Antidiabetic potential of methanol extracts from leaves of Piper umbellatum L. and Persea americana Mill.
2018-07-02GuySedarSingorNjatengSumeraZaibLarissaYetengeChimiCesaireFeudjioRaymondSimpliceMouokeuDonatienGatsingJulesRogerKuiateEzekielAdewoleJamshedIqbal
Guy Sedar Singor Njateng, Sumera Zaib, Larissa Yetenge Chimi, Cesaire Feudjio, Raymond Simplice Mouokeu, Donatien Gatsing, Jules-Roger Kuiate, Ezekiel Adewole,4, Jamshed Iqbal✉
1Laboratory of Microbiology and Antimicrobial Substances, Faculty of Science, University of Dschang, P.O. Box 67 Dschang, Cameroon
2Centre for Advanced Drug Research, COMSATS Institute of Information Technology, Abbottabad-22060, Pakistan
3Institute of Fisheries and Aquatic Sciences, University of Douala, P.O Box 7236, Douala, Cameroon
4Department of Chemical Sciences, Afe Babalola University Ado, P.M.B 5454, Nigeria
1. Introduction
Based on the most recent estimation of WHO, approximately 200 million individuals in the world are diabetics. By the year 2025,this value may rise to nearly 350 million and could lead to a severe consequence on human beings’ health[1,2]. Glycemic management is a long-term treatment for persons suffering from diabetes mellitus[3,4].Glycemic control is primarily focused on inhibitors designed to target glucosidases, members of hydrolases found at the level of gastro-intestinal tract and whose exo-acting abilities are necessary for carbohydrate digestion. These inhibitors of glucosidase are usually recommended to diabetics to diminish glucose flow into the bloodstream from dietary starch, reducing the postprandial effect of consumption of carbohydrates on glycemia[5]. Some leading inhibitors of glucosidase such as miglitol and acarbose are available on the market but they have been reported to cause diarrhea as well as other intestinal disturbances, with corresponding flatulence and intestinal pain[6,7]. Extended exposure to persistent hyperglycemia can result in several complications impeding the neurological, visual, renal,and cardiovascular systems[8]. The mechanisms by which diabetic complications arise are not yet totally known, several biochemical routes engaged in relation with hyperglycemia have been established[8].Of these pathways, polyol one was widely investigated[9].
Aldose reductase, an aldo-keto reductase, is the first enzyme of the polyol route. The rate of this latter is limited by that enzyme which acts as a cofactor to reduce glucose to sorbitol by utilizing nicotinamide adenine dinucleotide phosphate (NADPH)[9]. Sorbitol accumulation leads to modifications in the permeability of membranes, osmotic swelling, and oxidative stress resulting in tissue damage[10]. Experiments based on animal models showed that inhibition of aldose reductase could be efficient in the prevention of some complications[11].Aldehyde reductase is an isoform of aldose reductase responsible for aldehyde reduction as well as for synthesis of ascorbic acid in mammals by D-glucuronate reduction[12,13]. It is also responsible for the metabolism of methyl glyoxal and 3-deoxyglucosone. These aldehydes which are the result of oxidative stress under pathological conditions(hyperglycemia) arise in large quantities and act as an intermediate for advanced glycation end-products[14]. Some aldose reductase and aldehyde reductase inhibitors have been developed to reduce diabetic complications; however, because of undesirable side effects or limited efficacy, none of them has achieved worldwide use[15].
Medicinal plants may be a credible source of glucosidases, aldose reductase and aldehyde reductase inhibitors thanks to relative safety and low cost[16].Piper umbellatum(P. umbellatum) andPersea americana(P. americana) are traditionally used in Cameroon against diabetes.From the literature, three alkaloids named piperumbellactams AC isolated fromP. umbellatumbranches showed moderate inhibition of α-glucosidase[17]. Hypoglycemic activity of aqueous leaf extract as well as that of hydroalcoholic leaf extract ofP. americanaMill were reported by Muchandi[18] and Limaet al[19] in alloxan-induced and streptozotocin-induced diabetic rats respectively. But, to the best of our knowledge, the mechanisms by which they exert their activities have not yet been reported. Thus, this work aimed to determine the potential of methanolic leaf extract ofP. umbellatumandP. americanato inhibit α-glucosidase, β-glucosidase, glucoamylase, aldose reductase and aldehyde reductase activities. In addition, gas chromatography-mass spectrum (GCMS) analysis of these extracts was also performed in order to determine compounds that can be reponsible for their enzymes inhibition activity.
