Amide Alkaloids from the genus Piper:A Review of its Phytochemistry
2018-07-05WeiyuZhouMingBaiXiaoxiaoHuangShaojiangSong
Weiyu Zhou, Ming Bai, Xiaoxiao Huang*, Shaojiang Song*
Department of Natural Products Chemistry, Key Laboratory of Structure-Based Drug Design and Discovery, Ministry of Education, School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang 110016, PR China
1 Introduction
Plants have been the source of medicines for thousands of years and species of the genusPiperare among the important medicinal plants used in various systems of medicine [1]. The genus Piper belongs to the Piperaceae family and contains approximately 1000 species of herbs, shrubs, small trees and hanging vines[2]. Piper species such as Piper nigrum L. (pepper)and Piper methysticum (kava) are widely distributed in the tropical and subtropical regions of the world,which are used as food, spice and folk medicines on account of the physiological activities and thus possess a great commercial, economical and medicinal potential [3-5].
Numerous biologically active natural products,such as alkaloids, lignans, flavones, unsat -urated amides, aristolactams, monoterpenes, sesquiterpenes,ketones, propenylphenols, chalcones, long and short chain esters, arylpropanoids and aldehydes,were isolated from many Piper species through phytochemical investigations. Among the compounds,amide alkaloids are typical constituents in the genus Piper [6]. Generally speaking, the vast majority of the known amide alkaloids are based on monomeric and dimeric amide alkaloids.
This review was attempted to offer the timely and comprehensive information about chemical constituents of the genus Piper, to serve as a pointer to their diverse applications.
2 Research progress of amide alkaloids
The genus Piper belongs to the Piperaceae and has over 1000 species distributed in both hemispheres.Among them, the most well-known species arePiper nigrumL. (pepper) andPiper methysticum(kava).The ripened fruit of P.nigrum is the source of white pepper, while the unripened fruit of the same species is the source of black pepper. A narcotic beverage is produced from the roots of P.methysticum in Oceania.Several species of Piper are grown domestically as house plants for their foliage [2].
