Source and yearly distribution of PAHs in the snow from the Hailuogou glacier of Mountain Gongga,China
2018-07-04ChaoqiYuMeihanLiYinlingCaoXianHeHongZhouTingtingZhangChongyingLi
Chaoqi Yu•Meihan Li•Yinling Cao•Xian He•Hong Zhou•Tingting Zhang•Chongying Li
1 Introduction
Polycyclic aromatic hydrocarbons(PAHs)and their derivatives are associated with the incomplete combustion of organic material,arising from episodes of volcanic eruptions,forest fires(Bjorseth and Ramdahl 1985),and from human activities of burning fossil fuels(Baek et al.1991).Because of their inertness and volatility,PAHs can travel long distances in the air and be distributed in different environmental media,leading to widespread pollution(Wang et al.2006).
In high latitude or high-altitude areas,PAHs are transferred and trapped in the ice and snow via wet atmospheric deposition(rain,snow,etc).Over time,significant amounts of PAHscanbeaccumulated insuchlocations.Wheniceand snow melt as a result of seasonal temperature changes,the accumulated PAHs will be released to other environmental compartmentssuch assurrounding water,soil,and air,thus causing adetrimental impact to theecosystem of theregion.The general trend of global warming is making this dire situationeven morepressing.Therefore,itisvery important to investigate PAHs in ice and snow from glacier(Li et al.2010)and study theirorigin,distribution,and transport.Until now,research on organic pollutants,particularly PAHs in snow and ice,have been mainly focused on the north and southpoles(Kangetal.2012;Herbertetal.2005;Gregorand Gummer 1989;Halsall 2004;Jaffrezo et al.1993),Greenland(Jaffrezo etal.1994),and the Alpsregion(Carreraetal.2001;Villaet al.2006;Finizo et al.2006).Studiesof PAHs in the Qinghai Tibet Plateau region started fairly late and publications on the topic are still scarce(Li et al.2010)although the sediment core and road dusts were carefully studied(Han etal.2015;Weietal.2015).Wangetal.(2008)studied levels and distribution of organochlorine pesticides and PAHsin iceand snow from the Dasuopu glacier.Wang et al.(2007)reported concentrations of organochlorine pesticidesin new snow samplesat four different altitudesin east Rongbuk glacier of the Everest region and studied their correlationswith altitude.Lietal.(2010)investigated on the distribution and source of the PAHs in ice and snow from four glaciersincluding the Qilian Mountain Qiyiglacier and the Tanggula Dongkemadiglacier.More recently,Yu et al.(2014)studied thedistributionand thesourceof the PAHsin snow over a short period in the Hailuogou glacier,Mt.Gongga.
Inthiswork,snow samplesfromthe Hailuogou glacier of Mt.Gongga in China were collected over a 3-year period from 2012 to 2014,and their concentrations were analyzed for 16PAHs.Themainpurposeof thispreliminarystudywas to determine the levels of PAHs in this glacier and try to identify the distribution and source of these PAHs and‘to estimate their transport distance from origin.
1.1 Sample collection
In January of each year,three snow sampleswere collected from Hailuogou for a total of nine samples over the 3-year period from 2012 to 2014.The sampling sitesare shown in Fig.1.The thickness of snow cover was always greater than 25 cm.Snow samples were collected with a clean stainless-steel shovel and packed in a 10-L clean aluminum drum which was sealed with three layers of aluminum paper.The sample information is given in Table 1.The amount of each sample was equivalent to 3–4 L of water.
2 Experimental
2.1 Sample pretreatment
A solid phasemembraneextraction method wasadopted for the enrichment of PAHsfrom snow samples.The C18solid phase extraction disks membrane(Supelco Analytical,diameter 47 mm)was fixed on a sand core suction filter device.The membrane was activated by passing through 5.0 mL of cyclohexane,n-hexane,methanol,and purewater each in sequence.All of the organic reagents used were HPLC grade.The snow sample was melted at room temperature and the upper clear liquid was loaded on the activated C18membrane.The flow rate was regulated between 12 and 30 mL·min-1.The eluent was discarded,and 5.0 mL of n-hexane was then added to the C18membrane and let soaking for 10 min before being eluted.This step was repeated three times.The combined eluent was passed through a chromatography column filled with anhydrous sodium sulfate(activated in a muffle furnace at 400°C for 24 h),and the volumewasfurther reduced to 1.0 mL under astream of high purity nitrogen.A procedureblank(3 L of pure water)was processed along with the snow samples.
