Antiplasmodial activity of silver nanoparticles: A novel green synthesis approach
2018-06-29MadhurSardanaVarnikaAgarwalAkanshaPantVinitaKapoorKailashPandeySanjayKumar
Madhur Sardana, Varnika Agarwal, Akansha Pant, Vinita Kapoor, Kailash C. Pandey, Sanjay Kumar✉
1Department of Chemistry, Sri Venkateswara College, University of Delhi, Delhi 110021, India
2National Institute of Malaria Research, ICMR, New Delhi-110077, India
3Department of Biochemistry, National Institute for Research in Environmental Health, ICMR, Bhopal, India
1. Introduction
Nanotechnology is a versatile branch of development and research which is growing at a tremendous rate from the past two decades.Nanoparticles are known for their numerous physical, biological,and pharmaceutical applications[1]. Silver nanoparticles (AgNPs), in particular, are being used as antimicrobial agents[2-5] as they exhibit interesting antibacterial[6-8] and antiplasmodial[9,10] activities.Several chemical methods for synthesis of nanoparticles have been reported[11-14]. However, the green synthesis methods are considered to be more versatile[15,16].
The use of green techniques for synthesis of nanoparticles is a rapid, low cost and eco-friendly process. The advantage of using plant extracts for synthesis of nanoparticles is that each plant extract, by virtue of its unique metabolites such as polyphenols,terpenoids and thiols, gives rise to a wide diversity of unique microenvironments. This influences the physico-chemical and biological properties of the AgNPs which are hence formed. Several plants of medicinal importance are commonly prevalent and have been used over centuries[17,18]. Azadirachta indica (neem) and Ocimum sanctum (tulsi) plants are commonly available and each part of these plants has been used as a household remedy against various human diseases from historic times[19]. These plants have also been found to show antimalarial and antiplasmodial[20-22] properties.AgNPs prepared from neem and tulsi leaf extracts individually show good antibacterial and antimicrobial properties. However, the combinatorial properties of the above two extracts in unison are still an unexplored niche.
In the present work, we have studied the antiplasmodial activity of the AgNPs synthesized from the combination of neem and tulsi plant leaf extracts when taken in different proportions. The AgNPs synthesized by novel combination of neem and tulsi plant leaf extracts have proved to be potential antiplasmodial agents.The knowledge of antiplasmodial activities of AgNPs based on the calculated half maximal effective concentration (EC50) values would be helpful to understand their antimalarial properties.
2. Materials and methods
2.1. Materials
Fresh leaves of neem and tulsi were taken from the botanical garden of Sri Venkateswara College Campus. Silver nitrate was purchased from Merck India Ltd and 1 mM solution of the same was prepared.Other chemicals and solvents used were of analytical grade. All the glassware were washed with distilled water and dried in oven.Antiplasmodial activities were tested at National Institute of Malaria and Research, New Delhi.
2.2. Preparation of leaf extract
Collected leaves were first washed with distilled water and then air dried for 2 h. A total of 20 g of finely cut neem leaves were boiled in 150 mL distilled water for 30 min at 90 ℃ on an electric water bath and filtered through Whatman filter paper to obtain aqueous neem leaf extract. Also, 20 g of finely cut tulsi leaves were processed through the same procedure in order to obtain aqueous tulsi leaf extract. The tulsi extract obtained was centrifuged for 15 min at 5070 g. The prepared extracts were stored at 4 ℃.
2.3. Synthesis of silver nanoparticles
For synthesis of AgNPs, 3 mL aqueous extract of neem leaves was mixed with 27 mL of 1 mM silver nitrate and a vivid color change was observed indicating the formation of AgNPs. Similarly, other samples were prepared by mixing plant extracts of neem and tulsi in different compositions as 100% tulsi (0% neem), 100% neem(0% tulsi), 80% neem (20% tulsi), 60% neem (40% tulsi), 40%neem (60% tulsi) and 20% neem (80% tulsi) namely A, B, C, D, E,F respectively. Keeping the ratio of extract(s) to 1 mM silver nitrate equal to 1:9, the effects of various physico-chemical parameters were examined by varying the reactant concentration, pH and reaction time. Reduction of ionic silver to AgNPs was monitored after diluting a small amount of the sample 20 times. Absorption spectra were recorded with UV/VIS/NIR spectrometer (Systronics-P C Based Double Beam Spectrophotometer - 2202). The excitation source was a 450 Watt CW xenon lamp. Completion of the reaction indicated by a change in color from colorless solution to a brownyellow solution was observed, confirming the formation of AgNPs after 15 min in the presence of NaOH. Effect of pH was studied by varying the pH of all the samples by adding 2 drops of 1 M NaOH and adjusting its pH to 8. The pH of tulsi leaf extract was 7.0 and that of neem leaf extract was 6.5.