2. Materials and methods
2.1. Plant
Leaves ofP. umbellatumandP. americanawere harvested in February 2017 respectively from Bazou and Bangang-Fokam, west region in Cameroon. The authentification was done at the Cameroon National Herbarium in comparison to the registered specimen under reference 2854/SFR/Cm and 57756/HNC, respectively. After dryness, the leaves were ground for extraction.
2.2. Obtention of plant extracts
Powder from leaves ofP. umbellatumandP. americana(200 g) was macerated for 48 h at room temperature in methanol (600 mL) and filtered using Wattman paper No 1. Methanol was removed from the extract using a rotary evaporator at 45 ℃ under reduced pressure.
2.3. Exploration of extracts by GC-MS
GC-MS of extracts fromP. umbellatumandP. americanaleaves was done using TurboMass GC System, fitted with an Elite-5 capillary column (30 m long, 0.25 mm inner diameter, 0.25 μm film thickness and highest temperature of 350 ℃), combined with a Perkin Elmer Clarus 600C MS. The helium served as gas carrier at a steady flow rate of 1 mL/min. The injection, transfer line as well as ion source temperature were 280 ℃ while the energy of ionization was 70 eV. The oven temperature was adjusted from 40 ℃ (hold for 2 min) to 280 ℃(hold for 10 min) at a frequency of 5 ℃/min. The crude extracts were solubilised using ethyl acetate and filtered with syringe filter (Corning,0.45 μm). A volume of 1 μL of the crude extracts was injected with a split ratio of 1:20. The data were obtained by collecting mass spectrum within 50-550m/z. Chemical compounds present in analysed extracts were identified based on gas chromatography retention time and mass spectra matching those of standards available in NIST library 2014.
2.4. Antidiabetic activity
2.4.1. Isolation of intestinal maltase-glucoamylase enzyme
Intestinal maltase-glucoamylase was extracted following the literature reported procedure[20] with some modifications. The enzyme was extracted from white male rats (1-2 months) weighing 150-250 g and starved for 12 h before sacrifice. This latter was performed by cervical dislocation. Whole intestines were gently removed and washed with ice-cold 0.9% NaCl. For extraction, intestines were cleaned and cut longitudinally, and mucosal scrapings of 5-6 rats were combined and homogenized in 50 volumes of 5 mM EDTA; pH 7.0. The centrifugation of homogenized intestines was performed at 15 000 rpm at 4 ℃ for 45 min and the upper phase was thrown away. The obtained pellet was re-suspended in 90 mL of ice-cold water followed by addition of 5 mL of 0.2 M potassium phosphate (pH 7) containing 0.1 M EDTA, and 5 mL of 0.1 M cysteine. The incubation of the obtained mixture was done at 37 ℃ for 30 min. Pellet was collected following centrifugation at 15 000 rpm for 45 min while supernatant was discarded. Intestinal suspension was re-dissolved in potassium phosphate buffer (10 mM), pH 6.8, to which 4 mg of papain and 0.4 mg cysteine were added. Incubation was performed at 37 ℃ for 40 min with constant shaking. Afterward the mixture was centrifuged at 15 000 rpm for 90 min. The upper phase was collected and precipitated with ammonium sulphate to 80% saturation. After this process, mixture was centrifuged at 15 000 rpm for 30 min and the upper phase was thrown away while pellet was collected and redissolved in 4 mL of 10 mM-potassium phosphate (pH 7). This homogenate was dialyzed overnight against distilled water with three changes of water (40 vol every time).The extracted enzyme was kept at -80 ℃ until further use for total protein determination and inhibition studies.