In the genus Piper, amide alkaloids are the most typical constituents [6]. The vast majority of the known amide alkaloids are based on monomeric and dimeric amide alkaloids. Among them, most of the monomeric amide alkaloids are classified into types on the basis of their chemical structure characteristics and skeletons, including piperidine-,pyridone-, pyrrolidine-, isobutylamine-, aristololactamtype. Furthermore, the great mass of dimeric amide alkaloids possess two representative structural types,viz. cyclobutane- and cyclohexene- type. So far,various amide alkaloids which have been isolated from the genus Piper are listed in Table 1.
2.1 Monomeric amide alkaloids
2.1.1 Piperidine-type amide alkaloids
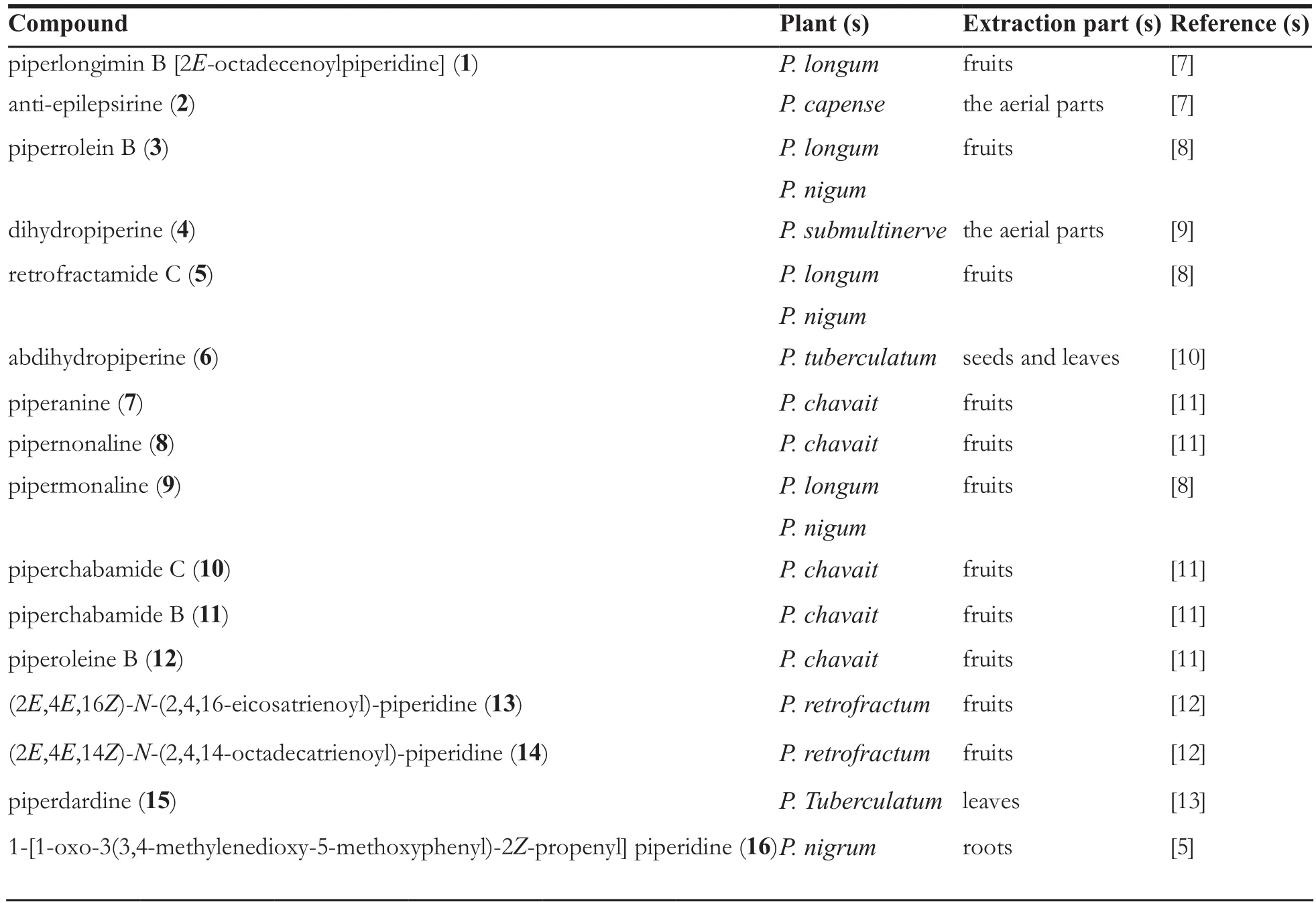
Table 1 Amide alkaloids from the genus Piper
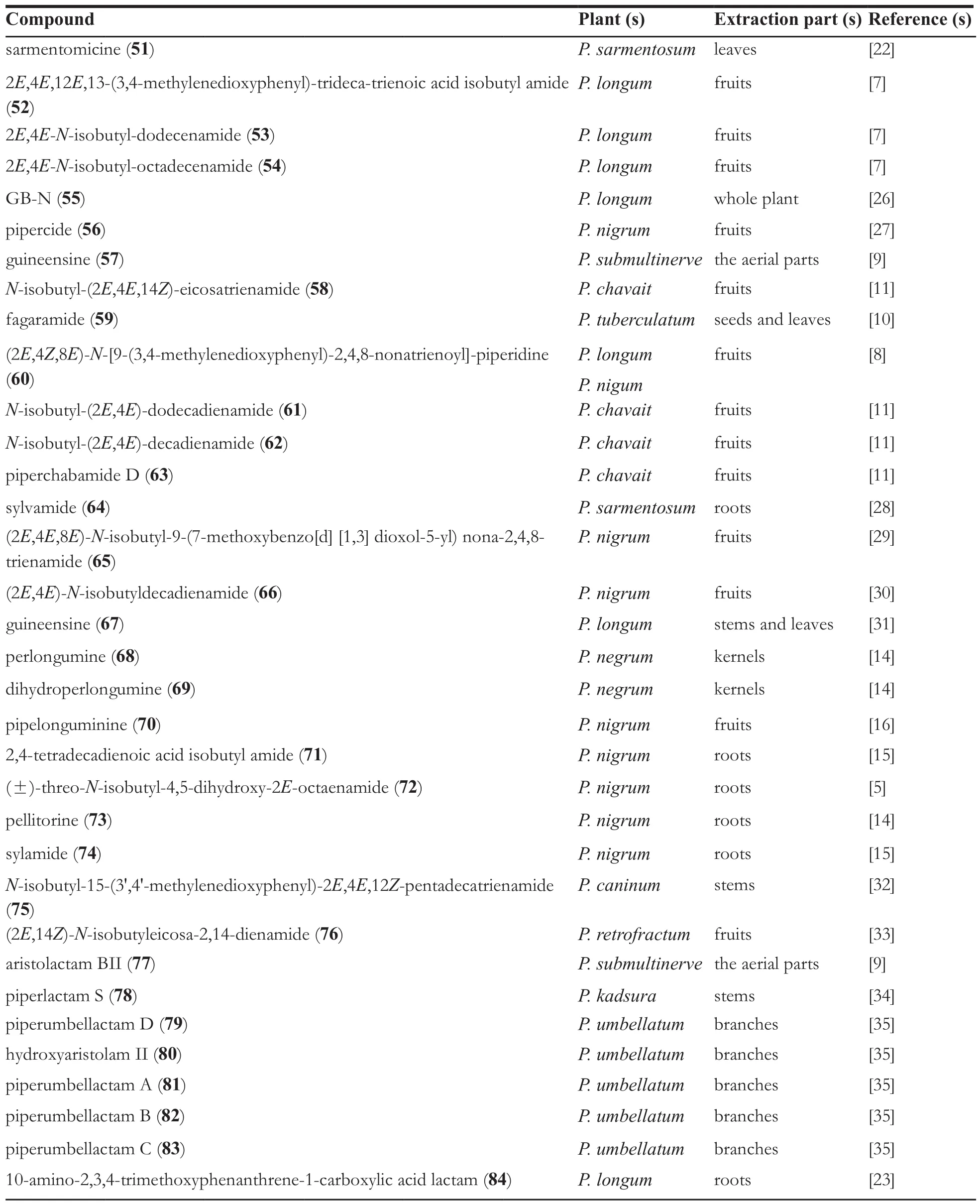
Continued table 1

Continued table 1
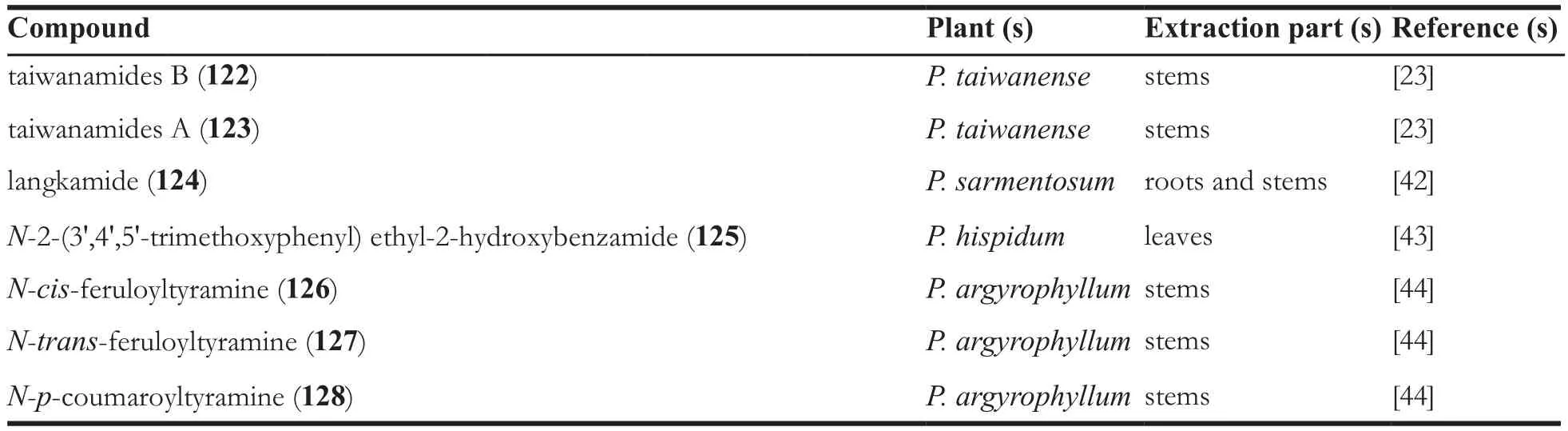
Continued table 1
About twenty-six piperidine-type amide alkaloids were isolated and identified from the genus Piper at present. They are piperlongimin B[2E-octadecenoylpiperidine] (1) [7], anti-epilepsirine(2) [7], piperrolein B (3) [8], dihydropiperine (4) [9],retrofractamide C (5) [8], abdihydropiperine(6) [10], piperanine (7) [11], pipernonaline (8) [11],pipermonaline (9) [8], piperchabamide C (10) [11],piperchabamide B (11) [11], piperoleine B (12) [11],(2E,4E,16Z)-N-(2,4,16-eicosatrienoyl)-piperidine(13) [12], (2E,4E,14Z)-N-(2,4,14-octadecatrienoyl)-piperidine (14) [12], piperdardine (15) [13], 1-[1-oxo-3(3,4-methylenedioxy-5-methoxyphenyl)-2Z-propenyl] piperidine (16) [5], dihydropiperine (17)[14], (E)-1-[3'-4'-(methylenedioxy)-cinnamoyl]piperidine (18) [15], chavicine (19) [16], piperanine(20) [16], piperine (21) [16], piperettine (22) [16],(±)-erythro-1-(1-oxo-4,5-dihydroxy-2E-decaenyl)-piperidine (23) [5], 1-(1,6-dioxo-2E,4E-decadienyl)-piperidine (24) [5], (±)-threo-1-(1-oxo-4,5-dihydroxy-2E-decaenyl) piperidine (25) [5], 1-[l-oxo-3(3,4-methylenedioxy-5-methoxyphenyl)-2Z- propenyl]piperidine (26) [5].
