest management strategy should be developed. At the same time, new insecticides of different modes of actions or no cross-resistance should be alternately used between emigration and immigration regions to slow down the resistance development and extend the working life of insecticides.
5. Conclusion
The present study demonstrates that the M. separata populations in five regions of Shaanxi and Shanxi provinces had various degrees of resistance to six insecticides, except emamectin benzoate. Compared with Lab-SS population,five field populations except the population of SY showed moderate to high levels of resistance to phoxim, ranging from 19.367- to 70.100-fold. Pair-wise correlation analysis among different insecticides indicated that chlorpyrifos had a positive and significant correlation with emamectin benzoate. Chlorantraniliprole had no significant correlation with emamectin benzoate, chlorpyrifos or phoxim. Our work found a widespread reduction in phoxim efficacy in controlling M. separata field populations. Implementing resistance management strategies to reduce the potential of progressive resistance development has a degree of urgency in light of these results.
Acknowledgements
The study was supported by the Special Fund for Agroscientific Research in the Public Interest of China (201403031)and the Research Project Program of Agricultural Science and Technology innovation Transformation in Shaanxi Province, China.
Abbott S W. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Ahmad M, Arif I, Ahmad M. 2007. Occurrence of insecticide resistance in field populations of Spodoptera litura(Lepidoptera: Noctuidae) in Pakistan. Crop Protection, 26,809–817.
Bi F C, Zhao J P. 2003. The outline of activities of emamectin benzoate against major insects of China. Modern Agrochemicals, 2, 34–36. (in Chinese)
Clark J K, Scott J G, Campos F, Bloomquist J R. 1995. Resistance to avermectins: Extent, mechanisms, and management implications. Annual Review of Entomology, 40, 1–30.
Che W N, Shi T, Wu Y D, Yang Y H. 2013. Insecticide resistance status of field populations of Spodoptera exigua(Lepidoptera: Noctuidae) from China. Journal of Economic Entomology, 106, 1855–1862.
Chen J Q, Huang S J, Qiu G H, Guan D Y, Qin W J, Chen Q,Qin H G. 2014. Comparison of susceptibility of Plutella xylostella to nine insecticides in different areas of Jiangxi Province. Acta Agriculturae Jiangxi, 26, 38–41. (in Chinese)
Daly J C. 1993. Ecology and genetics of insecticide resistance in Helicoverpa armigera: interactions between selection and gene flow. Genetica, 90, 217–226.
Dong J, Liu X X, Yue J, Qiao Y, Chu Y N, Wang P S, Zhang Q W. 2014. Resistance of Mythimna separata (Lepidoptera:Noctuidae) to five different types of insecticides in Beijing.Chinese Journal of Pesticide Science, 16, 687–692. (in Chinese)
Feng H Q, Zhao X C, Wu X F, Bo W, Wu K M, Cheng D F. 2008. Autumn migration of Mythimna separata(Lepidoptera: Noctuidae) over the Bohai Sea in northern China. Environmental Entomology, 37, 774–781.
Huang J, Wu S F, Ye G Y. 2011. Evaluation of lethal effects of chlorantraniliprole on Chilo suppressalis and its larval parasitoid, Cotesia chilonis. Journal of Integrative Agriculture, 10, 1134–1138.
Ishtiaq M, Saleem M A, Razaq M. 2012. Monitoring of resistance in Spodoptera exigua, (Lepidoptera: Noctuidae) from four districts of the Southern Punjab, Pakistan to four conventional and six new chemistry insecticides. Crop Protection, 33, 13–20.
Jiang X F, Luo L Z, Zhang L, Sappington T W, Hu Y. 2011.Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological,hormonal, genetic, and molecular factors. Environmental Entomology, 40, 516–533.
Jiang Y Y, Li CH G, Zeng J, Liu J. 2014. Population dynamics of the armyworm in China: A review of the past 60 years’research. Chinese Journal of Pesticide Science, 51,890–898. (in Chinese)
Jiang X F, Zhang L, Chen Y X, Luo L Z. 2014. Novel features,occurrence trends and economic impact of the oriental armyworm, Mythimna separata (Walker) in China. Chinese Journal of Applied Entomology, 51, 1444–1449. (in Chinese)
Lahm G P, Cordova D, Barry J D. 2009. New and selective ryanodine receptor activators for insect control. Bioorganic and Medicinal Chemistry, 17, 4127–4133.
Lai T, Li J, Su J. 2011. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pesticide Biochemistry and Physiology, 101, 198–205.
Lai T, Su J. 2011. Assessment of resistance risk in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae)to chlorantraniliprole. Pest Management Science, 67,1468–1472.