2.2 Sample analysis
2.2.1 Reference standards and reagents
Sixteen certified PAHs standards were purchased from AccuStandard(USA),including Naphthalene(Nap),Acenaphthylene(Ace),Acenaphthene(Acp),Fluorene(Fle),Phenanthrene(Phe),Anthracene(Ant),Fluoranthene(Fla),Pyrene(Pyr),Benzo(a)anthracene(BaA),Chrysene(Chry),Benzo(b)fluoranthene (BbF),Benzo [k]fluoranthene(BkF),Benzo[a]pyrene(BaP),Indeno[1,2,3-cd]pyrene(InP),Dibenz[a,h]anthracene(DahA),and Benzo(g,h,i)-perylene(BghiP).Individual stock solutions at concentrations of 100.00 μg·mL-1each were prepared in dichloromethane–acetone 50:50(v:v).The working standard solutions were prepared by mixing each of the PAH stock solutions and diluting with dichloromethane–acetone 50:50 for a final concentration of 2.00 μg·mL-1each.
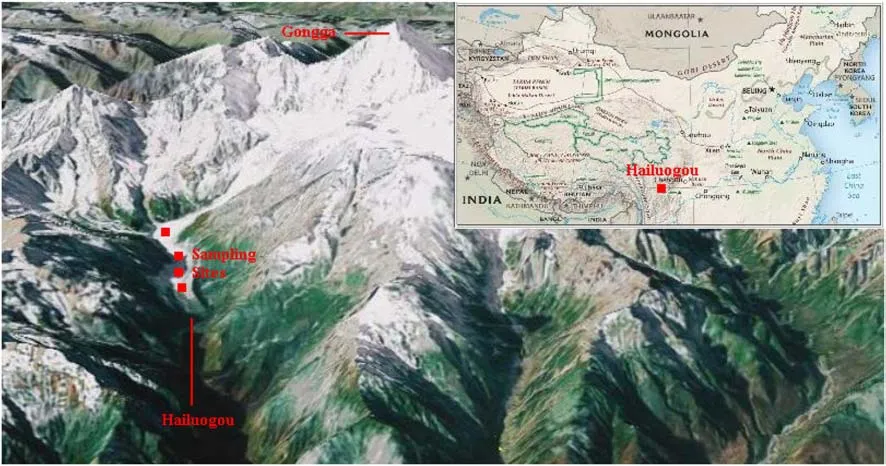
Fig.1 Map of sampling sites
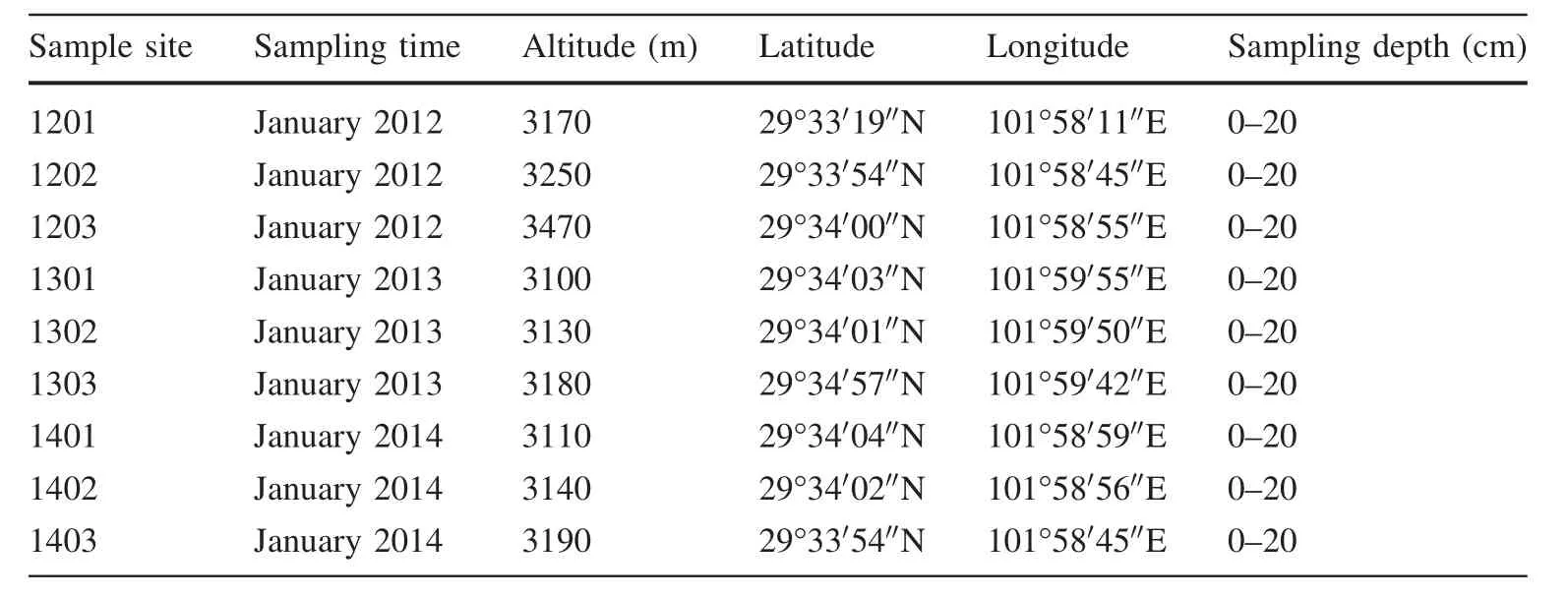
Table 1 Sample information
All organic solventswere HPLCgradefrom Changzheng Chemical Reagent Co.Ltd.(Chengdu,China).Milli-Qwater was used as pure water.Unless otherwise stated,all the reagentsused inthisstudy wereof analytical gradeor higher.
