A Double Network Hydrogel with High Mechanical Strength and Shape Memory Properties
2018-06-27LeiZhuChunmingXiongXiofenTngLijunWngKngPengHiyngYng
Lei Zhu,Chun-ming Xiong,Xio-fen Tng,Li-jun Wng,Kng Peng,Hi-yng Yng∗
a.CAS Key Laboratory of Soft Matter Chemistry,School of Chemistry and Materials Science,University of Science and Technology of China,Hefei 230026,China
b.Research Institute of Science and Technology,China National Petroleum Corporation,Beijing 100083,China
I.INTRODUCTION
As a kind of smart“soft and wet”materials with three-dimensional network structure,hydrogels have attracted great attention in many fields[1–5].Generally,hydrogels have high permeability to small molecules and can respond to various stimuli,such as temperature,pH,light,enzymes,etc.[6–10].However,the poor mechanical properties restrict their applications in more attractive area like tissue engineering and contact lenses[11].Previously,several strategies have been explored to enhance the mechanical strength of hydrogels,including topological gel[12],tetra-PEG gel[13],nanocomposite hydrogel[14],and double network(DN)gel[15].
Double network gels as one kind of extremely tough hydrogels have a very special interpenetrating network structure[16,17].The DN gels with an optimized structure exhibit high water content(90%),excellent strength(failure tensile stress 1−10 MPa,strain 1000%−2000%;failure compressive stress 20−60 MPa,strain 90%−95%),and toughness(tearing fracture energy of 100−1000 J/m2)[18].By altering the compositions of DN gels,the mechanical properties will change accordingly[17,19].
Generally,conventional double-network gels were prepared in two steps.The first polyelectrolyte gel was synthesized and further immersed in a solution of the second monomer for fully swollen,then the second neutral gel was obtained in the presence of the first gel[15,20].In particular,the double-network gels consist of two contrasting structures.The densely cross-linked first network in low concentrations was rigid and brittle,usually serving as a sacrificial bond that fractures at an early stage of deformation to dissipate a large amount of energy;while the sparsely cross-linked second network was flexible and concentrated,maintaining the integrity of the DN hydrogels[18,21,22].The fracture process of the first network enormously increased the toughness of DN gels.This DN concept was considered applicable to any chemical species of polymeric materials if the aforementioned contrasting DN structure was satisfied[19].
For extensive application,Gong and her coworkers synthesized various DN gels from kinds of chemical species[23,24].In addition,Chen et al.prepared a double network hydrogel with high mechanical strength by combining poly(N,N-dimethylacrylamide)(PDMAAm)and sodium hyaluronate(HA)[25].Tasuku Nakajima et al.found the first network can be not only polyelectrolytes but also a neutral network loaded with polyelectrolytes[19].Unfortunately,in order to construct the contrasting networks and make more seed monomers of the second network into the first network,the candidate species for the soft and ductile second network in these DN gels are neutral constituents.If electrolytes can be introduced into the second network of DN gels,some interesting functions might be introduced.As well known,acrylic acid,a weak electrolyte was extensively designed to synthesize redox or light responsive hydrogels based on the complexation between carboxyl group of acrylic acid and ferric ions,and these prepared hydrogels have sol-gel transition character[26,27].and shape memory behavior[28].Shape memory hydrogels have potential applications in soft robotics,shape memory toys,and smart textiles.If the neutral monomers of the second network can be replaced by acrylic acid and then polymerized in the presence of the first network,the obtained DN gels will possess both high mechanical strength and extraordinary responsiveness.