2.4.2. Maltase-glucoamylase inhibition assay
Maltase-glucoamylase inhibition assay was carried out usingp-nitrophenyl α-D-glucoside (substrate) based on reported procedure[21] with some modifications. Reaction mixture contained 70µL of phosphate buffer (70 mM, pH 6.8), 10 µL of extracted enzyme(25.0 µg of protein) and 10 µL of test extracts (1 mg/mL).. After incubation at 37 ℃ for 5 min, 10 µL of p-NPG (10 mM, prepared in assay buffer) was dispensed in all the wells of a 96 well, then incubation was done at 37 ℃ for 30 min. The activity of the test extracts against maltase-glucoamylase was noted by measuring increase in absorbance ofp-nitrophenol at a wavelength of 405 nm. Acarbose served as reference drug for positive control while negative control contained 10µL of dimethyl sulfoxide (DMSO) 10% instead of extracts. The percent inhibition was calculated as follows:
Percent inhibition (%) = [1-(Absorbancesample/Absorbancecontrol)]×100
2.4.3. Isolation of aldehyde reductase
Kidneys were separated from the calf soon after slaughtering. The cortex area of the kidney was dissected carefully and liquefied in 3 volumes of 10 mM sodium phosphate buffer at 7.2 pH containing 2.0 mM EDTA dipotassium salt, 0.25 M sucrose as well as 2.5 mM β-mercaptoethanol for dissolution and homogenization of tissue.The homogenate was further centrifuged at 12 000 ×gat 4 ℃ for 30 min, afterward the precipitate was thrown away as it contained some insoluble lipids. Collected supernatant was subjected to 40%salt (ammonium sulphate) saturation to isolate aldehyde reductase.This 40% saturated liquid was centrifuged in the same conditions as above. The precipitate was again discarded and the supernatant was subjected to 50% saturation with salt followed by centrifugation as described above. At last step of centrifugation, 75% saturation was obtained by adding powdered salt to the obtained supernatant followed by centrifugation as previously described, resulting in enzyme precipitation. The supernatant was thrown away while the pellet was collected and redissolved in 10 mM sodium phosphate (pH 7.2) containing 2.5 mM β-mercaptoethanol and 2.0 mM EDTA dipotassium salt. In the dialysis membrane, the obtained suspension was submitted to dialysis overnight using the above buffer. After that process, the extracted aldehyde reductase was aliquoted then stored(-80 ℃) until used for total protein determination and inhibition studies[22].
2.4.4. Isolation of aldose reductase
Isolation of aldose reductase was performed as described by Iqbalet al[22] with some changes. Briefly, aldose reductase was isolated from calf lenses. Calf lenses were detached from the eyes immediately after slaughtering and were frozen until use. A mass of 100 g of lenses was homogenized in 3 volumes of cold distilled water, afterward the homogenate was centrifuged at 10 000 ×gfor 15 min at 4 ℃ to remove insoluble constituents which was thrown away as it contained lipids.The supernatant was collected and ammonium sulphate salt was added to make 40% saturation. The mixture was centrifuged as previously decribed followed by discard of the precipitate. The ammonium sulphate concentration was increased up to 50% saturation in order to remove additional inert proteins. Aldose reductase was precipitated upon addition of ammonium sulfate salt to the previous obtained supernatant to reach 75% saturation. The obtained supernatant following centrifugation, was thrown away and the pellet (enzyme)was redissolved in 4 mL of 50 mM NaCl. The sample was submitted to dialysis overnight against 500 mL of 50 mM NaCl. The volume of the sample was measured after dialysis, kept in 1 mL aliquots (in eppendorf tubes) in freezer at -80 ℃ for total protein determination and inhibition studies[22].
