A New Species of Gracixalus (Anura: Rhacophoridae) from West Guangxi, China
2018-06-28WeicaiCHENYongjianBEIXiaowenLIAOShichuZHOUandYunmingMO
Weicai CHEN, Yongjian BEI, Xiaowen LIAO, Shichu ZHOU and Yunming MO*
1 Key Laboratory of Beibu Gulf Environment Change and Resources Utilization of Ministry of Education, Guangxi Teachers Education University, Nanning 530001, China
2 Guangxi Key Laboratory of Earth Surface Processes and Intelligent Simulation, Guangxi Teachers Education University, Nanning 530001, China
3 Natural History Museum of Guangxi. Nanning 530012, China
4 College of Biology and Pharmacy, Yulin Normal University, Yulin 537000, China
1. Introduction
The genusGracixaluswas originally treated as a subgenus ofAquixalus(Delormeet al., 2005). Liet al.(2008, 2009), however, suggested assigningGracixalusto a distinct genus based on their phylogenetic analyses and treatedGracixalusgracilipesas the type species of the genus. Currently, the species ofGracixalusoccurs in China, Vietnam, Laos, Thailand and Myanmar. Till date, the genusGracixaluscontains 13 recognized species, and most of them are described recently (Frost,2017). However, the specific composition of the genusGracixalusis still unknown. For instance, the group ofGracixalusjingxiuensiscontains cryptic species that are primarily based upon preliminary mitochondrial DNA analysis but their categorization far remains uncertain and unclassified (Rowleyet al., 2014; Matsuiet al., 2015,2017; Zenget al., 2017).
During recent fieldwork in western Guangxi Province, China, we discovered a small tree frog that closely resembles toG. jinggangensis,G. jinxiuensisandG. sapaensis, but the morphological characters,advertisement calls and genetic data differed from these and all other rhacophorids in China and adjoining countries. We here describe these specimens as a new discovered species.
2. Materials and Methods
All specimens were collected and deposited at the Natural History Museum of Guangxi (NHMG). We recorded morphological data from the specimens after fixing them in 10% formalin and then stored in 75%ethanol. Measurements were taken with digital calipers to the nearest 0.1 mm. Measurements included snout-vent length (SVL); head length from tip of snout to rear of jaws (HL); head width at the commissure of the jaws(HW); snout length from tip of snout to the anterior corner of eye (SNT); diameter of the exposed portion of the eyeball (ED); interorbital distance (IOD); horizontal diameter of tympanum (TD); upper eyelid width, greatest width of upper eyelids (UEW); distance from anterior edge of tympanum to posterior corner of the eye (TED);internarial space (IN); distance from front of eye to nostril(EN); tibia length with the hindlimb flexed (TIB); tibia width (TW); forelimb length from tip of disk of finger III to axilla (FLL); hand length from the base of outer palmar tubercle to the tip of third finger (HAL); maximal diameter of disc of third finger (FTD3); hindlimb length from tip of disk of fourth toe to groin (HLL); foot length from the base of inner metatarsal tubercle to the tip of fourth toe (FOL); and diameter of fourth toe tip, greatest diameter of disk on fourth toe (HTD4). The webbing formula is given as proposed by Myers and Duellman(1982).
We obtained comparative morphological data for the 13 recognized species in the genusGracixalufrom the literature as discussed in Boulenger (1893), Bourret(1937), Hu (1978), Orlov and Nguyen (2004), Nguyenet al.(2008, 2013), Feiet al.(2010), Moet al.(2013),Rowleyet al.(2011, 2014), Matsuiet al.(2015, 2017),and Zenget al.(2017).
Advertisement calls were recorded with an ICD recorder (Sony ICD-TX50). The calls were recorded at a distance about 0.1-0.3 m and ambient temperatures were taken with a TP-2200 (A-volt). Calls were analyzed with Raven Pro 1.3 beta version (http://www.birds.cornell.edu/raven) with the default settings.
A preliminary phylogenetic analysis was also reconstructed based on a fragment of the mitochondrial gene 16S (~530 bp). We used the primers 16Sar and 16Sbr(Palumbiet al., 1991) to amplify the fragment. Genomic DNA was extracted from tissue samples, using DNeasy tissue extraction kits (QIAGEN). PCR products were directly sequenced with an ABI 3730 automated DNA sequencer. Newly determined sequences were submitted for BLAST search in GenBank to ensure that the required sequences had been amplified (Altschulet al., 1997),and then deposited in GenBank (MH117960-61). Eleven out of 13 species ofGracixaluswere included in our phylogenetic analyses:Gracixalus gracilipes(Bourret,1937),Gracixalus jinggangensis(Zenget al., 2017),Gracixalus jinxiuensis(Hu, 1978),Gracixalus lumarius(Rowleyet al., 2014),Gracixalus nonggangensis(Moet al., 2013),Gracixalus quangi(Rowleyet al., 2011),Gracixalus quyeti(Nguyenet al., 2008),Gracixalus sapaensis(Matsuiet al., 2017),Gracixalus seesom(Matsuiet al., 2015),Gracixalus supercornutus(Orlovet al., 2004),Gracixalus waza(Nguyenet al., 2013). All butG. supercornutuscame from the type localities. The sequences ofGracixalus carinensis(Boulenger, 1893)andGracixalus medogensis(Ye and Hu, 1984) were unavailable. In addition, forG. jinxiuensisgroup, we also chose specimens from non-type localities, including Hunan and Yunnan Provinces, China, Lai Chau, Nghe An,Lao Cai, Vietnam, and Houapan, Laos (Table 1).