Li X C, Degain B A, Harpold V S, Marcon P G, Nichols R L,Fournier A J, Naranjo S E, Palumbo J C, Ellsworth P C.2012. Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Management Science, 68, 83–91.
Metcalf R L. 1989. Insect resistance to insecticides. Pesticide Science, 26, 333–358.
Pu X, Yang Y, Wu S, Wu Y. 2010. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Management Science, 66, 371–378.
Saleem M, Hussain D, Ghouse G, Abbas M, Fisher S W.2015. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Protection, 79, 177–184.
Sang S, Shu B, Yi X, Liu J, Hu M, Zhong G. 2016. Crossresistance and baseline susceptibility of Spodoptera litura(Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Management Science, 72,922–928.
Sattelle D B, Cordova D, Cheek T R. 2008. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebrate Neuroscience, 8, 107–119.
Shen J L, Wu Y D. 1995. Resistance and Management of Helicoverpa armigera. China Agriculture Press, China. pp.25–88. (in Chinese)
Shi T. 2011. Monitoring of insecticide resistance, mechanism and inheritance of resistance to emamectin and cypermethrin in field populations of Spodoptera Exigua (Hübner). MSc thesis, Nanjing Agricultural University, Nanjing. (in Chinese)
Sial A A, Brunner J F, Doerr M D. 2010. Susceptibility of Choristoneura rosaceana (Lepidoptera: Tortricidae) to two new reduced-risk insecticides. Journal of Economic Entomology, 103, 140–146.
Wang J, Li B L, Wu J X, Xu X L. 2016. Effects of fluctuating temperature on the reproduction and metabolism of primary energy substances in Mythimna separata (Lepidoptera:Noctuidae). Acta Entomologica Sinica, 59, 917–924. (in Chinese)
Wang S Y, Yu Y, Liu Y J. 2012. Cross-resistance and biochemical resistance mechanisms of emamectinbenzoate resistant population of Frankliniella occidentalis.Acta Phytophylacica Sinica, 39, 159–165. (in Chinese)
Wang X L, Li X Y, Shen A D, Wu Y D. 2010. Baseline susceptibility of diamondback moth (Lepidoptera:Plutellidae) to chlorantraniliprole in China. Journal of Economic Entomology, 103, 843–848.
Wang X L, Wu Y D. 2012. High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. Journal of Economic Entomology, 105, 1019–1023.
Yang C L, Gong G J. 1992. Selection of resistance strains of armyworm in laboratory to deltamethrin and dipterex.Agrochemicals, 31, 7–8. (in Chinese)
Yang C L, Gong G J, Tan F J, You Z P. 1995. Preliminary studies on monitoring and mechanisms of the insecticide resistance in Mythimna separata. Plant Protection, 21, 2–5.(in Chinese)
Yu G J. 1987. Monitoring of the insecticide resistance in Mythimna separata. Journal of Nanjing Agricultural University, Suppl., 86–92. (in Chinese)
Zeng J, Jiang Y Y, Liu J. 2013. Analysis of the armyworm outbreak in 2012 and suggestions of monitoring and forecasting. Plant Protection, 39, 117–121. (in Chinese)
Zhang Y H, Zhang Z H, Jiang Y Y, Zeng J, Gao Y, Cheng D.2012. Preliminary analysis of the outbreak of the thirdgeneration armyworm Mythimna separata in 2012 in China.Plant Protection, 38, 1–8.
Zhao Z H, Cheng D F. 2016. Analysis of the outbreak and suggestions of the armyworm Mythimna separata in part areas of China. Seed Science and Technology, 34, 89–90.(in Chinese)
Zheng X S, Ren X B, Su J Y. 2011. Insecticide susceptibility of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) in China.Journal of Economic Entomology, 104, 653–658.
杂志排行
Journal of Integrative Agriculture的其它文章
- Characterisation of pH decline and meat color development of beef carcasses during the early postmortem period in a Chinese beef cattle abattoir
- Effects of constant and stage-specific-alternating temperature on the survival, development and reproduction of the oriental armyworm, Mythimna separata (Walker) (Lepidoptera: Noctuidae)
- Migratory flight of insect pests within a year-round distribution:European corn borer as a case study
- Effects of temperatures on the development and reproduction of the armyworm, Mythimna roseilinea: Analysis using an age-stage,two-sex life table
- ldentification of summer nectar plants contributing to outbreaks of Mythimna separata (Walker) (Lepidoptera: Noctuidae) in North China
- Analysis on the migration of first-generation Mythimna separata(Walker) in China in 2013