2.4. Characterization of the synthesized AgNPs
Synthesis of AgNPs solution with leaf extract can be easily confirmed by UV–Vis spectroscopy. The bioreduction of the Ag+ions in solutions prepared by leaf extract was monitored after 20 times dilution and measuring the UV–Vis spectra of the solution.UV–Vis spectra of these samples was monitored as a function of time of reaction on a Systronics-P C Based Double Beam Spectrophotometer-2202 in the 350–600 nm range operated at a resolution of 1 nm. Further, the reaction mixture was subjected to sonicator for 15 min before transmission electron microscopy(TEM). A small drop of sample was poured on 300 mesh copper grids for TEM.
Sample for TEM was prepared by drop coating purified AgNPs on carbon-carbon coated 300 mesh copper TEM grids at AIIMS, New Delhi. TEM measurements were performed on Techai G2 20 S-Twin with accelerating voltage at 200 kV.
2.5. Antiplasmodial activity
In-vitro parasite cultivation and drug sensitivity assay were performed as follows. The parasite strain (3D7) was collected from NIMR parasite bank, and revived in vitro using Trager and Jensen method in RPMI 1640 medium for 7-8 cycles. The culture of Plasmodium falciparum 3D7 parasites were maintained in human erythrocytes (AB+) at 2% hematocrit in RPMI medium 1640 supplemented with 50 mg/L gentamicin, 5% sodium bicarbonate 10% human serum. Parasites were synchronized with 5% D-sorbitol and maintained in ring stage. ELISA plates were used in triplicates with 20 μM of each sample of AgNPs dissolved in water serially diluted to seven fold concentrations. A total of 180 μL/well of parasites with 0.5% synchronized ring stage parasitemia and 2%fresh RBC hematocrit was added. Further plates were incubated for 72 h at 37 ℃ in CO2incubator (Flow Laboratories).
ELISA plates were initially pre-coated with 100 μL of 1.0 μL/mL primary IgM antibody (MPFM-55A, Immunology Consultants Laboratories, Inc, Newberg, OR, USA) and incubated at 4 ℃overnight. The plates were further dried and blocked with 200 μL of blocking solution (2% bovine serum albumin in PBS) followed by three times washing with the PBS-Tween 20 (0.05%). The plates were stored at -20 ℃ till the experiment was conducted.
For HRPII ELISA assay, 100 μL of hemolysed lysate of 72 h post infection (hpi) of parasite was transferred to pre-dosed primary IgM antibody coated plate and incubated at RT in humid chamber for 1 h. The content was discarded, plate was washed thrice with PBS-Tween 20 (0.05%) and tap dried. Followed by addition of 100 μL of 0.2 μg/mL concentration of IgG secondary antibody(MPFG-55P, Immunology Consultants Laboratories, Inc. Newberg,OR, USA) dissolved in 2% BSA and 1% Tween 20, IgG added plate was incubated for 1 h at RT in humid chamber, washed three times in PBS/Tween and dried. Further 100 μL 3,3’,5,5’-Tetramethylbenzidine chromogen (Ameresco, US) was added and incubated for 10 min at RT in dark. The reaction was stopped by addition of 50 μL of 1M Sulphuric acid followed by absorbance recording of each plate using an ELISA plate reader (Spectrostar Nano, BMG LABTECH, Germany) at 450 nm[23,24]. EC50value was calculated by nonlinear regression analysis. The software used for the study was based on a polynomial regression model and was freely available from http://malaria.farch.net.
3. Results
3.1. UV-Vis spectra analysis
The UV-Vis spectrum of silver nanoparticles (Figure 1) of samples A-F was recorded as a function of concentration in the range of 350-550 nm. λmax(nm) range of the synthesized nanoparticles was found to be between 407-427 nm.