In this study, firstly the PEG gel is synthesized by the reaction of norbornene end-functionalized PEG with a tetra-functional thiol cross-linker PETMP(pentaerythritol tetra(mercaptopropionate)).Then we use the PEG gel as the first network and the second network is constructed by the copolymerization of acrylic acid(AAc),acrylamide(AAm)and N,N′-methylenebisacrylamide(MBAA).In order to make the PEG gel fully swollen and obtain a rigid and brittle first network,PDAC as polyelectrolyte is introduced into the first network before the PEG gel is immersed into the precursor solution of second network.The high swelling degree of the first network is mainly promoted by the increased osmotic pressure,which is caused by the dissociated counter-ions of polyelectrolyte[29].The dissociation of Cl−ions from PDAC will make the PDAC carry a large number of positive charges.However,the introduction of electrolytes with negative charges as the seed monomer for the second network will shield the positive charges of PDAC and the swelling degree of PEG gel will decrease.Accordingly,we use weak electrolyte AAc and copolymerize it with AAm to decrease the shielding effect caused by the interaction between dissociated PDAC and the dissociated acrylic acid.As a result,the first network is well extended and we obtained a high strength DN gel,of which the second network was copolymers of acrylic acid and acrylamide.The mechanical measurement exhibits the hydrogels have high tensile strength and toughness.The fracture stress and toughness of the DN gel(1 mol/L AAc and 3 mol/L AAm)reach up to 0.9 MPa and 3.8 MJ/m3,respectively.Furthermore,as for the extra application of the DN gels with polyelectrolytes(PAAc-co-PAAm)as the second network,the obtained hydrogels exhibited ascorbic acid activated shape memory properties.
II.MATERIALS AND METHODS
A.Materials
Polyethylene glycol 4000,2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone,N,N′-dicyclohexylcar bodiimide(DCC),5-norbornene-2-carboxylicacid(98%mixture of endo and exo),pentaerythritol tetrakis(3-mercaptopropionate)(PETMP),2-acrylamido-2-methylpropanesulfonic acid sodium(AMPS)and 4-(dime thylamino)pyridine(DMAP)werepurchasedfrom Sigma-Aldrich.Acryloyloxyethyltrimethyl ammoniumchloride(DAC),N,N′-methylenebisacrylamide(MBAA),acrylamide(AAm),acrylic acid(AAc),sodium chloride(NaCl),pyridine,ascorbic acid and iron(III)chloride hexahydrate were obtained from Sinopharm Chemical Reagent Co.Ltd.and used as received.
B.Synthesis of PEG-2norbornene(norbornene-PEG-norbornene)
Norbornene-functionalized PEG was prepared by a previous report[30].Brie fly,5 equivalent(equiv.)DCC with respect to PEG hydroxyls,was reacted at room temperature with 10 equiv.5-norbornene-2-carboxylic acid and the solvent was anhydrous dichloromethane.After a white by-product precipitate formed(dicyclohexylurea),indicating the formation of dinorbornene carboxylic acid anhydride,the solution was stirred for 30 min.In a separate vessel,PEG(Mn=4 kDa),5 equiv.(with respect to hydroxyls)pyridine and 0.5 equiv.DMAP was dissolved in anhydrous dichloromethane and then added into the solution containing dinorbornene carboxylic acid anhydride.The reaction was stirred overnight,after which the mixture was filtered.The filtrate was washed with 5%sodium bicarbonate solution and the product was precipitated in ice-cold diethyl ether twice.1H NMR spectra were recorded on a Bruker Advance 400 M NMR spectrometer with CDCl3as solvent.1H NMR(CDCl3)δ/ppm:5.91−6.21(m,4H),4.14−4.28(m,4H),3.46−3.83(m,364H),2.97−3.07(m,2H),2.87−2.95(m,2H),2.21−2.29(m,2H),1.46−1.57(m,2H).
C.Synthesis of PEG gels

FIG.1 Schematic illustration of the synthesis of PEG-PDAC/P(AAm-co-AAc)DN gels with a contrast double network structure.
The PEG gels were prepared by a previous method[31].The norbornene end-functionalized PEG(Mn=4 kDa)(100 mg,2.5×10−2mmol),PETMP(6.1mg,1.25×10−2mmol),and2-hydroxy-4′-(2-hydroxyethoxy)-2-methyl-propiophenone as photoinitiator(1 mg)were dissolved in DMF(0.9 mL).The precursor solution was put into the glass mold or syringe and then exposed to ultraviolet light with a wavelength of 365 nm for 1.5 h.The obtained gels(called as-prepared PEG gels)were removed from the mold and repeatedly washed with excess DMF to remove unreacted materials.Finally,the gels were immersed in excess deionized water,which was to remove DMF.