2.4.5. Inhibition assays of aldehyde and aldose reductases
UV spectrophotometer at 340 nm was used in order to evaluate the inhibition potential of aldehyde reductase and aldose reductase by measuring the decrease in NADPH absorbance. Each well of the 96-well microplate contained assay mixture which was made up of 10 μL of 100 mM phosphate buffer pH 6.2, 35 μL of enzyme (210 µg of protein), 10 μL of 1 mg/mL tested extracts and 20 μL of substrate(D,L-glyceraldehyde for aldose reductase or sodium glucuronate for aldehyde reductase). The preincubation of the mixture was performed for 5 min at 37 ℃ for the enzymatic reaction to run properly, afterward 25 μL of 0.1 mM NADPH (cofactor) was added. Reading using ELISA plate reader was immediately taken at 340 nm and the mixture was incubated and read in the same conditions as previously described.For positive and negative control, 10 μL of 10 mM valproic acid (for aldehyde reductase) or genistein (for aldose reductase) and DMSO 10% was used respectively[22]. The reaction was run in triplicate with a total volume of 100 μL per well. Absorbances were recorded and percentage of inhibition calculated as follows:
Percent inhibition (%) = [1-(Absorbance of test well/Absorbance of control)]×100
2.4.6. α-glucosidase inhibition studies
This assay was performed following the method used by Razaet al[23]with some changes. In short, solutions of commercial α-glucosidase obtained fromSaccharomyces cerevisiae(Sigma-Aldrich) as well asp-nitrophenyl α-D-glucopyranoside (substrate) were prepared in 70 mM phosphate buffer, pH 6.8 while extract solutions were prepared in DMSO 10%. The inhibition assay was performed by adding 10 μL of extract solution to 70 µL of buffer and 10 µL of 2.5 unit/mL enzyme solution followed by preincubation for 5 min at 37 ℃. After preincubation, 10 µL of 10 mM substrate was added to the mixture in order to start reaction. Incubation of the reaction mixture was done as indicated above for preincubation. Acarbose served as reference for positive control. Enzyme activity was evaluated by measuring increase in absorbance of p-nitrophenol released fromp-nitrophenyl α-D-glucopyranoside at 405 nm using an Elx 800 Micro plate reader.The percentage of inhibition was determined as follows:
Percent inhibition (%) = [1-(Absorbance of test well/Absorbance of control)]×100
2.4.7.β-glucosidase inhibition studies
This study was performed according to the previously described method[23] with some modifications. β-glucosidase extracted from sweet almonds (Sigma-Aldrich) andp-nitrophenyl β-D-glucopyranoside (10 mM) used as substrate were prepared in 0.07 M phosphate buffer, pH 6.8.The inhibition assay was carried out by adding extract solution (10 μL)to 70 µL of the above buffer and 10 µL of 2.0 unit/mL enzyme solution followed by preincubation for 5 min at 37 ℃. After preincubation, 10 µL of substrate was added to the mixture to start the reaction. The incubation of the reaction mixture was done for 30 min at 37 ℃. Castanospermine was considered as reference drug and was used for positive control, while 10 µL of DMSO 10% was used for negative control. Each test was carried out in triplicate. The percent inhibition was determined as follows:
Percent inhibition (%) = [(1-(Absorbancesample/Absorbancecontrol)]×100

Figure 1. GC-MS chromatogram of extract from leaves of P. umbellatum.
Figure 2. GC-MS chromatogram of extract from leaves of P. americana.
2.4.8. Ethical statement
All studies involving animals were conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration no. 173/CPCSEA, dated 28 January, 2000), Government of India, on the use of animals for scientific research.
2.4.9. Data analysis
Enzyme activity in presence of extracts was expressed as a percentage of uninhibited enzyme activity, and plotted versus inhibitor (extract)concentration. Enzyme inhibition was considered as the reciprocal value of the measured enzyme activity and was expressed as a percentage. The non-linear regression was done using GraphPad Prism software. To evaluate the inhibitory potency of extracts, concentration needed to inhibit 50% of enzyme activity (IC50) was determined.
2.5. Statistics analysis
Differences in calculated percent inhibition and IC50values were analyzed using unpairedt-tests as well as one-way ANOVA followed by Tukey-Kramerpost-hocanalysis in order to compare data sets.GraphPad Prism was used to this end. Differences among means were considered considerable at the probability threshold of 5%.
3. Results
3.1. GC-MS analysis
The chromatograms of methanol extract ofP. umbellatumandP. americanaleaves were presented in Figures 1 and 2. The relative retention time as well as mass spectra of different extract components were compared with those of standard from NIST library 2014. Ten[hentriacontane, 1-methylheptadecyl-benzene, 1-cyclohexylethylbenzene, decyl heptyl ether, carbonic acid decyl tetradecyl ester, 3,8-dimethyl-decane, carbonic acid octadecyl vinyl ester,2,6,10,15-tetramethyl-heptadecane , 2,4-di-tert-butylphenol, 3,5-bis(1,1-dimethylethyl)-phenol] and eight (carbonic acid dodecyl vinyl ester, 3-ethyl-3-methylheptane, benzoic acid 1-methoxy-1h-tetrazol-5-ylmethyl ester, estradiol benzoate, 1-cyclohexylethyl-benzene, 2-ethyl-2-methyl-tridecanol, 5-ethyl-5-methylnonadecane, heneicosane)compounds were identified fromP. umbellatumandP. americanaextract respectively. The identified compounds were classified as alkane, ester and compounds with benzene ring. InP. umbellatumandP. americanaextracts, four (two are phenolic compounds) and three compounds with benzene rings were identfied respectively.