2.1.2 Pyridone-type amide alkaloids
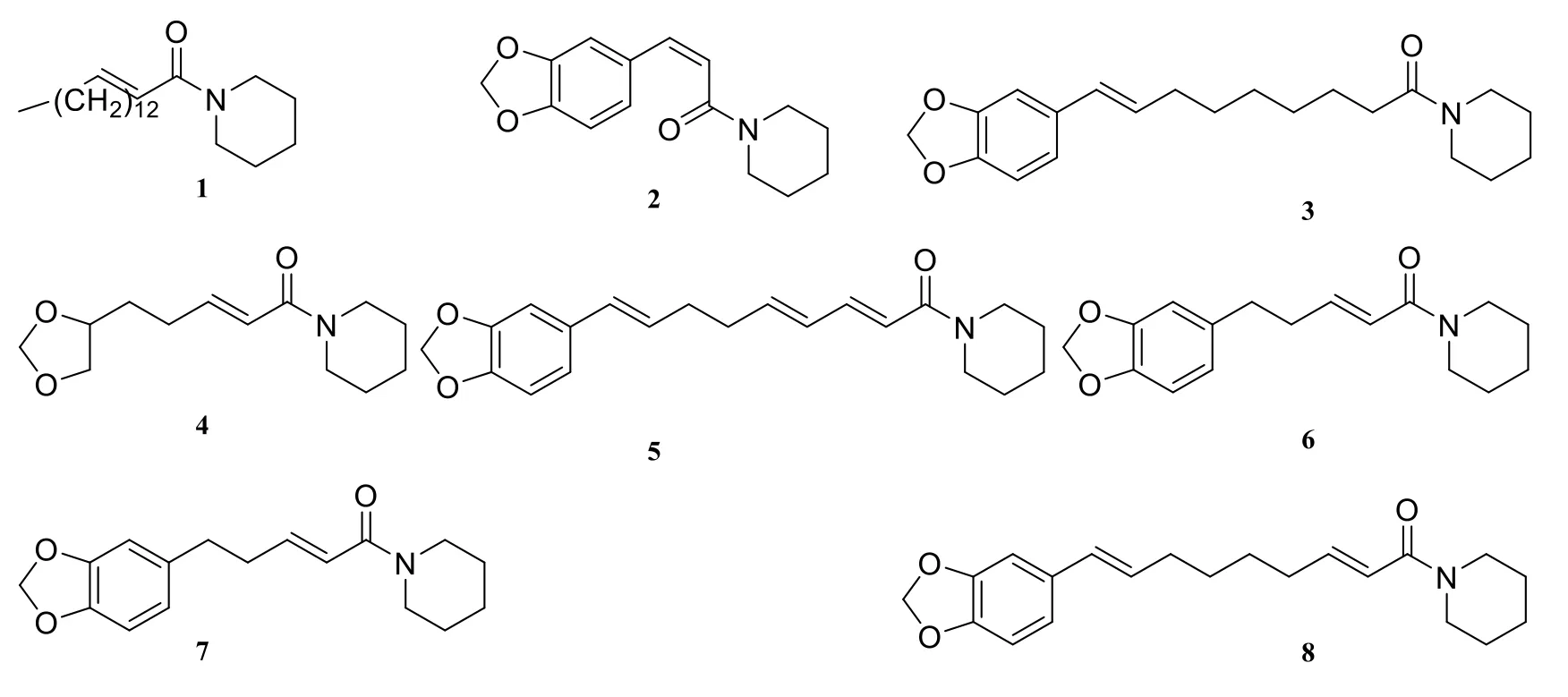
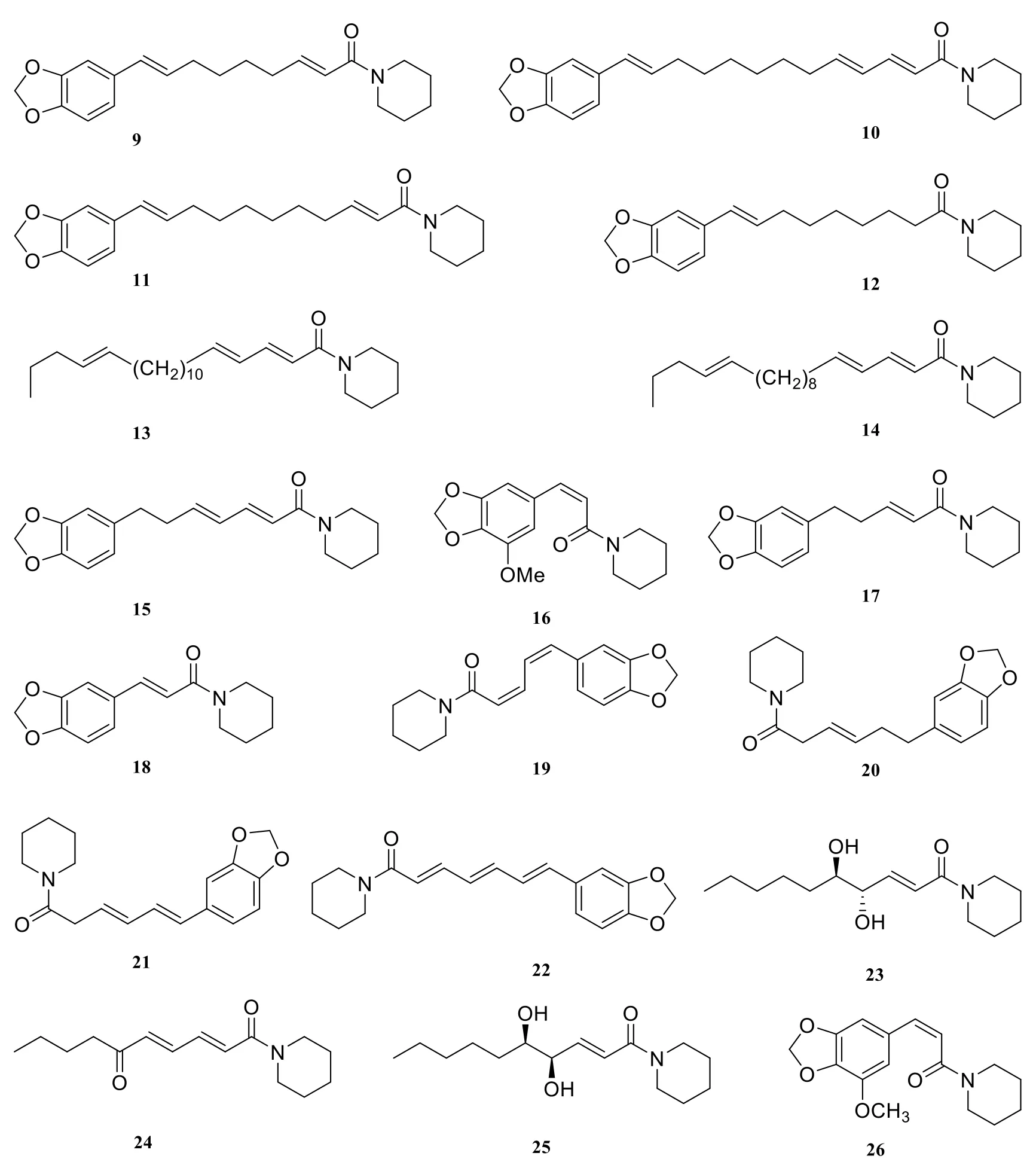
Fig. 1 Piperidine-type amide alkaloids in the genus Piper
At present, nine pyridone-type amide alkaloids were isolated from the genus Piper. They are piperme-thystine (27) [17], cis-piplartine (or 8(Z)-N-(12,13,14- trimethoxycinnamoyl)-pyridin-2-one) (28) [10], N-(3,4-dimethoxycinnamoyl)-Δ3-pyridin-2-one (29) [18], piplartine (30) [19],N-(3-methoxy-4,5-methylenedioxycinnamoyl)-Δ3-pyridin-2-one (31) [18], N-(3-methoxy-4,5-methylenedioxydihydrocinnamoyl)-Δ3-pyridin-2-one (32) [18], 3a,4a-epoxy-5b-piper-methystine(33) [20], awaine (34) [20], kaousine (35) [21].
2.1.3 Pyrrolidine-type amide alkaloids
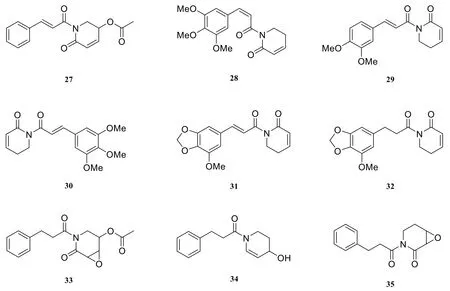
Fig. 2 Pyridone-type amide alkaloids in the genus Piper
There are about sixteen pyrrolidine-type amide alkaloids isolated and identified from the genus Piper, including pyrrolidine (36) [22],N-[10-(13,14-methylenedioxyphenyl)-7(E),9(E)-pentadienoyl]-pyrrolidine (37) [10], piperlotine E(38) [22], piperlotine D (39) [22], N-[10-(13,14-methylene- dioxyphenyl)-7(E)-pentaenoyl]-pyrrolidine(40) [10], piperlotine C (41) [22], piperlotine B(42) [22], N-[10-(13,14-methylenedioxyphenyl)-7(E),9(Z)-pentadienoyl]-pyrrolidine (43) [10],piperlotine A (44) [22], 1-(m-methoxycinnamoyl)pyrrolide (45) [23], 1-cinnamoyl pyrrolidine(46) [23], piperyline (47) [16], N-cinnamoyl-pyrrolidine(48) [23], peepuloidin (49) [24], 1-[1-oxo-5(3,4-methylenedioxyphenyl)-2Z,4E-pentadienyl] pyrrolidine (50)[25], sarmentomicine (51) [22].