D.Synthesis of PEG-PDAC gels
The PEG gels were immersed in 0.6 mol/L DAC solution and 0.1 mol%of 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone for 3 days.The gels were then sandwiched between two glass plates and wrapped by a plastic film.Finally,the gels were irradiated by 365 nm UV for 7 h under nitrogen atmosphere.The obtained PEG gels containing PDAC were called PEG-PDAC gels.
E.Synthesis of DN gels
A part of the PEG-PDAC gels was immersed in the second network precursor solution containing 2−4 mol/L of AAm,0−2 mol/L of AAc,0.02%of MBAA and 0.01%of 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone for about 18 h,and the total monomer concentration was 4 mol/L.Then the gels were sandwiched between two glass plates and wrapped by a plastic film.After that the gels were irradiated by 365 nm UV for 7 h to synthesize the second P(AAm-co-PAAc)networks within the PEG-PDAC gels.The obtained PEG-PDAC/P(AAm-co-AAc)gels were directly used for testing.In addition,the PEG-PDAC/P(AAmco-AMPS)gels were obtained by replacing AMPS with AAc.
The DN hydrogels were prepared by three steps as above through a molecular stent method[19],and the schematic of the synthesis is shown in FIG.1.
F.Swelling measurements
The one-dimensional swelling degree of the PEG gels is calculated by using the expression α=d/d0,where d is the thickness of PEG-PDAC gels after being immersed in the second monomer solution for 18 h and d0is the thickness of as-prepared PEG gels.
G.Mechanical measurements
Tensile tests were performed on cylindrical shaped P(AAm-co-AAc)gels,dumbbell shaped PEG gels and PEG-PDAC/P(AAm-co-AAc)gels.The tensile velocity was 100 mm/min.The tensile stress was defined as the force divided by the original cross-sectional area.The tensile stress was defined as the length of the deformed sample divided by that of the sample in the relaxed state.The fracture tensile stress and strain were determined by the breaking point of the stress-strain curve.The yield point was defined as where the slope of the stress-strain curve is zero.The work of extension W was determined as the area under tensile stress-strain curves.
H.Shape memory behavior
Firstly,a strip of hydrogel(width 3 mm,thickness 2.45 mm and length about 2.8 cm)was fixed at“U”shape and immersed into 0.5 mol/L FeCl3solution for 1 h to form the temporary“U”shape.Then the hydrogel was transferred into 0.5 mol/L ascorbic acid solution to recover the initial shape.The shape fixity ratio and recovery ratio were measured by a previous method[32].The shape fixity ratio(Rf)and recovery ratio(Rr)were calculated by the expression Rf=θt/θiand Rr=(θi−θf)/θi,where the θiis the given angle,θtis the temporarily fixed angle,and θfis the final angle.
III.RESULTS AND DISCUSSION
A.Swelling properties of PEG gels
The swelling ability of the first network in DN hydrogel system is an important criterion in the final stage of the DN hydrogel preparation.Enough swelling ability facilitates the extension of the first PEG network,which increases the PEG network’s rigidity,and enriches the concentration of second network seed monomer inside the hydrogel before final polymerization.However,the neutral PEG gel as the first network shows poor swelling ability in the second monomer solution containing AAm and AAc.To overcome this circumstance,polyelectrolytes,PDAC,are introduced into the DN hydrogel system,which significantly increase the swelling degree of neutral PEG gel.The volume of PEG gel containing PDAC increases dramatically after immersed in the second monomer solution,for comparison,the volume of the neutral PEG gel has little change for 18 h.FIG.2(a)shows the one-dimensional swelling degree,α,of the PEG gels with various AAc and AAm concentrations.Here,the one-dimensional swelling degree of PEG gels means the ratio of the thickness of PEG-PDAC gels after being immersed in second monomer solution for 18 h to the thickness of the as-prepared PEG gels.And it was used to measure the extension of the PEG network.Obviously,swelling degree of PEG-PDAC gels will decrease with the increase of AAc concentration.The reason ascribed to the swelling of gels is an extra osmotic pressure,which is induced by the dissociated counter-ions[29],and the increased osmotic pressure can promote the swelling of gels[33,34].Due to the high dielectric constant of water,when the PEG gels containing PDAC were immersed in the distilled water or AAm aqueous solution,the Cl−ions tied with CH2N+(CH3)3ions will dissociate from PDAC and then the osmotic pressure will increase,which will lead the gels fully swollen.The acrylic acid as a weak electrolyte in the second monomer solution will decrease the swelling ratio of PEG gels.We conjecture that this phenomenon is a result of partial ionization of AAc.And the dissociation of Cl−ions from PDAC will make the field of the PDAC positive and a number of ionized acrylic acid will be attracted.Subsequently,the net charge of the polycation might be reduced and the osmotic pressure may also be reduced.With the increase of AAc concentration,the content of ionized AAc will increase and more positive charge of PDAC will be shielded.Thus the swelling degree of PEG gels will decrease.