3.2. Antidiabetic activity
Table 1 showed the inhibition potency of crude extracts against α-glucosidase, maltase-glucoamylase and β-glucosidase. From Table 1, it can be noted that the tested extracts were highly potent inhibitors of α-glucosidase and maltase-gucoamylase. Among these extracts,P.americanaextract appeared to be the most active compared with IC50values ofP. umbellatumextract. To check the selectivity of these two extracts,they were also tested against β-glucosidase. Both extracts showed less than 16% inhibition when tested at 0.1 mg/mL end concentration,meaning that they did not almost inhibit β-glucosidase (Table 1).However, both extracts were significantly (P<0.05) more active against all the enzymes when compared to the tested positive controls.
The inhibition potency of crude extracts against aldose reducase and aldehyde reductase was demonstrated in Table 2. From Table 2,it appeared that both extracts were potent inhibitors of two enzymes,P. americanaextract of which was the most active (Table 2). Against aldose reductase, both extracts showed better activity than the reference drug (Genistein) while onlyP. americanaextract exerted significantly higher inhibitory effect against aldehyde reductase when compared to sodium valproic acid (The reference substance).

Table 1Inhibition efficiency of crude extracts against α-glucosidase, maltaseglucoamylase and β-glucosidase (mean±SD).
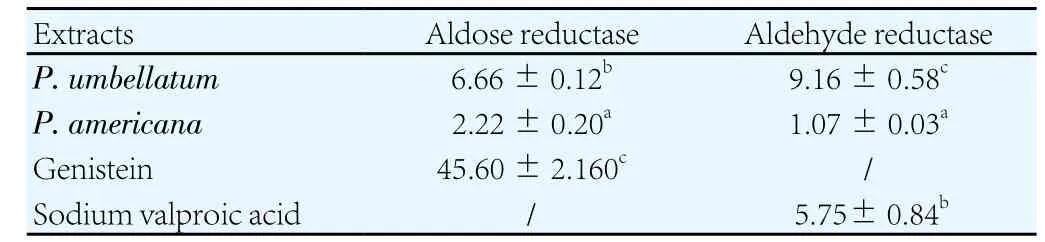
Table 2Inhibition potency of crude extracts against aldose reducase and aldehyde reductase (mean ± SD) (µg/mL).
4. Discussion
Many natural substances have been tested for glucosidase, aldose reductase and aldehyde reductase inhibitory activities[7,13,24]. In the present study, the tested extracts showed inhibitory effects against α-glucosidase and maltase-glucoamylase. This could be due to the presence of antidiabetic compounds in these extracts. In fact, from GCMS analysis of extracts, it appeared that both extracts contain aromatic compounds and according to Craneet al[25]; and Yinet al[6], currently available treatments for diabetes and particularly type 2 diabetes include the administration of insulin through sub-cutaneous route and the use of various oral agents among which were benzoic acid derivatives.Moreover, Patelet al[26] showed that the electron donating groups on sterically hindered benzene aromatic ring, the effector region,increase antidiabetic activity of compounds. In our study, the aromatic identified compounds contain releasing groups and this could justify the important activity observed in these crude extracts.P. americanaextract appeared to be the most important inhibitor of both glucosidases and maltase-glucoamylase. Knowing that both extracts possess aromatic compounds, the difference in their inhibitory effects may be due to a difference in the concentration of active principles among benzene aromatic compounds in these extracts or to antagonistic effects among compounds in the less active extract[27]. Both extracts showed more significant inhibitory effects compared to the tested acarbose. This suggests that these plant extracts, especiallyP. americanaextract, can be a promising source of antidabetic drug. Both extracts showed less than 16% inhibition effect against β-glucosidase, which indicated the selectivity of their activities.