2.2.2 GC–MSconditions
Analyses were performed using a 7890-5975 Gas Chromatography–Mass Spectrometer(GC–MS)(Agilent Technologies,Santa Clara,CA)equipped with an autosampler(Triplus Co.USA).Separations were facilitated using an HP-5MS analytical column,30 m×0.25 mm×0.25μm(SN:USB439554H,Agilent Technologies).
Thecarrier gaswashelium(99.999%purity)with aflow rate of 2.0 mL·min-1at 164.6 kPa.Injections were made in the splitless mode with an injection volume of 2.00μL.The injector temperature was 290°C.The temperature program was as follows:holding initial temperature at 100 °C for 1 min,ramping to 240 °C at 10 °C·min-1(linear),holding for 5.0 min,ramp to 280°C at 20 °C·min-1(linear)and holding for 8.0 min.
The mass spectrometry measurements were carried out through an electron impact(EI,70 eV,230°C)coupled with a full scan mode.Other parameters included scanning range of 0–500 amu,transmission temperature of 150 °C and solvent delay time of 2.0 min.
3 Results and discussion
3.1 Qualification of the method
The analysis was performed using a five-point standard calibration curve.Linear correlation coefficients of the 16 PAHs varied from 0.9975 to 0.9998.Recoveries and relative standard deviations were 75.9%–99.2%and 2.8%–15.8%,respectively.Detection limits were ranged from 0.001 to 0.010 μg·L-1.
3.2 Distribution of PAHs in sample area
The results for individual 16 PAHs over the 3-year period from 2012 to 2014 are listed in Tables 2,3 and 4 of Appendix 1,and they are plotted in Fig.2.The amount of individual PAHs varies widely,ranging from non-detectable(DahA and BghiP)to~100 ng·L-1(Phe).Among the 16 PAHs that are reported here,nine PAHs(Nap,Ace,Acp,Fle,Phe,Fla,Pyr,BaA,BkF)were highest for the year 2012,four(Chry,BbF,BaP,INP)for 2013 and one(Ant)for 2014.This change in dominance possibly indicates a change in the source of PAHs over the 3-year period.
The total concentration of the 16 measured PAHs was 452 ± 31 ng·L-1for 2012,305 ± 54 ng·L-1for 2013 and 290 ± 30 ng·L-1for 2014(Fig.3),seemingly suggesting a downward trend which would be in synchronization with the Chinese government’s environmental protection policies installed in energy-saving and emission-reduction.Although the results of year 2014 cannot be considered as significantly different from thoseof year 2013,thefact that apart from 2 exceptions,all other PAHs are lower in 2014 seems suggesting the downward trend of PAHs emission.Regardless of the trend,measured PAH concentrations are still much higher in the Hailuogou glacier in comparison to those from the Qinghai-Tibet Plateau glacier between 20.45 and 60.57 ng·L-1(Li et al.2010)and some remote mountains in Europe between 5.6 and 81 ng·L-1(Carrera et al.2001).
3.3 Origin of PAHs
3.3.1 Source of PAHs
In spite of its limitations,many researchers have used ratiosof PAHsto tentatively identify their sourcesin whichthe same molecular weight but different structure(i.e.,isomers)are used in the calculation.Among them Phenanthrene(Phe)/Anthracene(Ant),Fluoranthene(Fla)/Pyrene(Pyr),Benzo[a]Anthracene(BaA)/Chrysene(Chry),and Benzo[b]Fluoranthene(BbF)/Benzo[k]Fluoranthene(BkF)are included(Guinan et al.2001;Lee et al.1977;Yunker et al.2002;Colmsjo et al.1986;Simoneit et al.1993;Dominguez et al.1996).It isimportant to select PAH isomerswhoseratiosare stable during their emission,migration,and deposition.In their simulating studies of atmospheric particles,Behymer and Hites(1985)showed that Fluoranthene(Fla)and Pyrene(Pyr),and Benzo[a]Anthracene(BaA)and Chrysene(Chry)have very similar half-lifes and are highly stable,and therefore they can be used reliably for pollution source identification.