Phylogenetic analyses were done using Bayesian inference (BI) implemented in MrBayes 3.12 (Ronquist and Huelsenbeck, 2003) and maximum likelihood (ML)on the RAxML Web server (http://phylobench.vitalit.ch/raxml-bb/; Stamatakiset al., 2008). The optimal nucleotide substitution model (GTR+I+G) was selected based on the Akaike Information Criterion (Posada and Buckley, 2004) as implemented in MrModeltest 2.3 (Nylander, 2004). Two independent runs with four Markov Chain Monte Carlo simulations were performed for 10 million iterations and sampled every 1000thiteration and the first 25% of samples were discarded as burn-in.Rhacophorus borneensis(Matsuiet al., 2013)andKurixalus odontotarsus(Ye and Fei, 1993) were used as outgroups following Matsuiet al.(2017). Uncorrected pairwise (p-distance) sequence divergence was calculated using MEGA version 7 (Tamuraet al., 2016).
3. Results
Gracixalus tianlinensis sp. nov.
HolotypeNHMG1706002 (Figure 1 A-C), adult male,from Cenwanglaoshan National Nature Reserve, Tianlin County, Guangxi, China (24.4883º N, 106.3947º E, 1858 m), collected by Yunming Mo, Xiaowen Liao and Shichu Zhou on 8 June 2017.
ParatypesNHMG1705009-11, 1706001-2, 1706006-8,adult males, and 1705008, 1706003-5, adult females from the same site as the holotype, collected by Yunming Mo,Shichu Zhou, Zhiming Xie and Xueqiang Lei, on 23 May and 8 June 2017.
DiagnosisG. tianlinensissp. nov. is assigned to the genusGracixalusbased on molecular data and morphological characters. Morphologically, the new species presents an intercalary cartilage between the terminal and penultimate phalanges of digits, tips of digits expanded into large disks with circummarginal grooves and also the vomerine teeth were absent with the pupil being horizontal.Gracixalus tianlinensissp. nov. is distinguished from allother rhacophorids by a combination of (1) SVL 30.3-35.9 mm in male, 35.6-38.7 mm in female, (2) head length less than head width, (3) vomerine teeth absent,(4) supratympanic fold distinct, (5) axilla and posterior surface of flanks pale yellow, (6) nuptial pads distinct on the first finger and slightly visible on the second finger,(7) dorsum brown to beige, with an inverse Y-shaped dark brown marking, (8) single subgular vocal sac.

Table 1 Samples and sequences used in this study. TL= type locality. The new determined sequences in this study were shown in italic bold. Generic allocation according to Frost (2017). Voucher abbreviations: AMS: Australian Museum; BORN: BORNEENSIS, University Malaysia, Sabah; CIB: Chengdu Institute of Biology; CUMZ: Chulalongkorn University Museum of Natural History; IEBR: Institute of Ecology and Biological Resources, Hanoi; KIZ: Kunmin Institute of Zoology; KUHE: Graduate School of Human and Environmental Studies, Kyoto University; MNHN: Muséum national d’Histoire naturelle, Paris; NHMG: Natural History Museum of Guangxi; SYS: Sun Yat-sen University; VNMN: Vietnam National Museum of Nature; VNUH: Vietnam National University, Hanoi; ZFMK; Zoologisches Forschungs-museum Alexander Koenig.
Description of holotypeBody dorsoventrally compressed; head length less than head width (HL/HW=0.9); snout rounded in dorsal view and in profile,projecting beyond margin of the lower jaw; canthus rostralis distinct and rounded; loreal region sloping and concave; interorbital region flat; nostril oval, laterally positioned, slightly protuberant, nearer tip of snout than eye; internarial distance greater than eye to nostril distance (IN/EN=1.5); pupil horizontal; eye diameter greater than eye to nostril distance (ED/EN=1.5);interorbital width greater than upper eyelid width (IOD/UEW=1.2); tympanic diameter less than eye diameter(TD/ED=0.5); supratympanic fold distinct and extending to axilla; vomerine teeth absent; choanae small and oval;tongue attached anteriorly, deeply notched posteriorly;external single subgular vocal sac.