Chronic secondary complications are the main cause of morbidity and mortality in diabetic patients[28]. Structurally different compounds such as phenols, spirohydantoins, flavonoids, benzopyrans, quinones,and alkaloids have all been highlighted as having the ability to inhibit aldehyde and aldose reductases with distinct degrees of efficacy and specificity[7,10,29]. Sorbinil, alrestatin, tolrestat, statil, ALO1576 and epalrestat are some of those enzymes inhibitors that have been wellinvestigated and clinically tested. However, up to date, none of the available synthetic aldose and aldehyde reductases has proved clinically effective and in fact some have had severe side effects. Moreover, in recent times, there is an increased interest to plants as natural sources of antidiabetic substances, especially because most of the medicinal plants and medicinal plant products are free from adverse side effects and are being used as a source of diet or traditional medicine[24]. The medicinal use ofP. umbellatumandP. americanahas a very long tradition. Results of the current study that show interesting inhibition of aldose and aldehyde reductases by these extracts could be ascribed among others to benzene aromatic compounds identified in these extracts. Substances that can considerably delay or prevent the diabetic complications onset and development would offer many advantages when involved in glycemic control. In principle,P. umbellatumandP.americanaextracts may be included in this category. The results of the present work are a step forward in the developpment of such agents.
This work along with findings strongly provides basis for developing good alternatives to available synthetic drugs for glycemic management fromP. umbellatumandP. americana. Further work is still needed for the identification of specific anti-hyperglycemic constituents in these extracts and evaluation of their pharmacological potentials.
Conflict of interest statement
The authors have no competing interests.
Acknowledgments
This work was supported by the 2016 CIIT-TWAS Postdoctoral Fellowship (grant number: 3240293205).
[1] Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes:Estimates for the year 2000 and projections for 2030.Diabetes Care2007;27(5): 1047-1053.
[2] Oh J, Jo SH, Kim JS, Ha KS, Lee JY, Choi HY, et al. Selected tea and tea pomace extracts inhibit intestinal α-glucosidase activityin vitroand postprandial hyperglycemiain vivo.Intern J Mol Sci2015;16(4): 8811-8825.
[3] Blonde L. Benefits and risks for intensive glycemic control in patients with diabetes mellitus.Am J Med Sci2012;343(1): 17-20.
[4] Sonmez H , Kambo V, Avtanski D, Lutsky L, Poretsky L. The readmission rates in patients with versus those without diabetes mellitus at an urban teaching hospital.J Diabetes Complicat2017;31: 1681-1685.
[5] Gourgari E, Wilhelm EE, Hassanzadeh H, Aroda VR , Shoulson I. A comprehensive review of the FDA-approved labels of diabetes drugs:Indications, safety, and emerging cardiovascular safety data.J Diabetes Complicat2017;31: 1719-1727.
[6] Yin Z, Zhang W, Fenga F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants.Food Sci Human Wellness2014;3(3-4): 136-174.
[7] Yilmazer-Musa M, Griffith AM, Michels AJ, Schneider E, Frei B. Inhibition of α-amylase and α-glucosidase activity by tea and grape seed extracts and their constituent catechins.J Agric Food Chem2012;60(36): 8924-8929.
[8] Brownlee M. Biochemistry and molecular cell biology of diabetic complications.Nature2001;414(6865): 813-820.
[9] Kinoshita JH. A thirty year journey in the polyol pathway.Exp Eye Res1990;50(6): 567-573.
[10] Bhatnagar A, Srivastava SK. Aldose reductase: Congenial and injurious profiles of an enigmatic enzyme.Biochem Med Met Biol1992;48(2): 91-121.
[11] Kador PF, Kinoshita JH, Sharpless NE. Aldose reductase inhibitors: A potential new class of agents for the pharmacological control of certain diabetic complications.J Med Chem1985;28(7): 841-849.
[12] Carbone V, Giglio M, Chung R, Huyton T, Adams J, Maccari R, et al.Structure of aldehyde reductase in ternary complex with a 5-arylidene-2, 4-thiazolidinedione aldose reductase inhibitor.Eur J Med Chem2010;45(3):1140-1145.
[13] Takahashi M, Miyata S, Fujii J, Inai Y, Ueyama S, Araki M, et al.In vivorole of aldehyde reductase.Biochimica et Biophysica Acta2012;1820(11): 1787-9176.