2.1.4 Isobutylamine-type amide alkaloids
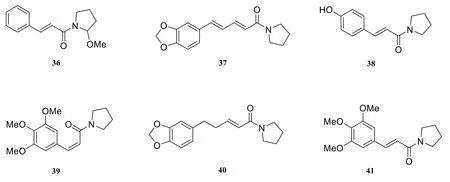
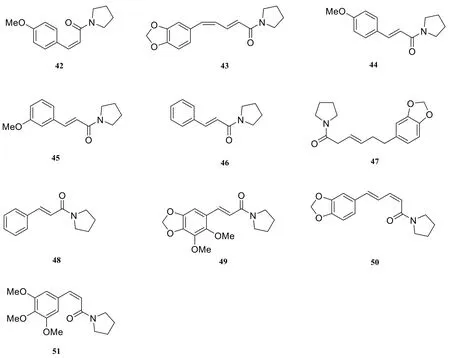
Fig. 3 Pyrrolidine-type amide alkaloids in the genus Piper
It is reported that there are a number of isobutylamine-type amide alkaloids in the genus Piper, they are 2E,4E,12E,13-(3,4-methylenedioxyphenyl)-trideca-trienoic acid isobutyl amide (52) [7], 2E,4E-N-isobutyl-dodecenamide(53) [7], 2E,4E-N-isobutyl-octadecenamide(54) [7], GB-N (55) [26], pipercide (56) [27],guineensine (57) [9], N-isobutyl-(2E,4E,14Z)-eicosatrienamide (58) [11], fagaramide (59) [10],(2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)-2,4,8-nonatrienoyl]-piperidine (60) [8], N-isobutyl-(2E,4E)-dodecadienamide (61) [11], N-isobutyl-(2E,4E)-decadienamide (62) [11], piperchabamide D (63) [11], sylvamide (64) [28], (2E,4E,8E)-N-isobutyl-9-(7-methoxybenzo [d] [1,3] dioxol-5-yl)nona-2,4,8-trienamide (65) [29], (2E,4E)-N-isobutyldecadienamide (66) [30], guineensine(67) [31], perlongumine (68) [14], dihydroperlongumine (69) [14], pipelonguminine (70) [16],2,4-tetradecadienoic acid isobutyl amide (71) [15],(±)-threo-N-isobutyl-4,5-dihydroxy-2E-octaenamide(72) [5], pellitorine (73) [14], sylamide (74) [15],N-isobutyl-15-(3',4'-methylenedioxyphenyl)-2E,4E,12Z-pentadecatrien amide (75) [32], (2E,14Z)-N-isobutyleicosa-2,14-dienamide (76) [33].
2.1.5 Aristololactam-type amide alkaloids
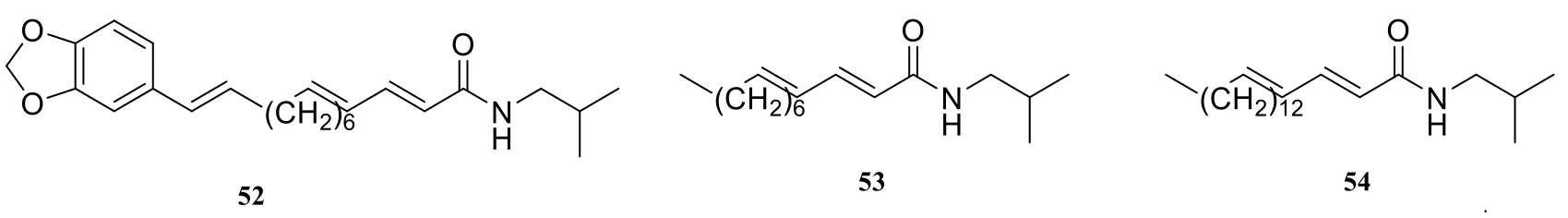
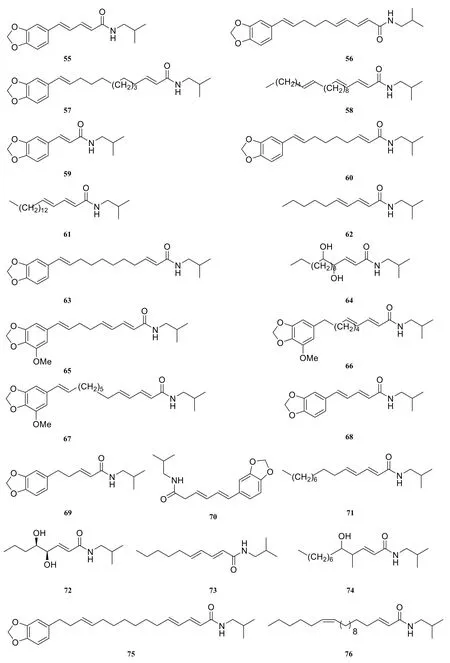
Fig. 4 Isobutylamine-type amide alkaloids in the genus Piper
Till now, thirteen aristololactam-type amide alkaloids were reported in the genus Piper, including aristolactam BII (77) [9], piperlactam S (78) [34],piperumbellactam D (79) [35], hydroxyaristolam II (80)[35], piperumbellactam A (81) [35], piperumbellactam B (82) [35], piperumbellactam C (83) [35], 10-amino-2,3,4-trimethoxyphenanthrene-1-carboxylic acid lactam (84) [23], cepharadione A (85) [36],cepharanone B (86) [37], (Z)-(E)-N-formouragines(87) [38], (Z)-(E)-formylnornuciferines (88) [38],piperolactam D (89) [39].
2.2 Dimeric amide alkaloids
2.2.1 Cyclobutane-type amide alkaloids
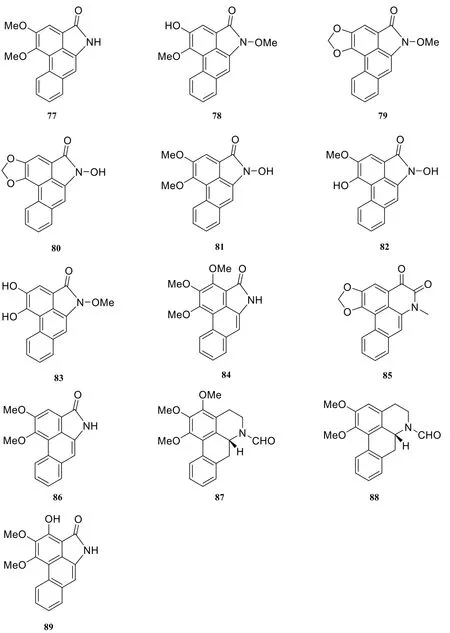
Fig. 5 Aristololactam-type amide alkaloids in the genus Piper
About seven cyclobutane-type amide alkaloids were isolated and identified from the genus Piper at present. They are piplartine dimer A (90) [18],nigramide T (91) [40], nigramide S (92) [40],nigramide Q (93) [40], nigramide R (94) [40],dipiperamides F (95) [33], dipiperamides G(96) [33].
In 2004, four new dimeric alkaloids (91-94)possessing a cyclobutane ring were isolated from the root of Piper nigrum by Kun [16] et al. In 2015, two new dimeric alkaloids (95-96) of the cyclobutane type were isolated from the fruit of Piper nigrum.