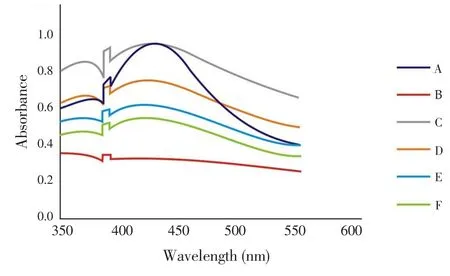
Figure 1. UV-Vis spectra of silver nanoparticles synthesized from aqueous leaf extracts of neem and tulsi in different combinations.
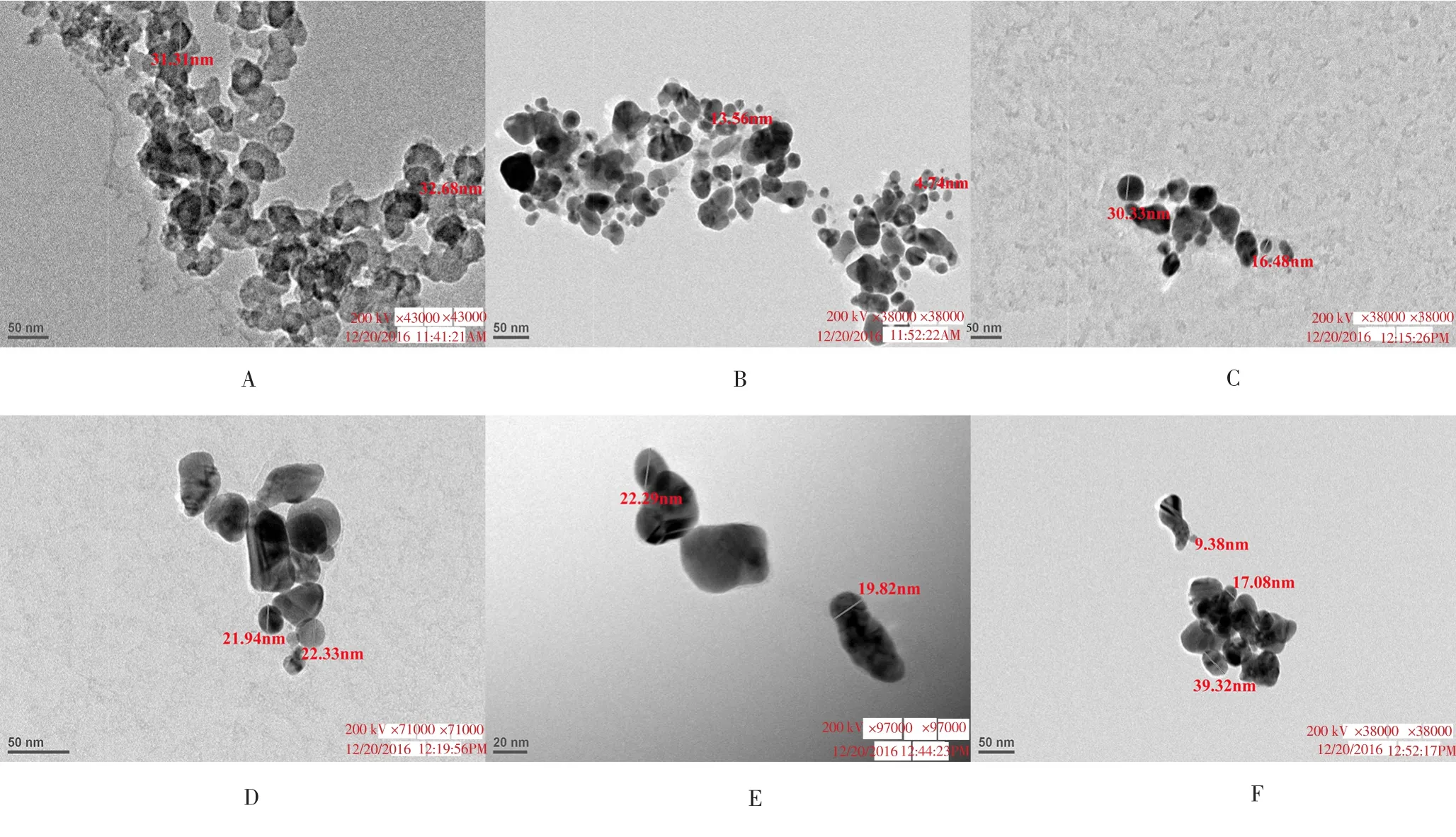
Figure 2. TEM images of AgNPs formed from different combinations of neem and tulsi extracts: Sample A (100% tulsi, 0% neem), B (100% neem, 0% tulsi), C (80%neem, 20% tulsi), D (60% neem, 40% tulsi), E (40% neem, 60% tulsi), and F (20% neem, 80% tulsi).