FIG.2 One-dimensional swelling degree,α,of the PEG gels with(a)various AAc and AAm concentrations and(b)various NaCl concentrations.Data are given as average±SD(standard deviation)from three independent measurements.
In order to further prove the effect of ions on the swelling degree of PEG-PDAC gels,parts of the gels are immersed in various concentration of NaCl solution.As shown in FIG.2(b),the one-dimensional swelling degree of PEG gels decreased with the increase of NaCl concentration.Compared to the AAc,a little content of NaCl can make the swelling degree of PEG gels decrease a lot.For example,after the PEG-PDAC gel was immersed in 1 mol/L AAc and 3 mol/L AAm mixed solution for 18 h,the one-dimensional swelling degree of PEG gel was about 2.47.While only 0.001 mol/L NaCl solution can make the swelling degree of PEG gel decrease to 2.466.It is clearly that the existence of ions in the second network precursor solution will decrease the swelling degree of PEG gels.But AAc as a weak electrolyte,and it can only ionize a little amount and shield little ions in the system.Despite of the moderate concentration of AAc,the one-dimensional swelling degree is still large.At large α,the first network PEG chains are in extended state.With the decrease of α,the chains are gradually coiled[35].
B.Mechanical properties of PEG-PDAC/P(AAm-co-AAc)DN gels
The extensive swelling of PEG-PDAC gels facilitates the preparation of a typical double-network hydrogel,resulting in the significant improvement of mechanical properties subsequently.The prepared PEGPDAC/P(AAm-co-AAc)DN gels show high mechanical performance.FIG.3(a)shows the tensile stress-strain curves of the PEG-PDAC/P(AAm-co-AAc)DN gel(1 mol/L AAc and 3 mol/L AAm),the single-network PEG gel and P(AAm-co-AAc)gel(1 mol/L AAc and 3 mol/L AAm).The single-network PEG gel was too weak,which suffered fracture at low stress and strain.From FIG.3(a),although the single-network P(AAmco-AAc)gel shows much higher fracture stress than the single-network PEG gel,also suffered fracture under low stress.For comparison,the PEG-PDAC/P(AAm-co-AAc)gel(1 mol/L AAc and 3 mol/L AAm)had excellent mechanical strength(high fracture stress(0.9 MPa)and fracture strain(8.3)).It is obvious that the DN gel has higher mechanical strength than single network gels.
As mentioned before,AAc units in the second network serve as the physical crosslinking point for further coordinating with Fe3+.For this consideration,the concentration of AAc should be above a certain value to maintain an enough crosslinking density with Fe3+.But in previous swelling section,the data and analysis show that too high AAc concentration results in limited swelling degree of PEG-PDAC gels,which has huge influence on the mechanical performance of the final DN hydrogel.This might be attributed to the following reason.Different swelling degree leads to different extension ratios of PEG network which acts as the first network.Extensive studies showed that the high mechanical strength and excellent toughness was related to the extension and further fracture of the brittle first network[36–39].The extension and fracture of the first network dissipated a lot of energy to toughen the DN gel[17,18,40].Under the above consideration,the ratio of concentration of AAc and AAm monomers should be set at a suitable range.The ideal ratio for the two monomers should balance the strength of the hydrogel with enough concentration of AAc monomer.As a consequence,it is necessary to research the relationship between the swelling degree of PEG gels and the mechanical strength of DN gels.Subsequently,we synthesized a series of PEG-PDAC/P(AAm-co-AAc)DN gels with various AAc concentration and evaluated their strength and toughness.