2.2.2 Cyclohexene-type amide alkaloids
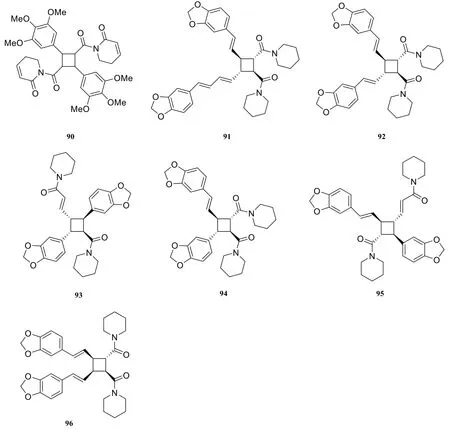
Fig. 6 Cyclobutane-type amide alkaloids in the genus Piper
About seventeen piperidine-type amide alkaloids were isolated and identified from the genus Piper at present. They are nigramide P (97) [40],nigramide O (98) [40], nigramide L (99) [40], nigramide K (100) [40], nigramide N (101) [40], nigramide M (102) [40], nigramide J (103) [40], nigramide D(104) [40], nigramide H (105) [40], nigramide B(106) [40], nigramide I (107) [40], nigramide F (108)[40], nigramide A (109) [40], nigramide C (110) [40],nigramide G (111) [40], nigramide E (112) [40],chabamine (113) [33].
In 2004, sixteen new dimeric alkaloids (97-112)of the cyclohexene type were isolated from the root of Piper nigrum by Kun [16] et al.
2.3 Other amide alkaloids
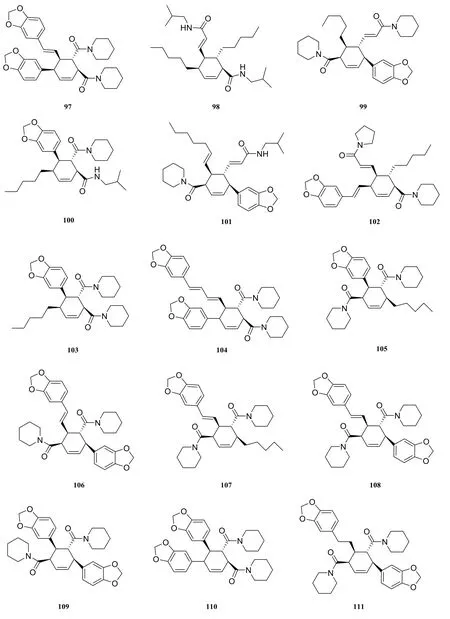
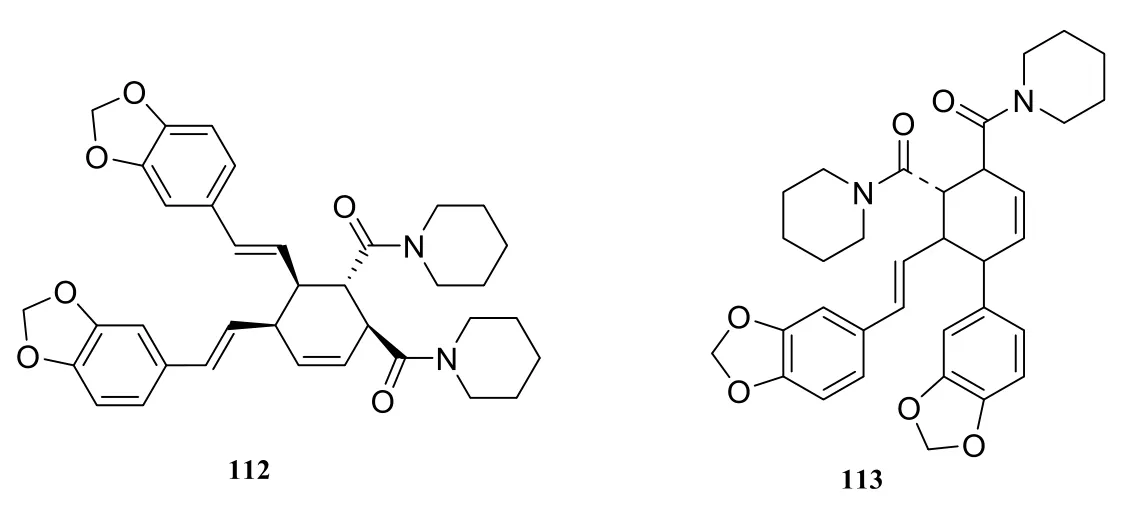
Fig. 7 Cyclohexene-type amide alkaloids in the genus Piper
In addition to different types of amide alkaloids stated above, there are also some other amide alkaloids, they are pipyahyine (114) [41],N-benzylcinnamide (115) [9], submultinamide A(116) [10], arboreumine (117) [10], retrofractamide B (118) [11], retrofractamide A (119) [11],piperchabamide E (120) [11], piperlotine J(121) [22], taiwanamides B (122) [23], taiwanamides A (123) [23], langkamide (124) [42], N-2-(3',4',5'-trimethoxyphenyl)ethyl-2-hydroxybenzamide(125) [43], N-cis-feruloyltyramine (126) [44], N-transferuloyltyramine (127) [44], N-p-coumaroyltyramine(128) [44].