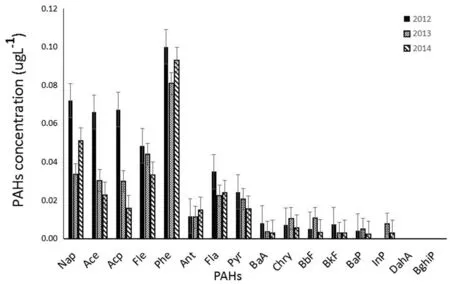
Fig.2 Concentration distribution of PAHs in snows
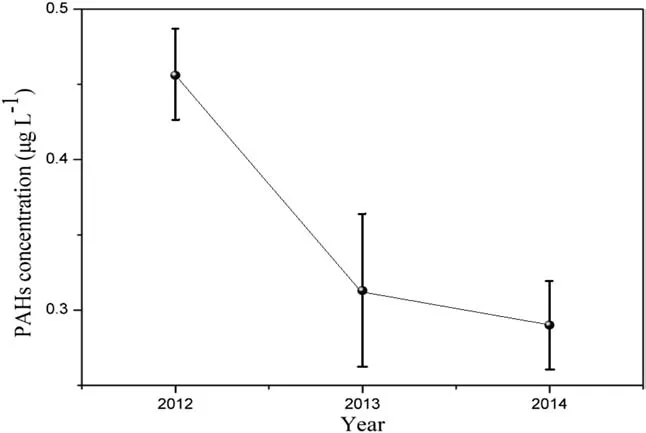
Fig.3 Inter-annual distribution and trend of total PAHs in snow
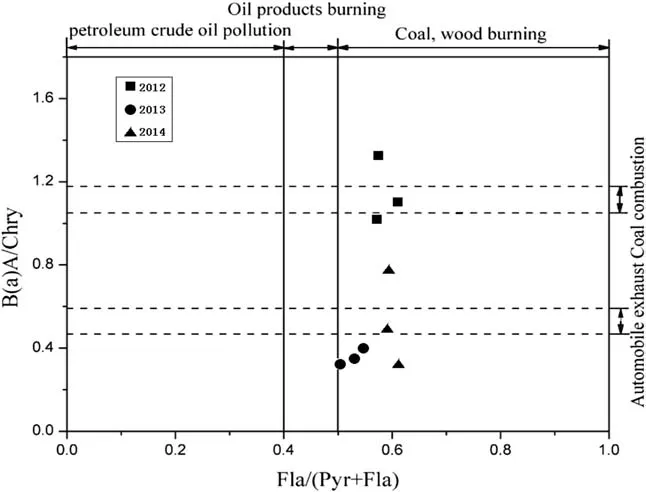
Fig.4 Cross chart of PAHs ratio
The isomer ratio characteristics in ice and snow from Hailuogou are shown in Table 5.Research conducted by Yunker et al.(2002)has indicated that the ratio of Fla/(Pyr+Fla)from petroleum crude oil pollution istypically less than 0.4;this ratio gets greater than 0.5 for wood and coal burning and between 0.4 and 0.5 for petroleum refinery products.The ratio value from Hailuogou was greater than 0.5(see Table 2;Fig.4),suggesting that the PAHs in ice and snow from Hailuogou are mainly from coal and timber burning.
According to Colmsjo et al.(1986),Simoneit et al.(1993),and Dominguez et al.(1996),theratio of BaA/Chry can be used to differentiate PAHsfrom automobileexhaust and coal combustion produces.The values are typically 0.53±0.06 and 1.11±0.06 for automobile exhaust and coal burning,respectively.Hailuogou snow samples show that the ratio of BaA/Chry in 2012 averaged 1.16,but decreased at 0.44 for 2013 and 2014,respectively(Table 5 in Appendix 1;Fig.4),thus suggesting an increase contribution from automobile activitiesover the 3-year period.
In summary,datafrom Hailuogou snow samplesseem to suggest that the PAHswere mainly coming from wood and coal burning early on(2012),and automobile activities contributed more significantly in 2013 and 2014.Pollution from petroleum industries was much less than expected in the study area.This conclusion fits well with the characteristics of local industry,residence,and recent development of tourism in the surrounding areas.The Hailuogou glacier is located in the Ganzi district of the southeast Sichuan Province and surrounded by mining industry.It is also close to several largest cities in Western China,including Chengdu and Chongqing.Pollution from industrial emissions,mining in particular,has become a serious concern.In addition,it is estimated more than 1 million touriststravel to the Hailuogou glacier by automobileseach year,and this number has been steadily increasing in the last years(Administration of Hailuogou scenic spot 2015).Several hundred restaurants and hotels have been built recently,and nearly half of them burn coal and wood as their energy sources.Almost all local residents use coal and timber for their cooking and heating needs on an everyday basis.All these factors are leading to a much higher amount of PAHs as compared to Qinghai-Tibet Plateau glacier and the characteristic PAH ratio patterns in snow from the Hailuogou glacier.
3.3.2 Estimation of distance from emission source
In this study,2,3,and 4-ring PAHs have high loadings in snow from the Hailuogou glacier,and the sum of them accounted for 96.4%,91.6%,and 96.0%of total PAHs in 2012,2013,and 2014,respectively.The distance of migration,or mobility of PAHs is directly related to their molecular weights,as with a lower molecular weight,a PAH likely migrating further in the atmosphere.