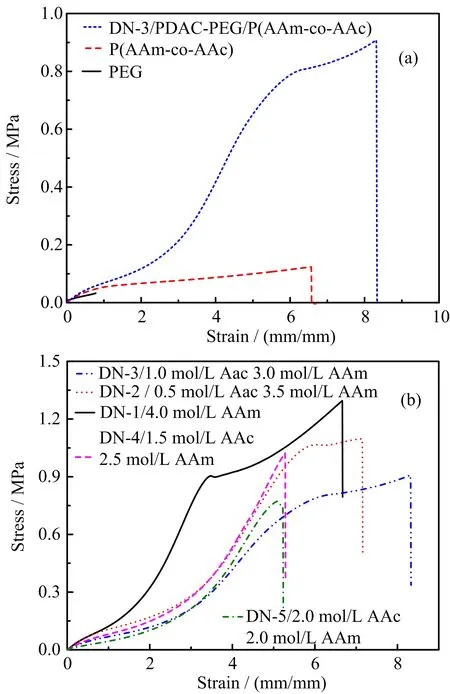
FIG.3 (a)Tensile stress-strain curves of the PEG gel,P(AAm-co-AAc)gel(1 mol/L AAc and 3 mol/L AAm)and PEG-PDAC/P(AAm-co-AAc)DN gel(1 mol/L AAc and 3 mol/L AAm).(b)Tensile stress-strain curves of PEGPDAC/P(AAm-co-AAc)DN gels with various AAc concentrations.
FIG.3(b)shows the tensile stress-strain curves of the PEG-PDAC/P(AAm-co-AAc)DN gels with various AAc concentrations.In addition,the σfand W values of PEG-PDAC/P(AAm-co-AAc)DN gels with various AAc concentrations are listed in Table I.Here,the tensile fracture stress σfwas usually regarded as the index of strength.The work of extension W defined as the area under the tensile stress-strain curves,which is the total work required to break a unit volume of a material and was used to measure the toughness of material[19].The result shows that the PEG-PDAC/P(AAmco-AAc)DN hydrogel exhibits high mechanical strength and toughness at low AAc concentration and shows poor mechanical strength and toughness at high AAc concentration.Moreover,the PEG-PDAC/P(AAm-co-AAc)DN gels show yielding-like behavior,which has been generally observed in tough DN gels previously[19,36].In contrast,when AAc concentration was more than 1.5 mol/L,the disappeared yielding-like behavior limit the materials mechanical performance,especially for the work of extension W.Based on the swelling degree of PEG-PDAC gels,mechanical and toughness of PEG-PDAC/P(AAm-co-AAc)DN gels,and tensile stress-strain curves of the DN gels,we found that when the swelling degree of PEG gels was high,the mechan-ical strength and toughness of DN gels were excellent.

TABLE I Compositions and the mechanical properties of the P(AAm-co-AAc)and PEG-PDAC/P(AAm-co-AAc)DN gels.DN-i represent PEG-PDAC/P(AAm-co-AAc)DN gels with various AAm and AAc concentration(i=1,2,3,4,5).CAAcand CAAm(in mol/L)are the concentration of AAc and AAm respectively.σf(in MPa)is the fracture stress of gels.W(in MJ/m3)is the total work required for the fracture of a unit volume of a material.
Takahiro Matsuda et al.proposed that when the DN gels are subjected to force,the brittle first network will break and dissipate energy,which will increase the energy required to completely fracture DN gels[35].In our DN gel system,when the swelling degree of PEG gel is higher,the PEG chains are more brittle and easier to break,which will increase the energy to break the PEG-PDAC/P(AAm-co-AAc)DN gel.Eventually,the mechanical strength and toughness of the DN gel will get enhanced.