3.2. TEM analysis
TEM technique was employed to visualize the size and shape of AgNPs. Table 1 showed the corresponding sample combinations,particles size obtained, λmax(nm), EC50and R2values for the synthesized AgNPs. The optimum particle size was found to be < 40 nm. The TEM images of different samples were illustrated in Figure 2, confirming the formation of AgNPs between size range 4.74-39.32 nm. In addition, the TEM images showed that the nanoparticles were circular and spherical in shape (Figure 2).

Table 1 Particles size, λmax(nm), EC50 and R2 values of AgNPs synthesized from samples A-F.
3.3. Antiplasmodial studies
To determine the efficacy, the nanoparticles of neem and tulsi alone and in combination were incubated with synchronized ring stage Plasmodium falciparum 3D7 parasites. Each nanoparticle was screened in triplicates till 72 h post infection (hpi) using HRPII ELISA assay.The result indicated that 100% and 80% neem contained nanoparticles showed better value of EC50about 0.3 μM among other combinations while other showed moderate activity. The EC50values were mentioned in the Table 1 and belonged to 0.313-1.692 range.
4. Discussion
Reduction of silver ions into metallic silver when exposed to the plant extracts depicted a color change. The Surface Plasmon Resonance phenomena is accountable for the dark yellowish - brown color of AgNPs. For characterisation of the nanoparticles, UV-Vis spectroscopy substantiated to be a suitable technique for the analysis of nanoparticles. Reduction of Ag+ions in the aqueous medium to atomic silver in the presence of plant leaf extracts was correlated with the UV-Vis spectra.
The effect of AgNPs of neem and tulsi alone and in combination suggested that 100% and 80% neem exhibited EC50value of about 0.3 μM. The observed anti-plasmodial activity could be due to presence of steroids, terpenes, coumarins, flavonoids, phenolic acids,lignans, xanthones and anthraquinones[25]. A simple green synthesis of AgNPs prepared from leaf extract showed effective antiplasmodial properties at concentrations 100% neem with 0% tulsi (Sample B)and 80% neem with 20% tulsi (Sample C). Pure neem and tulsi leaf extract have been reported[20,26] to show insignificant antiplasmodial activity with IC50values ranging from 35-40 μg/mL whereas AgNPs synthesized by our group shows a manifold increase in antiplasmodial activity. This is probably because of the fact that AgNPs can penetrate more against Plasmodium falciparum owing to their small size and spherical shape. Further, a lot of clinical trials[1,27] are being carried out on antimalarial activity of AgNPs and our results would be helpful to understand the mechanism of their action.
In the present study we have found that plant extracts of neem and tulsi, when taken in different proportions in combination,give rise to novel AgNPs that are potential antiplasmodial agents.This knowledge of antiplasmodial activity of AgNPs based on the reported EC50values would be helpful in understanding their antimalarial properties as well.
Conflict of interest statement
We declare that we do not have any conflict.
Acknowledgements
The authors are thankful to the University of Delhi for their financial assistance through the Innovation project (SVC 311) and Principal, Sri Venkateswara College for her valuable advice. KCP is thankful to Dept. of Science and Technology for extramural grant(SR/SO/BB-0092/2013).
[1] Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 2005; 3: 6.
[2] Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Corrigendum to“Antimicrobial effects of silver nanoparticles”. Nanomed Nanotechnol Biol Med 2007; 1: 95-101.
[3] Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci 2016; 6: 399-408.
[4] Das MR, Sarma RK, Borah SCh, Kumari R, Saikia R, Deshmukh AB, et al. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity.Colloids Surf B Biointerfaces 2013; 105: 128-136.
[5] Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent:A case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 2004; 275(1): 177-182.