3 Conclusion
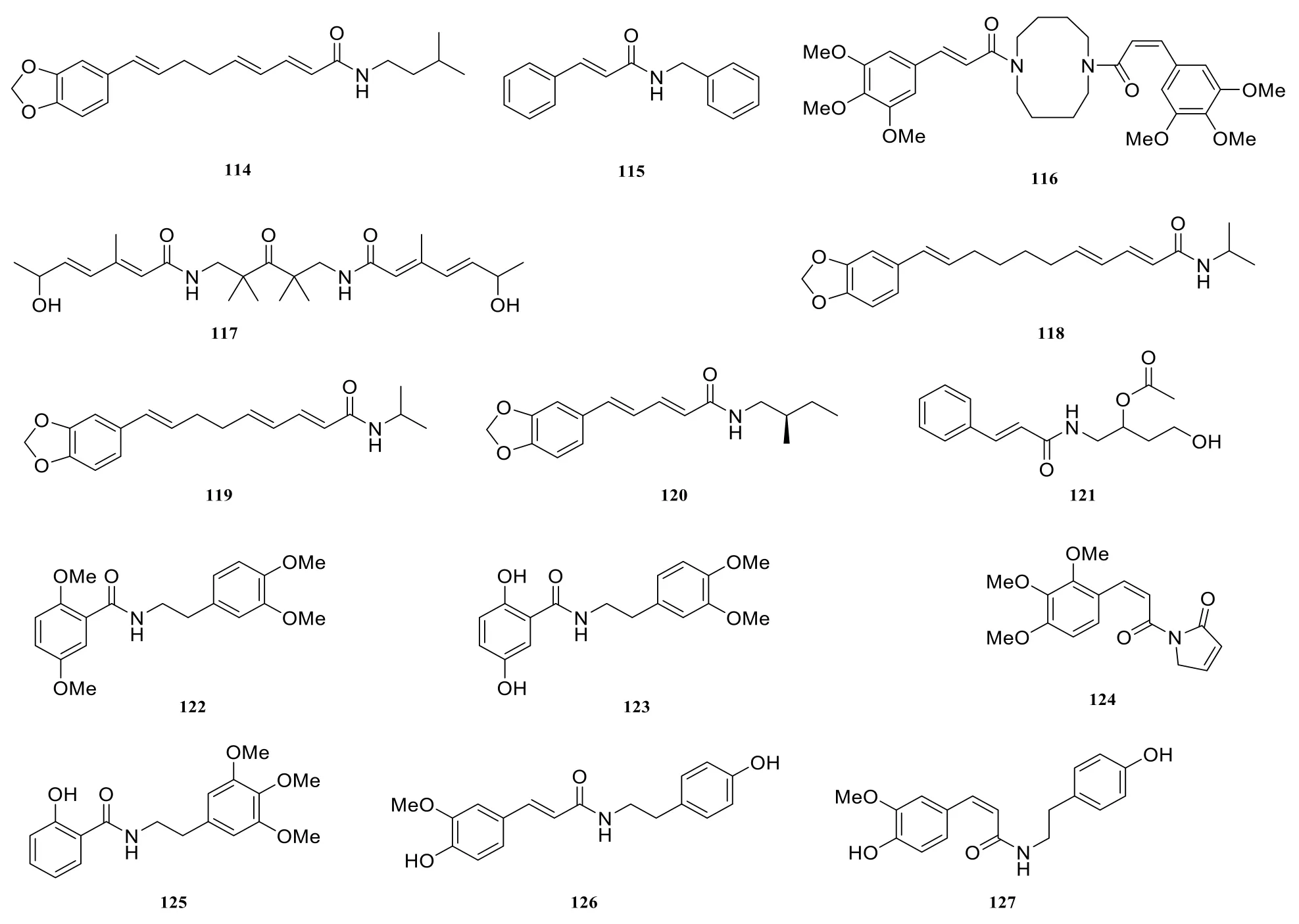
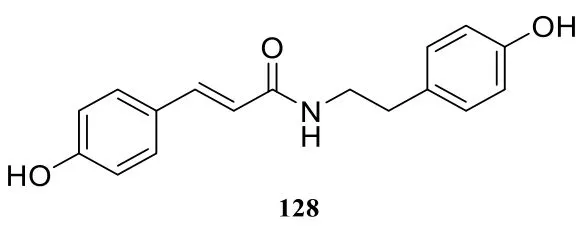
Fig. 8 Other amide alkaloids in the genus Piper
Phytochemical investigations showed that the constituents of the Piper genus included alkaloids,lignans, flavones, unsaturated amides and other constituents. This paper summarizes that the most significant chemical constituents of this genus are amide alkaloids and their different types on the basis of their chemical structure characteristics and skeletons which lead to distinct pharmacological activities. In this way, we can establish the corresponding relationship between chemical structures and pharmacological effects better. It’s reported that these compounds have different degrees of antioxidant, antitumor, anti-inflammatory,antihypertensive, antidepressant, antifungal,antibacterial, immuno-modulatory and hepatoprotective activities [45]. This paper has certain reference significance for the further development and utilization of the genus Piper.
Reference
[1] Hwang KS, Kim YK, Park KW, et al. Piperolein B and piperchabamide D isolated from black pepper[Piper nigrum L.] as larvicidal compounds against the diamondback moth [Plutella xylostella]. Pest Management Science, 2017, 73: 1564-1567.
[2] Simpson BB, Ogorzaly MO. Economic Botany: plants in our world. 2nd edn. New York: McGraw-Hill, 1995, 742.
[3] Kirtikar KR, Basu BD. Indian Medicinal Plants (Vol. III),1933: 2128.
[4] Siddiqui BS, Gulzar T, Mahmood A, et al. New Insecticidal Amides from Petroleum Ether Extract of Dried Piper nigrum L. Whole Fruits. Cheminform, 2005, 36: 1349-1352.
[5] Wei K, Li W, Koike K, et al. New amide alkaloids from the roots of Piper nigrum. Journal of Natural Products, 2004,67: 1005-1009.
[6] Parmar VS, Jain SC, Bisht KS, et al. Phytochemistry of the genus Piper. Phytochemistry, 1997, 46: 597-673.
[7] Ghosal S, Deb A, Mishra P, et al. Leishmanicidal compounds from the fruits of Piper longum. Planta Medica, 2012, 78: 906-908.
[8] Lee SW, Rho M, Park HR, et al. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. Journal of Agricultural and Food Chemistry, 2006, 54: 9759-9763.
[9] Nobsathian S, Tuchinda P, Soorukram D, et al. A new conjugated amide-dimer from the aerial parts of Piper submultinerve. Natural Product Research, 2012, 26: 1824-1830.
[10] Mujumdar AM, Dhuley JN, Deshmukh VK, et al. Antiinflammatory activity of piperine. Japanese journal of medical science & biology, 2010, 43: 95-100.
[11] Matsuda H, Ninomiya K, Morikawa T, et al. Protective effects of amide constituents from the fruit of Piper chaba on d-galactosamine/TNF-α-induced cell death in mouse hepatocytes. Bioorganic & Medicinal Chemistry Letters,2008, 18: 2038-2042.
[12] Jongwoong A, Mija A, Okpyo Z, et al. Piperidine alkaloids from Piper retrofractum fruits. Phytochemistry, 1992, 31:3609-3612.
[13] De Araujojunior JX, Dacunha EV, Chaves MC, et al. Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry, 1997, 44: 559-561.
[14] Scott IM, Puniani E, Jensen HR, et al. Analysis of piperaceae germplasm by HPLC and LC/MS: A method for isolating and identifying unsaturated amides from Piper spp extracts. Journal of Agricultural and Food Chemistry,2005, 53: 1907-1913.