An accurate estimate of the distance travelled by PAHs isan important step in determining thelocation of emission and deciphering their origin to better protecting the environment.Li et al.(2014)have established a model to estimate PAH travel distance in the atmosphere,based on factors such as the ratio between the concentrations of Phenanthrene(Phe)and Anthracene(Ant)in the samples collected at the destination and at the emission source the concentration of OH free radicals and wind speed.The travel distance for Hailuogou was then estimated as follows:

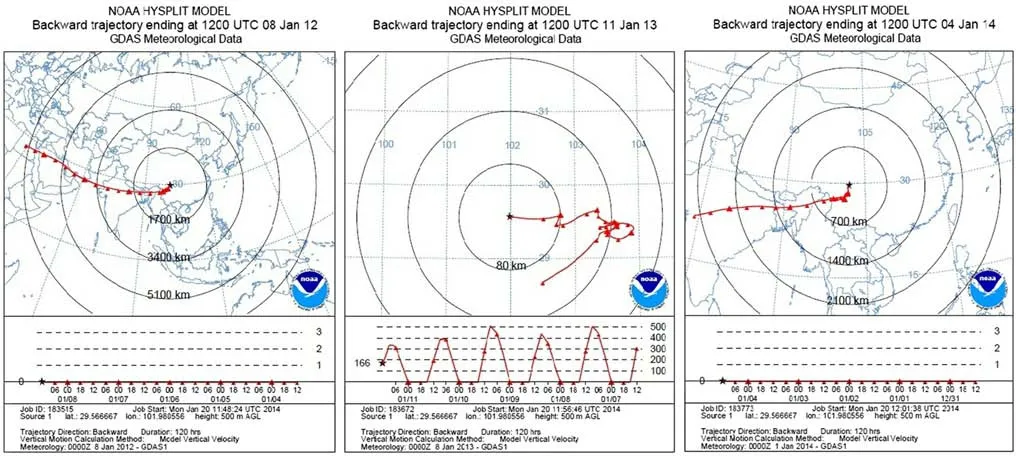
Fig.5 Backward trajectories for Hailuogou(left 2012;middle 2013;right 2014)

Fig.6 The maximum range of emission sources of the Hailuogou snow PAHs
where Dt(km)is the longest possible distance that a PAH can travel in the atmosphere;COH(mol·cm-3)is the average concentration of OH free radical in the atmosphere;SW(m·s-1)is the wind speed;is the concentration ratio of phenanthrene and anthracene at the site of emission;is the concentration ratio of anthracene and phenanthrene in snow samples(destination).
The wind speed,Sw,used in our calculation was the maximum possible wind speed of 60 m·s-1(Gatey and Miller 2007)instead of the actual wind speed,since we intended to estimate the longest possible travel distance.Also,the lowest possible OH radical concentration of 0.3×106molecules cm-3in the atmosphere(Hewett and Harrison 1985)was used for the same reason.The value ofwastaken from coal burning,which isestimated at 5.67(Galarneau 2008,US EPA).The average values ofat the Hailuoguo sampling sites were 0.117,0.131,and 0.125 for 2012,2013,and 2014,respectively.The maximum distance between the emission source and Hialuogou was then estimated to be 492,357,and 413 km for 2012,2013,and 2014,respectively.
The 120 h backward trajectories were calculated using the hybrid single-particle lagrangian integrated trajectory model and the NOAA data downloaded from http://ready.arl.noaa.gov/hypub-bin/traj1.pl.The trajectory end points were set at 500 m above the sampling site.Back trajectories showed that the air mass originates from different directions(Fig.5).
Therefore,the concentrations of PAHs in the snow samples should reflect the PAHs emissions surrounding Hailuogou.Figure 6 shows areas and cities within 500 km radius of Hailuoguo,which cover part of Sichuan,Yunnan,Chongqing and Tibet.
4 Conclusions
Analyses of snow samples from the Hailuogou glacier revealed high concentrations of 16 PAHs,ranging from 452 ± 31 to 290 ± 30 ng·L-1over the years from 2012 to 2014,demonstrating a remarkable decreasing trend,which may suggest the possible consequence of implementation of more strict air pollution law(Air pollution prevention action plan,2013).Compounds with 2–4 rings are accounted for more than 90%of the total PAHs.The maximum travel distance of these PAHs was estimated to be~500 km.The main source of the PAHs is likely coming from coal combustion with increasing contributions from automobile emissions in more recent years.This conclusion is in agreement with the characteristics of coal as a main energy source and recent development in tourism around the Hailuogou area.
AcknowledgementsThis study was supported by the National Natural Science Foundation of China(41073085,41573014)and the programof Sichuan Provincefor researchinnovationteamof universities(12TD001).The authors thank Prof.Belzile N and Chen YW at Laurentian University(Canada)and Dr.S.Huang at Mallinckrodt Biopharmaceuticals(USA)for the helpful edits and valuable discussions.
Appendix 1:GC/MSresults of 16 PAHs in Hailuoguo snow samples
See Tables 2,3,4 and 5.