Figure 1 (A) Dorsolateral, (B) dorsal and (C) ventral view of the holotype in life, (D) ventral view of the hand and (E) ventral view of the foot of the holotype, (F) showing nuptial pads on Finger I and Finger II.
Forelimb relatively robust, relative length of fingers I < II < IV < III; tips of all fingers with well-developed disks with horizontal circummarginal grooves, disks relatively wide compared to finger width, third finger disk width greater than tympanic diameter (FTD3/TD=1.2); fingers webbing absent; subarticular tubercles prominent, rounded, formula 1, 1, 2, 2; accessory palmar tubercles indistinct; nuptial pads present on posterolateral surface of Finger I and on basis of Finger II; nuptial pads of Finger I strongly dilated, and of Finger II small and slightly dilated. Relative length of toes I < I < III <V < IV; tips of toes disks with distinct circummarginal grooves; disks smaller than those of fingers; toes moderately webbed, webbing formula: I 1–1-II 1+–1+III 2+–2 IV 2+–2 V; subarticular tubercles rounded, distinct,formula 1, 1, 2, 3, 2; inner metatarsal tubercle oval,elongated; outer metatarsal tubercle and supernumerary tubercle absent (Figure 1 D-F). Dorsal surface of head,body and limbs rough, scattered tubercles, ventral surface of limbs smooth; chest, throat and belly granular; tarsal fold absent.
Measurements of holotype (in mm)SVL 33.6, HL 11.3,HW 12.3, SNT 5.3, ED 4.2, IOD 3.9, TD 2.0, UEW 3.2,TED 0.9, IN 4.2, EN 2.8, TIB 16.1, TW 5.3, FLL 17.5,HAL 11.0, FTD32.4, HLL 50.1, FOL 15.8, HTD42.1.
Color of holotype in lifeDorsal surface was found to be ranging from brown to beige, with an inverse Y-shaped dark brown marking across back, covering interorbital region and eyelids, bifurcating into two branches on the shoulder, and reaching the posterior of the back. Axilla and posterior surface of flanks pale yellow. Dorsal surface of arms with two brown transverse bands, and dorsal surface of limbs with three brown transverse bands.Ventral surface of the throat, chest gray with small dark specks. Belly creamy white. Ventral surface of thighs halftransparent creamy yellow. Iris bronze with a network of fine brown reticulations; pupil black.
Color of holotype in preservativeDorsal surface of head, body and limbs brown; Y-shaped marking and transverse bands black; ventral surface of chest and belly pale white and throat gray with variable brown marbling.
VariationMeasurements of the type series are shown in Table 2. All males have nuptial pads on Finger I and Finger II. On Finger I, the nuptial pads are strongly dilated, but small or just rudimentary on Finger II in some specimens. In life, dorsal coloration varied among individuals and within individuals over time and may be brown to beige or dark brown. The ventral surface of the throat varied from gray to brown.
EtymologyThis species is named after the locality in which it was collected. The suggested English name is Tianlin small tree frog (田林纤树蛙 in Chinese).
Advertisement callAmbient air temperature was 18.0 °C when the male calls were recorded. The male advertisement call of the new species consists of one note with two pules per note. The interval of calls varies from 2 to 5s. The dominant frequency spectrum of the calls lays between 2.0 and 3.0 kHz, and harmonic presents at 4.5-5.5 kHz (Figure 2).
Molecular relationshipsOur preliminary phylogenetic trees were like Rowleyet al.(2014), Zenget al.(2017)and Matsuiet al.(2017) with the exception of the generic placement ofG. lumarius. In our results, the generic placement ofG. lumariuswas treated as Clade III. Then,we identified three supported clades within species that is currently proposed within the genusGracixalus, which is similar to Rowleyet al.(2014), Zenget al.(2017) and Matsuiet al.(2017). Clade I consists ofG. gracilipes,G.quangi,G. quyeti,G. seesomandG. supercornutus.Clade II consists ofG. jinggangensis,G. jinxiuensisgroup,G.nonggangensis,G. sapaensis,Gracixalussp.,G. wazaandG. tianlinensissp. nov.Gracixalus tianlinensissp.nov. is sister toG. sapaensiswith well-supported values(BP=0.99; PP=96) (Figure 3). Uncorrected pairwise distance (p-distance) betweenG. tianlinensissp. nov.andG. sapaensisis 2.3-3.2%.Gracixalus jinxiuensisgroup did not form a monophyletic group.Gracixalus jinxiuensis(TL) is close toG. jinggangensis, and both are close toG. jinxiuensis(Hunan) with a large distance(p-distance=5.8%). AmongG. jinxiuensis(China-Laos-Vietnam) group, three sequences share one haplotype(~450 bp) and are close toGracixalussp. with a large distance (p-distance=5.0-5.1%) (Table 3).