In addition,some researches[17,18]reveal that more entanglement between the second network and the first network will help transfer force,and the first network is more prone to fracture to dissipate energy,which is helpful for strengthening DN gels.In our DN gels,the high swelling degree of the PEG gels leads to abundant seed monomers of the second network penetrating into the PEG-PDAC gels.After polymerization,the entanglement between PEG networks and P(AAm-co-AAc)networks will be high enough to enhance the strength and toughness of the DN gels.In contrast,the poor swelling degree of PEG gels has much soft PEG chains,which is hard to break to dissipate energy.Also,this will weaken the entanglement between PEG networks and P(AAm-co-AAc)networks,leading to a poor mechanical performance.All these results and theories demonstrate the importance of the swelling degree of the first network in achieving a high mechanical performance DN hydrogel.

FIG.4 (a)A strip of the representative sample with a width of 2.5 mm and a thickness of 2.42 mm lifts up a steel block of 500 g in weight;and(b)The representative sample is elongated to several times of its original length.
Furthermore,FIG.3(b)shows that the fracture stress of the PEG-PDAC/P(AAm-co-AAc)gels with moderate AAc concentrations remains a high level and the fracture strain exceeds 800%(DN-3,AAc concentration is 1 mol/L).According to Table I,the PEGPDAC/P(AAm-co-AAc)DN gels show higher σfand W than the single network PEG gel and P(AAm-co-AAc)gel.When the AAc concentration is 1 mol/L,the PEG-PDAC/P(AAm-co-AAc)DN gel exhibit high σf(0.9 MPa)and excellent W(3.8 MJ/m3),which is comparable to those of the DN gels prepared by molecular stent method[19].As shown in FIG.4,a strip of the representative sample with a width of 2.5 mm and a thickness of 2.42 mm could lift up a steel block of 500 g in weight,and could be elongated to several times of its original length.Moreover,the PEG-PDAC/P(AAmco-AAc)gel with 1 mol/L AAc concentration has shape memory effect.Therefore,the PEG-PDAC/P(AAm-co-AAc)DN gel with 1 mol/L AAc and 3 mol/L AAm was selected as a representative sample for further study.
C.Effects of swelling degree of the first network on the mechanical strength of DN hydrogels
It is worth noting that the introduction of AAc into the second network also introduced ion interaction between linear PDAC and P(AAm-co-AAc)network.But the ion interaction has little contributions to the improvement of the mechanical strength of DN gels.On one hand,the high toughness and excellent mechanical strength of DN gels come mainly from the combination of brittle and ductile networks[17,24,40].On the other hand,the pKaof AAc in water is 4.25 at 25◦C[41],which means only small amount of AAc will be ionized in water,and hence the ion interaction between linear PDAC and P(AAm-co-AAc)network is weak.On top of that,experiments above demonstrate that the introduction of AAc does not increase the mechanical strength and toughness of DN gels,instead,the high concentration of AAc decreases those of DN gels.Obviously,it is the swelling degree of the first network that has great influence on the mechanical properties of PEG-PDAC/P(AAm-co-AAc)DN gels.
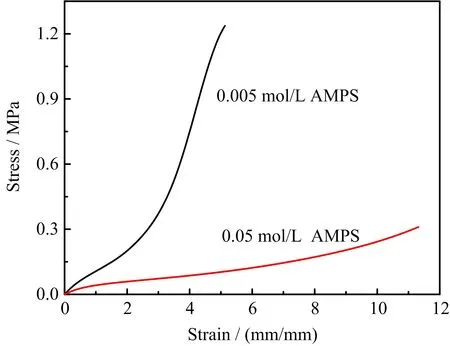
FIG.5 Tensile stress-strain curves of PEG-PDAC/P(AAmco-AMPS)DN gels with various AMPS concentrations.