Table 2 PAH results for 2012

Table 3 PAH results for 2013
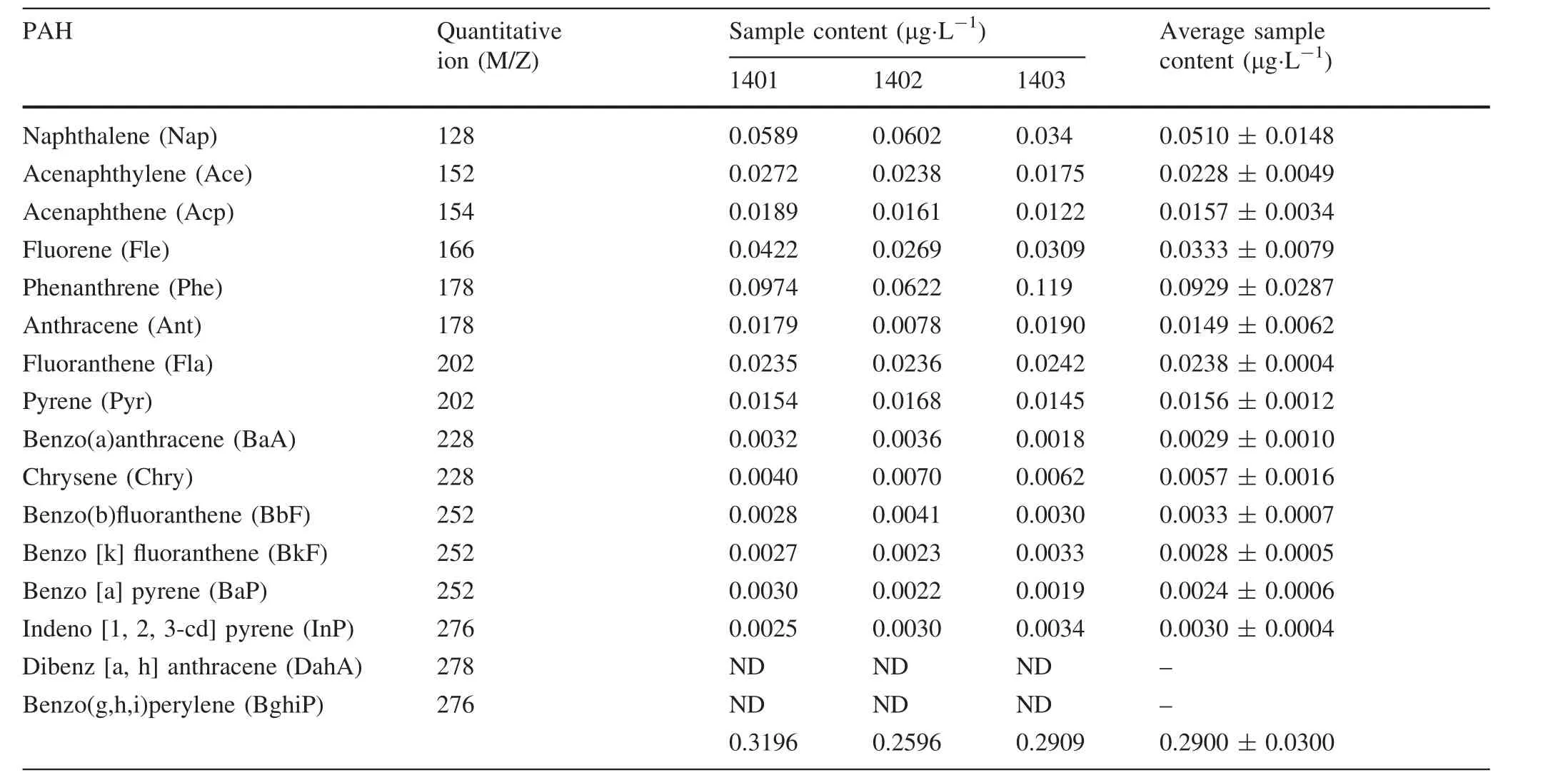
Table 4 PAH results for 2014
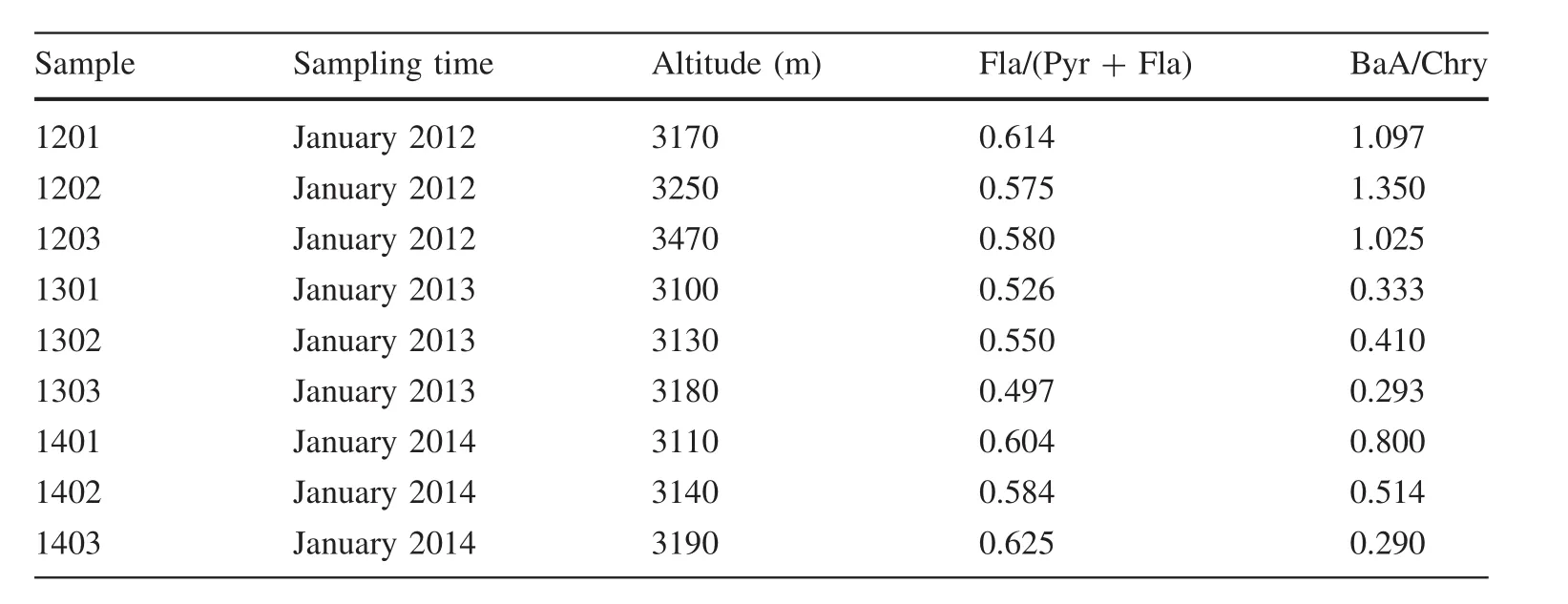
Table 5 PAH isomer ratios in snow from Hailuogou
Administration of Hailuogou scenic spot(2015)http://www.hailuo gou.com/html/info/about_us/
Air pollution prevention action plan(2013)http://www.gov.cn/jrzg/2013-09/12/content_2486918.htm
Baek SO,Field RA,Goldstone ME,Kirk PW,Lester JN,Perry R(1991)A review of atmospheric polycyclic aromatic hydrocarbons:sources,fate and behaviour.Water Air Soil Pollut 60:273–300
Behymer TD,Hites RA(1985)Photolysis of polycyclic aromatic hydrocarbons adsorbed on simulated atmospheric particulates.Environ Sci Technol 19(10):1004–1006
Bjorseth A,Ramdahl T(1985)Source and emissions of PAH,handbook of polycyclic aromatic hydrocarbons,vol 2.Marcel Dekker Inc,New York
Carrera G,Fernandez P,Vilanova RM,Grimalt JO(2001)Persistent organic pollutants in snow from European high mountain areas.Atmos Environ 35(2):245–254
Colmsjo AL,Ostman CE,Zebuhr YU,Soderstrom H,Wadding A(1986)Polynuclear aromatic compounds in the ambient air of Stockholm.Chemosphere 15(2):169–182
Dominguez A,Alvarez R,Blanco CG,Diez MA(1996)Chromatographic evaluation of some selected polycyclic aromatic hydrocarbons of coal tars produced under different coking conditions and pitches derived from them.JChromatogr A 719(1):181–194
Finizo A,Villa S,Raffaele F,Vighi M(2006)Variation of POP concentrations in fresh–fallen snow and air on an Alpine glacier(Monte Rosa).Ecotoxicol Environ Saf 63(1):25–32
Galarneau E(2008)Source specificity and atmospheric processing of airborne PAHs:implications for source apportionment.Atmos Environ 42(35):8139–8149
Gatey DA,Miller CA(2007)An investigation into 50-year return period wind speed differences for Europe.J Wind Eng Ind Aerodyn 95:1040–1052
Gregor DJ,Gummer WD(1989)Evidence of atmospheric transport and deposition of organochlorine pesticides and polychlorinated biphenyls in Canadian Arctic snow.Environ Sci Technol 23(5):1528–1531
Guinan J,Charlesworth M,Service M,Oliver T(2001)Sources and geochemical constraints of polycyclic aromatic hydrocarbons(PAHs)in sediments and mussels of two Northern Irish Sealoughs.Mar Pollut Bull 42(11):107–108
Halsall CJ(2004)Investigating the occurrence of persistent organic pollutants(POPs)in the arctic:their atmospheric behaviour and interaction with the seasonal snow pack.Environ Pollut 28(1–2):163–168
Han YM,Wei C,Bandowe BAM,Wilcke W,Cao JJ,Xu BQ,Gao SP,Tie XX,Li GH,Jin ZD,An ZS(2015)Elemental carbon and polycyclic aromatic compounds in a 150-year sediment core from Lake Qinghai,Tibetan Plateau,China:influence of regional and local sources and transport pathways.Environ Sci Technol 49(7):4176–4183
Herbert BMJ,Halsall CJ,Villa S,Jones KC,Kallenborn R(2005)Rapid changes in PCB and OC pesticide concentration in arctic snow.Environ Sci Technol 39(9):2998–3005
Hewett CN,Harrison RM(1985)Tropospheric concentrations of the hydroxyl radical–a review.Atmos Environ 19:545–554
Jaffrezo JL,Masclet P,Clain MP,Wortham H,Beyne S,Cachier H(1993)Transfer function of polycyclic aromatic hydrocarbons from the atmosphere to the polar ice.I:determination of atmospheric concentrations at dye 3,Greenland.Atmos Environ 27(17):2781–2785
Jaffrezo JL,Clain MP,Masclet P(1994)Polycyclic aromatic hydrocarbons in the polar ice of Greenland,geochemical use of these atmospheric tracers.Atmos Environ 28(6):1139–1145
Kang JH,Son MH,Hur SD,Hong SM,Motoyama H,Fukui K,Chang YS(2012)Deposition of organochlorine pesticides into the surface snow of East Antarctica. Sci Total Environ 433(1):290–295
Lee ML,Prado GP,Howard JB,Hites RA(1977)Source identification of urban airborne polycyclic aromatic hydrocarbons by chromatographic mass spectrometry and high resolution mass spectrometry.Biomed Mass Spectrom 4(3):182–185
Li QL,Wang NL,Wu XB,Pu JC,He JQ,Zhang CW(2010)Distribution characteristics and sources of PAHs in snow from the Qinghai-Tibet Plateau.Sci Sin Terrae 40(10):1399–1409
Li CY,Yu CQ,Li MH,Yin G(2014)Modelling the atmospheric transport distance of polycyclic aromatic hydrocarbons based on the photochemical breakdown.Int J Environ Eng Nat Resour 5:240–246
Simoneit BR,Cass GR,Hildemann LM,Rogge WF,Mazurek MA(1993)Sources of fine organic aerosol.2.Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks.Environ Sci Technol 27(4):636–651