[15] Ee GC, Lim CM, Lim CK, et al. Alkaloids from Piper sarmentosum and Piper nigrum. Natural Product Research, 2009, 23: 1416-1423.
[16] Friedman M, Levin CE, Lee S, et al. Analysis by HPLC and LC/MS of Pungent Piperamides in Commercial Black,White, Green, and Red Whole and Ground Peppercorns.Journal of Agricultural and Food Chemistry, 2008, 56:3028-3036.
[17] Teschke R, Qiu SX, Xuan TD, et al. Kava and kava hepatotoxicity: requirements for novel experimental,ethnobotanical and clinical studies based on a review of the evidence. Phytotherapy Research, 2011, 25: 1263-1274.
[18] Duh C, Wu YC, Wang S, et al. Cytotoxic pyridone alkaloids from the leaves of Piper aborescens. Journal of Natural Products, 1990, 53: 1575-1577.
[19] Bezerra DP, De Castro FO, Alves AP, et al. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. Journal of Applied Toxicology, 2008, 28:156-163.
[20] Dragull K, Yoshida WY, Tang C, et al. Piperidine alkaloids from Piper methysticum. Phytochemistry, 2003, 63: 193-198.
[21] Kaou AM, Mahiou-Leddet V, Canlet C, et al. New amide alkaloid from the aerial part of Piper capense, L.f.(Piperaceae). Fitoterapia, 2010, 81: 632-635.
[22] Li CY, Tsai W, Damu AG, et al. Isolation and identification of antiplatelet aggregatory principles from the leaves of Piper lolot. Journal of Agricultural and Food Chemistry,2007, 55: 9436-9442.
[23] Chen I, Chen Y, Liao C, et al. Amides with anti-platelet aggregation activity from Piper Taiwanense. Fitoterapia,2007, 78: 414-419.
[24] Dhar KL, Shah S, Prakhakar A, et al. New pyrrolidinamide dimers from Piper peepuloides. Fitoterapia, 1995, 66: 390-392.
[25] Wei K, Li W, Koike K, et al. Nigramides A—S, Dimeric Amide Alkaloids from the Roots of Piper nigrum. Journal of Organic Chemistry, 2005, 70: 1164-1176.
[26] Bao L, Bai S, Borijihan G, et al. Hypolipidemic effects of a new piperine derivative GB-N from Piper longum in highfat diet-fed rats. Pharmaceutical Biology, 2012, 50: 962-967.
[27] Yang Y, Lee S, Lee H, et al. A piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. Journal of Agricultural and Food Chemistry, 2002, 50: 3765-3767.
[28] Ee GC, Lim CM, Lim CK, et al. Alkaloids from Piper sarmentosum and Piper nigrum. Natural Product Research, 2009, 23: 1416-1423.
[29] Ngo QM, Tran PT, Tran MH, et al. Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO-1 Pathway. Phytotherapy Research, 2017,31: 663.
[30] Ohigashi H, Nishimuro S, Koshimizu K. Larva-Development Inhibitors of Black Pepper(Commemoration Issue Dedicated to Professor Yuzo Inouye on the Occasion of his Retirement). Bulletin of the Institute for Chemical Research Kyoto University, 1983,61.
[31] Parmar VS, Jain SC, Gupta S, et al. Polyphenols and alkaloids from Piper species. Phytochemistry, 1998, 49:1069-1078.
[32] Wan S, Ahmad F, Yen K. Chemical constituents from Piper caninum and antibacterial activity. Journal of Applied Pharmaceutical Science, 2015, 5: 020-025.
[33] Muharini R, Liu Z, Lin W, et al. New amides from the fruits of Piper retrofractum. Tetrahedron Letters, 2015,56: 2521-2525.
[34] Terreaux C, Gupta MP, Hostettmann K, et al. Antifungal benzoic acid derivatives from Piper Dilatatum in honour of Professor G. H. Neil Towers 75th birthday.Phytochemistry, 1998, 49: 461-464.
[35] Tabopda TK, Ngoupayo J, Liu J, et al. Bioactive aristolactams from Piper umbellatum. Phytochemistry,2008, 69: 1726-1731.
[36] Jaggy H, Achenbach H. Cepharadione A from Piper methysticum. Planta Medica, 1992, 58: 111.
[37] Desai SJ, Prabhu BR, Mulchandani NB, et al. Aristolactams and 4,5-dioxoaporphines from Piper longum.Phytochemistry, 1988, 27: 1511-1515.
[38] Prasad AK, Kumar V, Arya P, et al. Investigations toward new lead compounds from medicinally important plants.Pure and Applied Chemistry, 2005, 77: 25-40.
[39] Shi Y, Liu F, Jacob MR, et al. Antifungal Amide Alkaloids from the Aerial Parts of Piper flaviflorum and Piper sarmentosum. Planta Medica, 2016: 143-150.
[40] Dragull K, Yoshida WY, Tang C, et al. Piperidine alkaloids from Piper methysticum. Phytochemistry, 2003, 63: 193-198.
[41] Scott IM, Jensen HR, Philogene BJ, et al. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochemistry Reviews, 2007, 7: 65-75.
[42] Bokesch HR, Gardella RS, Rabe DC, et al. A New Hypoxia Inducible Factor-2 Inhibitory Pyrrolinone Alkaloid from Roots and Stems of Piper sarmentosum. Chemical &Pharmaceutical Bulletin, 2011, 59: 1178-1179.
[43] Ruiz C, Haddad M, Alban J, et al. Activity-guided isolation of antileishmanial compounds from Piper hispidum.Phytochemistry Letters, 2011, 4: 363-366.
[44] Singh SK, Ashok PK, Olsen CE, et al. Neolignans and alkaloids from Piper argyrophylum. Phytochemistry, 1996,43: 1355-1360.
[45] Sanjay Kumar Chelak. Preformulation and Formulation Study of Anticancer Principle of Piperine. World Journal of Pharmaceutical Research. 2015, 4: 722-737.
杂志排行
Asian Journal of Traditional Medicines的其它文章
- Screening of potential active compounds in Eurycoma longifolia against hepatic carcinoma by Network pharmacology
- Molecular targets of Gelsemium elegans: a study of hepatoma based on network pharmacology
- Research review on the main chemical constituents and pharmacological effects of the samara derived from Ailanthus altissima (Mill.) Swingle