[6] Paosen S, Saising J, Septama AW, Voravuthikunchaia SP. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater Lett 2017; 209: 201-206.
[7] Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles – A review on biomolecules involved, characterisation and antibacterial activity. Chem-Biol Interact 2017; 273: 219-227.
[8] Li WR, Sun TL, Zhou SL, Ma YK, Shi QS, Xie XB, et al. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int Biodeter Biodegr 2017; 123: 304-310.
[9] Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed 2012; 2: 574-580.
[10] Mishra A, Kaushik NK, Sardar M, Sahal D. Evaluation of antiplasmodial activity of green synthesized silver Nanoparticles. Colloids Surf B Biointerfaces 2013; 111: 713-718.
[11] Mousavi-Kamazani M, Salavati-Niasari M, Goudrazi M, Zarghami Z.Hydrothermal synthesis of CdIn2S4 nanostructures using new starting reagent for elevating solar cells efficiency. J Mol Liq 2017; 242: 653-661.
[12] Goudrazi M, Mir N, Mousavi-Kamazani M, Bagheri S, Salavati-Niasari M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci Rep 2016; 6: 32539.
[13] Goudrazi M, Salavati-Niasari M. Controllable synthesis of new Tl2S2O3nanostructures via hydrothermal process; characterization and investigation photocatalytic activity for degradation of some anionic dyes. J Mol Liq 2016; 219: 851-857.
[14] Wang Y, Liu W, Liu W, He P, Fan Z, Wang X, et al. Synthesis of SnAgCu nanoparticles with low melting point by the chemical reduction method.Microelectron Reliab 2017; 78: 17-24.
[15] Mallikarjuna K, Narasimha G, Dillip GR, Praveen B, Shreedhar B,Lakshmi CS, et al. Green synthesis of silver nanoparticles using ocimum leaf extract and their characterisation. Dig J Nanomater Bios 2011; 6:181-186.
[16] Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Mishra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surface A 2009; 339: 134-139.
[17] Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles:Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 2016; 17(9): 1534.
[18] Rafique M, Sadaf I, Ra fique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 2017; 45: 1272-1291.
[19] Verkerk RHJ, Wright DJ. Biological activity of neem seed kernel extract and synthetic azadirachtin against larvae of Plutella xylostella. Pestic Sci 1993; 37: 83-91.
[20] Mishra A, Kaushik NK, Sardar M, Sahal D. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf B Biointerfaces 2013; 111: 713-718.
[21] Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus and their antiplasmodial activities. Asian Pac J Trop Biomed 2012; 2: 574-580.
[22] Kaur R, Kaur H. Plant derived antimalarial agents. J Med Plants Stud 2017; 5: 346-363.
[23] Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. Histidinerich protein Ⅱ: A novel approach to malaria drug sensitivity testing.Antimicrob Agents Chemother 2002; 46:1658-1664.
[24] Sharma S, Mishra N, Valecha N, Anvikar AR. Comparison of WHO Mark Ⅲ and HRP Ⅱ ELISA for in vitro sensitivity of P. falciparum. J Vector Borne Dis 2016; 53: 341-347.
[25] Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, et al. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol 2004; 93(1): 27-32.
[26] Inbaneson SJ, Sundaram R, Suganthi P. In vitro antiplasmodial effect of ethanolic extracts of traditional medicinal plant Ocimum species against Plasmodium falciparum. Asian Pac J Trop Med 2012; 5: 103-106.
[27] Chung I, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G. Plantmediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res Lett 2016; 11(1): 40.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Molecular methods for detection of pathogenic viruses of respiratory tract-A review
- Phytochemical bioprospecting, antioxidant, antimicrobial and cytotoxicity activities of saline extract from Tithonia diversifolia (Hemsl) A. Gray leaves
- Hepatoprotective effect of Opuntia dillenii seed oil on CCl4 induced acute liver damage in rat
- Combination treatment of bisphosphonate (pamidronate) and Quercus infectoria semipurified fraction promotes proliferation and differentiation of osteoblast cell via expression of Osterix and Runx2 marker
- Larvicidal efficacy of crude and fractionated extracts of Dracaena loureiri Gagnep against Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles minimus mosquito vectors
- Co-detection and isolation of Leishmania and Crithidia among naturally infected Tatera indica (Rodentia: Muridae) in Fars province, southern Iran