[14] Chen X, Yang Y, Ma B, Zhang S, He M, Gui D, et al. Design and synthesis of potent and selective aldose reductase inhibitors based on pyridylthiadiazine scaffold.Eur J Med Chem2011;46(5): 1536-1544.
[15] Kirkman MS. Dual oral agent therapy for type 2 diabetes: Why don’t our patients stick with it?J Diabetes Complicat2016;30: 1417-1418.
[16] Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors.Curr DiabetesRev2010;6(4): 247-254.
[17] Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. Antioxidant constituents in the dayflower (Commelina communisL.) and their α-glucosidase-inhibitory activity.J Nat Med2008;62(3): 349-353.
[18] Muchandi IS. Hypoglycemic activity of aqueous leaf extract ofPersea americanaMill.Indian J Pharmacol2005;37(5): 325-326.
[19] Lima CR, Vasconcelos CF, Costa-Silva JH, Maranhão CA, Costa J, Batista TM, et al. Anti-diabetic activity of extract fromPersea americanaMill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats.J Ethnopharmacol2012;141(1): 517-525.
[20] Lee LMY, Salvatore AK, Flanagan PR, Forstner GG. Isolation of a detergentsolubilized maltase/glucoamylase from rat intestine and its comparison with a maltase/glucoamylase solubilized by papain.Biochem J1980;187(2): 437-446.
[21] Tanaka A, Ohya M, Yammato T, Nakagawa C, Tsuji T, Senoo K, et al.Steady-state inhibitory kinetic studies on the ligand binding modes ofAspergillus nigerglucoamylase.Biosci Biotechnol Biochem1999;63(9):1548-1552.
[22] Iqbal Z, Hameed S, Ali S, Tehseen Y, Shahid M, Iqbal J. Synthesis,characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro [fluorene-9, 5′-imidazolidine]-2′, 4′-diones.Eur J Med Chem2015;98: 127-138.
[23] Raza R, Ilyas Z, Ali S, Nisar M, Khokhar MY, Iqbal J. Identification of highly potent and selective α-glucosidase inhibitors with antiglycation potential,isolated fromRhododendron arboreum.Nat Prod2015;9(2): 262-266.
[24] Xua X, Shan B, Liao CH, Xie JH, Wen PW, Shi JY. Anti-diabetic properties ofMomordica charantiaL. polysaccharide in alloxan-induced diabetic mice.Intern J Biol Macromol2015;81: 538-543.
[25] Crane L, Anastassiadou M, El Hage S, Stigliani JL, Baziard-Mouysset G,Payard M, et al. Design and synthesis of novel imidazoline derivatives with potent antihyperglycemic activity in a rat model of type 2 diabetes.Bioorg Med Chem2006;14(22): 7419-7433.
[26] Patel KD, Patel CN, Patel GM. Microwave assisted synthesis and antidiabetic activity of novel 5-[4-(substituted) benzylidine]thiazolidine-2,4-dione.Med Chem2016;6: 10.
[27] Teke GN, Lunga KP, Wabo HK, Kuiate JR, Vilarem G, Giacinti G, et al.Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark ofEntada abyssinicaStend exA. Satabie.BMC Complement Altern Med2011;11: 57.
[28] Liu F, Yang X, Li J, Cao J, Chen J, Li Y, et al. Association of fasting glucose levels with incident atherosclerotic cardiovascular disease: An 8-year followup study in a Chinese population.J Diabetes2017;9: 14-23.
[29] Raskin P, Rosenstock J. Aldose reductase inhibitors and diabetic complications.American J Med1987;83(2): 298-306.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antioxidant and antiglycation properties of two mango (Mangifera indica L.) cultivars from Senegal
- Synthesis of silver and gold nanoparticles from leaf of Litchi chinensis and its biological activities
- NO-cGMP-K channel-dependent anti-nociceptive activities of methanol stem bark extract of Piptadeniastrum africanum (Mimosaceae) on rats
- Diet containing seeds of Buchholzia coriacea accelerates healing of acetic acid induced colitis in rats
- Synsepalum dulcificum extracts exhibit cytotoxic activity on human colorectal cancer cells and upregulate c-fos and c-jun early apoptotic gene expression
- A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases