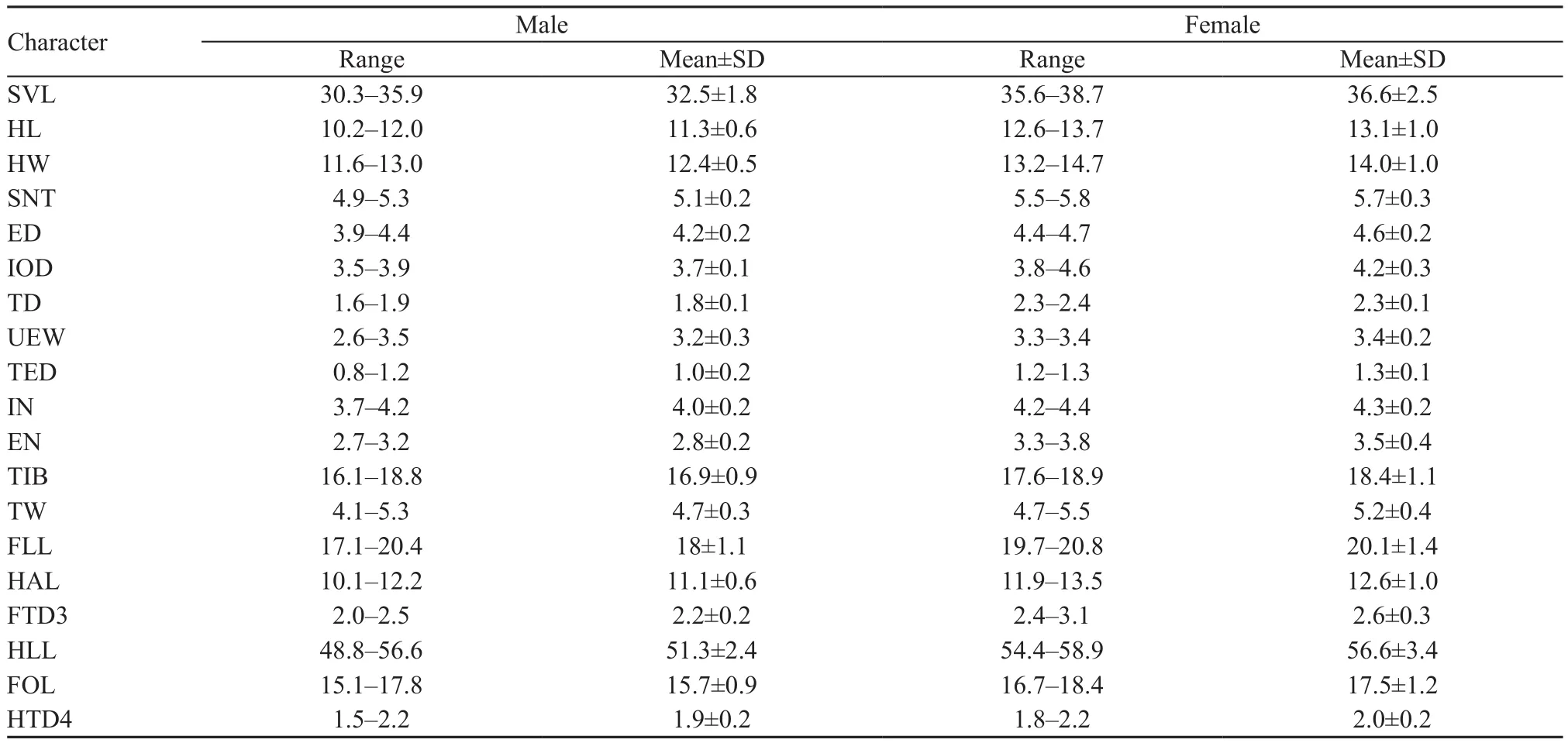
Table 2 Measurements (mm) of Gracixalus tianlinensis sp. nov. Abbreviations defined in text.
ComparisonsGracixalus tianlinensissp. nov. can be distinguished from most of the species ofGracixalusby having a brown to beige dorsum with an inverse Y-shaped dark brown marking, rounded snout in dorsal view and in pro file, axilla and posterior surface of flanks pale yellow,distinct nuptial pads on Finger I and slightly visible on basis of Finger II, with the exception ofG. jinggangensis,G. jinxiuensisandG. sapaensis. Considering advertisement calls,G. tianlinensissp. nov. just has one note and the dominant frequency spectrum of the calls lay between 2.5 and 3.1 kHz. ButG. jinggangensisandG.jinxiuensishave more than two notes of the advertisement calls (Huet al., 1978; Zenget al., 2017). Morphologically,G. tianlinensissp. nov. differs fromG. jinggangensisby having axilla and posterior surface of flanks pale yellow(vs.pale yellow absent inG. jinggangensis); finger webbing absent (vs. finger webbing rudimentary inG.jinggangensis); temporal region and corner of the mouth without large tubercles (vs.densely covered with large tubercles inG. jinggangensis); webbing formula, I 1–1-II 1+–1+III 2+–2 IV 2+–2 V (vs.I 2–2+II 1+–3 III 2–3 IV 3–2 V inG. jinggangensis).Gracixalus tianlinensissp.nov. differs fromG. jinxiuensisby having a larger size in adults (SVL 30.3-35.9 mm in male and 35.7-38.7 mm in femalevs. 24.2-26.3 mm in males and 28.0-29.2 mm in females inG. jinxiuensis); nuptial pads on Finger I and Finger II of males (vs.only on Finger I inG. jinxiuensis);axilla and posterior surface of flanks pale yellow (vs.pale yellow absent inG. jinxiuensis).Gracixalus sapaensishas dermal fringe along fifth toe, from tip of toe to base of metatarsal; nuptial spines absent on Finger I; a pair of long vocal slits on both sides of mouth floor well anterior to jaw commissure, dorsal surface golden ochre with a darker brownish marking on upper eyelid and across back, forming X marking, webbing yellowish; ventral surface of throat, chest, belly, and forelimb light yellow,hindlimb darker yellow; third finger disk width less than tympanic diameter; webbing formula: I 2–22/3II 11/2–3 III 2–31/3IV 22/3–11/2V; inner palmar tubercle flat, outer palmar tubercle divided into two, and iris golden with greenish reflection (vs. dermal fringe absent, external single subgular vocal sac, dorsal surface brown to beige,webbing gray, ventral surface of the throat, chest gray with small dark specks, belly creamy white, accessory palmar tubercles indistinct, third finger disk width greater than tympanic diameter (FTD3/TD=1.2), webbing formula: I 1–1-II 1+–1+III 2+–2 IV 2+–2 V, iris bronze with a network of fine brown reticulations, and nuptial pads on Finger I and Finger II inG. tianlinensissp. nov.).

Figure 2 Oscillogram and corresponding audiospectrogram of two advertisement calls of a paratypic male G. tianlinensis sp. nov.(NHMG1706006). Ambient air temperature was 18.0 °C.
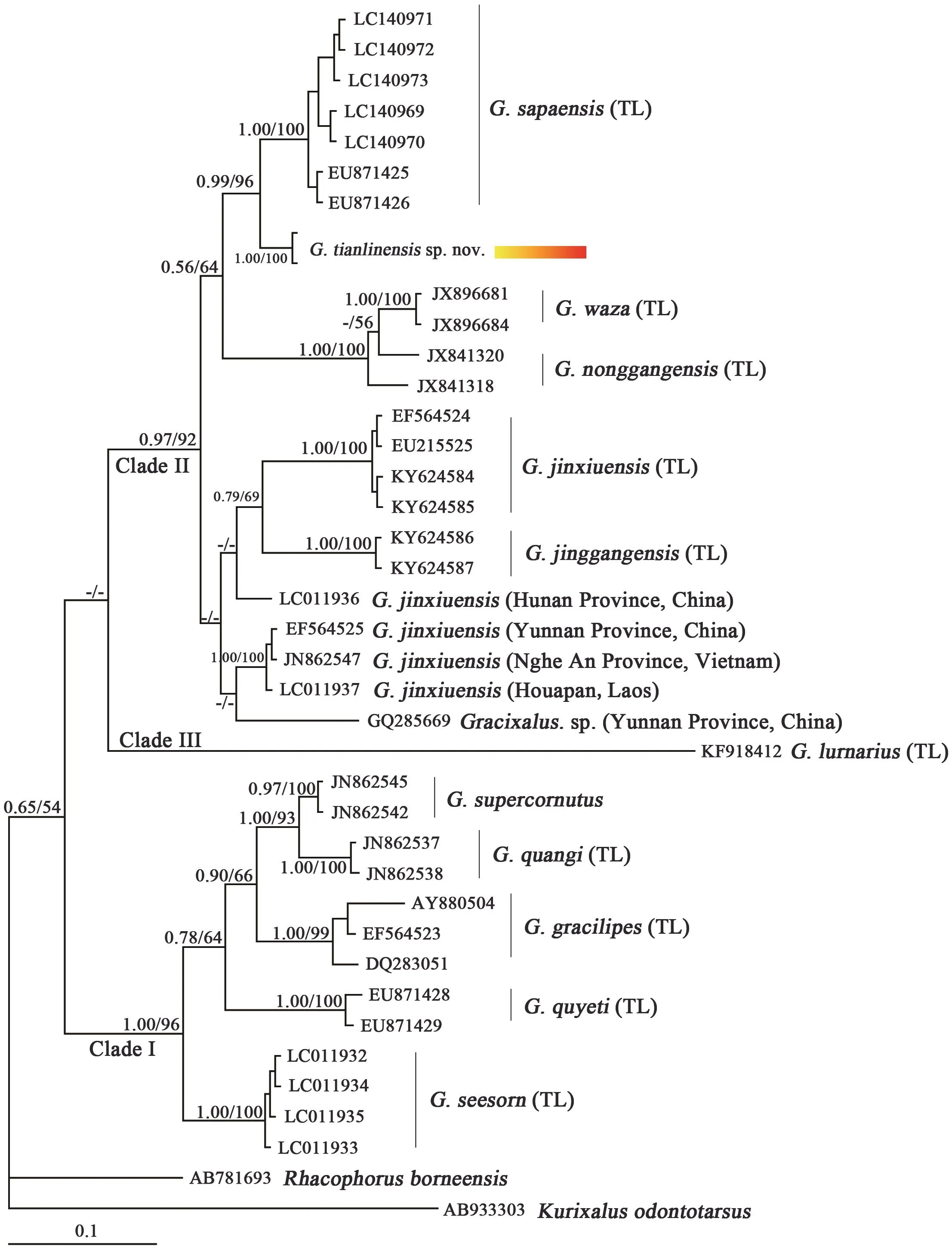
Figure 3 Bayesian inference tree reconstructed from 16S rRNA mitochondrial gene with Kurixalus odontotarsus and Rhacophorus borneensis as outgroups. Numbers above branches represent bootstrap supports for Bayesian posterior probabilities (BPP) ( > 0.5 retained) /bootstrap support for maximum likelihood analyses ( > 50 retained); ‘-’ represents BPP and bootstrap proportions lower than 50% and 50%,respectively.

Table 3 Uncorrected p-distances (in %) in the genus Gracixalus based on mitochondrial 16S rRNA sequences (~530bp). TL =type locality, C-L-V= China-Laos-Vietnam.
Gracixalus tianlinensissp. nov. differs fromG.carinensisas it has SNT longer than ED (vs. SNT less than ED inG. carinensis), ventral surface of chest and belly pale white and throat gray with variable brown marbling (vs. immaculate white inG. carinensis), nuptial pads on Finger I and Finger II (vs. on Finger I inG.carinensis) (Boulenger, 1893; Zenget al., 2017; Matsuiet al., 2017).Gracixalus nonggangensisandG. wazadiffer fromG. tianlinensissp. nov. by having yellow-green dorsum, supratympanic fold indistinct (vs. brown dorsum and supratympanic fold distinct in the new species), dorsal skin smooth (vs. rough in the new species), outer palmar tubercle divided into two (vs. palmar tubercles indistinct in the new species), and nuptial pads on Finger I (vs. on Finger I and Finger II in the new species).Gracixalus gracilipes,G. quangi,G. quyeti, andG. supercornutusdiffer fromG. tianlinensissp. nov. by having a greenish dorsum and spines on upper eyelid present (vs. brown to beige dorsum and spines on upper eyelid absent in the new species).Gracixalus gracilipes,G. quangi,andG.supercornutushave a triangular pointed snout (vs.snout rounded in the new species). Furthermore,G. quyeti, andG. supercornutushave a tibiotarsal projection (vs. absent in the new species).Gracixalus quangidiffers fromG.tianlinensissp. nov. by having the greenish translucent skin, triangularly pointed snout and the presence of a tibiotarsal projection (vs. brown dorsum, rounded snout and tibiotarsal projection absent in the new species).Gracixalus lumariusdiffers fromG. tianlinensissp.nov. by having venter pink (vs. creamy white in the new species), supratympanic fold indistinct (vs. supratympanic fold distinct and extending to axilla in the new species),dorsum with distinctive white conical tubercles in adult males (vs. absent white conical tubercles in the new species), nuptial pads on inner surface of the prepollex(vs. nuptial pads on Finger I and Finger II in the new species).Gracixalus medogensishas smaller body size,SVL 26.5 mm in male; dorsal skin smooth; nuptial pads on the first fingers in male present (vs.SVL, 30.3-35.9 mm in male and 35.7-38.7 mm in female; dorsal skin rough; nuptial pads on Finger I and Finger II in male present in new species).Gracixalus tianlinensissp. nov.differs fromG. seesomby having a larger SVL, 30.3-35.9 mm in male and 35.7-38.7 mm in female (vs.21.6-23.0 mm in males, 23.2-25.4 mm in females inG. seesom), a single subgular vocal sac in male (vs.a pair of vocal slits inG. seesom), rounded snout (vs. triangular snout inG.seesom), brown dorsum with an inverse Y-shade marking(vs. light yellowish brown dorsum without an inverse Y-shade marking inG. seesom), nuptial pads on Finger I and Finger II (vs. nuptial pads absent inG. seesom).
4. Discussion

Figure 4 Map of the type localities of Gracixalus.
Given the morphological distinctness, advertisement calls, and genetic evidence, we describedG. tianlinensissp. nov. as a new member ofGracixalus. AlthoughG.tianlinensissp. nov. is similar toG. jinggangensisandG. jinxiuensis, their advertisement calls and genetic variation are distinct from each other (Table 3, Figure 2 and Figure 3).
The correct identification of the taxa studied is essential for phylogenetic hypothesis. Matsuiet al.(2017) pointed out thatG. jinxiuensis(GenBank no.EU871425-26, from Lai Chau, Vietnam),G. carinensis(LC011938-39, LC140969-70, Lao Cai, Vietnam)andAquixalus odontotasrus(AY880503, Lao Cai,Vietnam) were mis-identified. Above three species were assigned toG. sapaensisbased on their molecular and morphological data. In our phylogenetic analyses, most of sequences either were holotype and paratype, or from topotype, which ensured the correct identification.In our preliminary results, we detected three distinct clades, which were similar to Rowleyet al.(2014) and Matsuiet al.(2015, 2017) (Figure 3). Clade III just includedG. lumariuswith weakly supported values.Position ofG. lumariuswas enigmatic and needs to be tested further. In Clade I, five species,G. gracilipes,G.quangi,G. quyeti,G. supercornutus,G. seesomform five evolutionary branches with relatively robust values(Figure 3). Interestingly, in Clade II, the individuals ofG. jinxiuensisformed the non-monophyly.Gracixalus jinxiuensis(TL) formed a monophyly and was close toG.jinggangensiswith a large distance (p-distance=6.9%).The genetic distance betweenG. jinxiuensis(TL) andG.jinxiuensis(Hunan) reached 5.8%,G. jinxiuensis(TL)andG. jinxiuensis(China-Laos-Vietnam) is 5.8-6.2%(Table 3). Considering relative large genetic variation and phylogenetic analyses, we suggest thatG. jinxiuensis(Hunan) andG. jinxiuensis(China-Laos-Vietnam) are two unknown members ofGracixalus.The genetic distance betweenGracixalussp. (Yunnan, GQ285669)andG. jinxiuensis(TL),G. jinxiuensis(Hunan) andG.jinxiuensis(China-Laos-Vietnam), all are larger than 5.0% (Table 3). Hence, we suggest thatGracixalussp. (Yunnan, GQ285669) is an unknown member ofGracixalustoo.Figure 4 showed most type locality of the genusGracixalus.
So far,G. tianlinensissp. nov. is only found in Cenwanglaoshan National Nature Reserve and restricted to high-elevation evergreen forest with montane bamboo.More distribution of the new species is unknown.
Acknowledgments The work was generously funded by Guangxi Natural Science Foundation under Grant No.2016GXNSFAA380007. The authors wish to thank Vivek SRIVASTAVA from University of British Columbia for his English revision and the anonymous reviewers for valuable suggestions.
Altschul S. F., Madden T. L, Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSI BLAST: a new generation of protein database search programs.Nucl Acid Res, 25: 3389–3402
Boulenger G. A. 1893. Concluding report on the reptiles and batrachians obtained in Burma by Signor L. Fea dealing with the collection made in Pegu and the Karin Hills in 1887-88. Ann Mus Civ Stor Nat Genova, 13: 304– 347
Bourret R. 1937. Notes herpétologiques sur lIndochine française.XIV. Les batraciens de la collection du Laboratoire des Sciences Naturelles de lUniversité. Descriptions de quinze especes ou variétés nouvelles. Annexe Bull GenInstr Publique, 1937: 5–56
Delorme M., Dubois A., Grosjean S., Ohler A. 2005. Une nouvelle classification générique et subgénérique de la tribu des Philautini(Amphibia Anura Rhacophorinae). Bull Mens Soc Linn Lyon,74: 165–171
Fei L., Ye C. Y., Jiang J. P. 2010. Colored Atlas of Chinese Amphibians. Sichuan Publishing Group Sichuan Publishing House of Science Technology Sichuan, 519 pp
Frost D. R. 2017. Amphibian Species of the World: an Online Reference. Version 6.0 (accessed August 23, 2017) Electronic Database accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History,New York, USA
Hu S. Q., Fei L., Ye C. Y. 1978. Three new amphibian species in China. Material Herpetol Res, 1978: 4
Kumar S., Stecher G., Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets.Mol Bio Evol, 33(7): 1870–1874
Li J. T., Che J., Bain R. H., Zhao E. M., Zhang Y. P. 2008.Molecular phylogeny of Rhacophoridae (Anura): a framework of taxonomic reassignment of species within the generaAquixalus Chiromantis RhacophorusandPhilautus. Mol Phylogenet Evol,48: 302–312
Li J. T., Che J., Murphy R. W., Zhao H., Zhao E. M., Rao D. Q.,Zhang Y. P. 2009. New insights to the molecular phylogenetics and generic assessment in the Rhacophoridae (Amphibia:Anura) based on five nuclear and three mitochondrial genes with comments on the evolution of reproduction. Mol Phylogenet Evol, 53: 509–522
Matsui M., Khonosue W., Panha S., Eto K. 2015. A new tree frog of the genusGracixalusfrom Thailand (Amphibia:Rhacophoridae). Zool Sci, 32: 204–210
Matsui M., Ohler A., Eto K., Nguyen T. T. 2017. Distinction ofGracixalus carinensisfrom Vietnam and Myanmar with description of a new species. Alytes, 33: 25–37
Mo Y. M., Zhang W., Luo Y., Zhou S. C., Chen W. C. 2013.A new species of the genusGracixalus(Amphibia: Anura:Rhacophoridae) from Southern Guangxi China. Zootaxa, 3616(1): 61–72
Myers C. W., Duellman W. E. 1982. A new species ofHylafrom Cerro Colorado and other tree frog records and geographical notes from western Panama. Am Mus Nat His Novit, 2752: 1–32
Nguyen T. Q., Hendrix R., Böhme W., Vu T. N., Ziegler T.2008. A new species of the genusPhilautus(Amphibia: Anura:Rhacophoridae) from the Truong Son Range Quang Binh Province central Vietnam. Zootaxa, 1925: 1–13
Nguyen T. Q., Le M. D., Pham C. T., Nguyen T. T., Bonkowski M., Ziegler T. 2013. A new species ofGracixalus(Amphibia:Anura: Rhacophoridae) from northern Vietnam. Org Div Evol,13: 203–214
Nylander J. A. A. 2004. MrModeltest v2. Program Distributed by the Author. Evolutionary Biology Centre Uppsala University
Orlov N. L., Ho T. C., Nguyen Q. T. 2004. A new species of the genusPhilautusfrom Central Vietnam (Anura: Rhacophoridae).Russ J Herpetol, 11: 51–64
Palumbi S. R., Martin A., Romano S., McMillan W. O., Stice L., Grabowski G. 1991. The simple fool’s guide to PCR.Department of Zoology University of Hawaii Honolulu, 47pp
Posada D., Buckley T. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Bio, 53:793–808
Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19:1572–1574
Rowley J. J. L., Dau Q. V., Nguyen T. T., Cao T. T., Nguyen S. N.2011. A new species ofGracixalus(Anura: Rhacophoridae) with a hyperextended vocal repertoire from Vietnam. Zootaxa, 3125:22–38
Rowley J. J. L., Le D. T. T., Dau V. Q., Hoang H. D., Cao T. T.2014. A striking new species of phytotelm breeding tree frog(Anura: Rhacophoridae) from central Vietnam. Zootaxa, 3785(1): 25–37
Stamatakis A., Hoover P., Rougemont J. 2008. A Rapid Bootstrap Algorithm for the RAxML Web-Servers. Syst Bio, 75: 758–771
Yu G. H., Rao D. Q., Zhang M. W., Yang J. X. 2009. Reexamination of the phylogeny of Rhacophoridae (Anura) based on mitochondrial and nuclear DNA. Mol Phylogenet Evol, 50:571–579
Zeng Z. C., Zhao J., Chen C. Q., Chen G. L., Zhang Z., Wang Y. Y. 2017. A new species of the genusGracixalus(Amphibia:Anura: Rhacophoridae) from Mount Jinggang southeastern China. Zootaxa, 4250 (2): 171–185
猜你喜欢
杂志排行
Asian Herpetological Research的其它文章
- Effects of Increased Salinity on Growth, Development and Survival in Early Life Stages of the Green Toad Bufotes variabilis (Anura:Bufonidae)
- Sexual Dimorphism, Female Reproductive Characteristics and Egg Incubation in an Oviparous Forest Skink (Sphenomorphus incognitus) from South China
- Amphibian Species Contribute Similarly to Taxonomic, but not Functional and Phylogenetic Diversity: Inferences from Amphibian Biodiversity on Emei Mountain
- A Rapid, Non-invasive Method for Anatomical Observations of Tadpole Vertebrae in Vivo
- Three New Ranidae Mitogenomes and the Evolution of Mitochondrial Gene Rearrangements among Ranidae Species
- A New Species of the Genus Sinomicrurus Slowinski, Boundy and Lawson, 2001 (Squamata: Elapidae) from Hainan Province, China