Villa S,Negrelli C,Maggi V,Finizio A,Vighi M(2006)Analysisof a firn core for assessing POPseasonal accumulation on an Alpine glacier.Ecotoxicol Environ Saf 63(1):17–24
Wang XP,Yao SD,Cong ZY,Yan XL,Kang SC,Zhang Y(2006)The content and altitudinal gradient distribution of polycyclic aromatic hydrocarbons in soil and vegetation in the Everest region.Chin Sci Bull 51(21):2517–2524
Wang F,Zhu T,Xu BQ,Kang SC(2007)Organochlorine pesticides in new-fallen snow from East Rongbuk Glacier.Mt.Everest.Sci China(Ser D Earth Sci)37(5):670–675
Wang XP,Yao TD,Wang PL,Wei Y,Tian LD(2008)The recent deposition of persistent organic pollutants and mercury to the Dasuopu glacier,Mt.Xixiabangma,central Himalayas.Sci Total Environ 394(1):134–143
Wei C,Bandowe BAM,Han YM,Cao JJ,Zhan CL,Wilcke W(2015)Polycyclic aromatic hydrocarbons(PAHs)and their derivatives(alkyl-PAHs,oxygenated-PAHs,nitrated-PAHs and azaarenes)in urban road dusts from Xi’an,Central China.Chemosphere 134:512–520
Yu CQ,He X,Cao YL,Zhou H,Liu B,Li CY(2014)Short-term distribution and sourceapportionment of PAHsin thesnow from Hailuogou.Gongga Mt.Geochim 43(4):358–364
Yunker MB,Macdonald RW,Vingarzan R,Mitchell RH,Goyette D,Sylvestre S(2002)PAHs in the Fraser River basin:a critical appraisal of PAH ratios as indicators of PAH source and composition.Org Geochem 33(4):489–515
杂志排行
Acta Geochimica的其它文章
- Geochronological and geochemical constraints on the Cuonadong leucogranite,eastern Himalaya
- Geochemistry and geochronology of Late Jurassic and Early Cretaceous intrusions related to some Au(Sb)deposits in southern Anhui:a case study and review
- Re–Os dating of molybdenite and in-situ Pb isotopes of sulfides from the Lamo Zn–Cu deposit in the Dachang tin-polymetallic ore field,Guangxi,China
- Major Miocene geological events in southern Tibet and eastern Asia induced by the subduction of the Ninetyeast Ridge
- Using electrogeochemical approach to explore buried gold deposits in an alpine meadow-covered area
- U–Pb zircon age of the base of the Ediacaran System at the southern margin of the Qinling Orogen