As mentioned before,the effect of strong electrolytes on the swelling properties of PEG-DAC gels was tremendous.A little strong electrolytes can make the swelling properties of PEG-DAC gels decrease a lot.To further illustrate the influence of the swelling degree of the first network on the mechanical properties of DN gels,AMPS as strong electrolytes were introduced into the second network of DN gels.On one hand,there exists ion interaction between negative charged AMPS and positive charged PDAC,on the other hand,the ion interaction will lower the swelling degree of the first network.FIG.5 shows the tensile stress-strain curves of PEG-PDAC/P(AAm-co-AMPS)gels.The total monomer concentration of the second network precursor solution was 4 mol/L.When the AMPS concentration was 0.005 mol/L,the fracture stress of the PEGPDAC/P(AAm-co-AMPS)DN gel was about 1.23 MPa.While the AMPS concentration reached 0.05 mol/L,the fracture stress of the DN gel was only 0.31 MPa.It is obvious that the high AMPS concentration lowered the fracture stress of the DN gel.Therefore,it is necessary to observe the swelling degree of the PEG gels with various AMPS and AAm concentrations.When the AMPS concentration was 0.005 mol/L,the swelling degree of PEG gel was about 2.38,which is beneficial to the permeation of the substantial monomers of the second network into the PEG gels.Therefore,the relatively high swelling degree of PEG gel and the entanglement between PEG networks and P(AAm-co-AMPS)networks both will strengthen the PEG-PDAC/P(AAmco-AMPS)DN gels a lot.However,when the AMPS concentration reaches 0.05 mol/L,the swelling degree of PEG gel was about 1.9.The poor swelling ability of the PEG gel makes the PEG network soft and seriously prevents more monomers of the second network into the PEG gel.Thus,the tensile stress of the PEGPDAC/P(AAm-co-AMPS)gels decreased a lot when the AMPS concentration increased.The results also indicate that the high swelling degree of the first network was the main reason for the mechanical improvement of DN gels.
D.Redox-responsive shape memory performance
Fe3+had been introduced into networks of gels and it could coordinate with carboxyl groups as a crosslinking points[26,42].The coordination interactions between Fe3+and carboxyl group behave as reversible sacrificial bonds to dissipate energy,which enhanced the mechanical properties of gels[43].Nevertheless,a previous research reported that the carboxyl group could form much weaker complexation with Fe2+than Fe3+[27].Since the Fe3+-carboxyl complexation is redox-sensitive,the ascorbic acid can reduce Fe3+to Fe2+,and the gels can exhibit ascorbic acid-responsive shape memory behavior.After immersed into FeCl3solution,the Fe3+ions are introduced into the hydrogel and complexed with carboxyl groups.Therefore,a temporary shape can be fixed by the additional crosslinking points,and the gel can be switched from a temporary shape to the original shape through weakening or breaking the cross-linking.As shown in FIG.6(a),after immersing the“U”shaped representative sample into 0.5 mol/L FeCl3solution for 1 h,the original straight hydrogel was fixed at a“U”shape as a result of the introduction of coordination interaction into the hydrogel.Meanwhile,endowed by Fe3+ions,its color changed from transparent to brown.In order to recover the“U”shape to the original shape,the hydrogel was immersed into 0.5 mol/L ascorbic acid solution.With the color of the gel changing from brown back to transparent,the temporary“U”shape is unable to be sustained and its original straight shape will recover in 1 h,which indicates that the Fe3+is reduced to Fe2+and Fe3+-carboxyl complexation is broken.Then the gel performed a shape memory behavior again.Tensile tests of the representative sample with and without Fe3+are used to demonstrate the mechanical enhancement of the complexation between Fe3+ions and carboxyl groups for the hydrogel.FIG.6(b)shows the stress-strain curves of the representative sample with and without Fe3+.When introducing Fe3+ions into gels,the fracture stress will increase from 0.9 MPa to 2.7 MPa.This mechanical enhancement made the fixity of the temporary shape possible.During the whole process,the shape fixity ratio(Rf)reached 85%and the shape recovery ratio(Rr)could be above 95%(FIG.6(c)).Actually,the PEG-PDAC/P(AAm-co-AAc)DN gels have no shape memory effect when the AAc concentration was 0 and 0.5 mol/L.
IV.CONCLUSION
In conclusion,we have prepared a PEG-PDAC/P(AAm-co-AAc)DN hydrogel via a double network gels preparation method.Compared to the conventional double-network gels,of which the second network is neutral polymer,the PEG-PDAC/P(AAm-co-AAc)DN hydrogels use P(AAm-co-AAc),a weak polyelectrolyte,as the second network.The PEG-PDAC/P(AAm-co-AAc)DN hydrogel exhibits high mechanical strength as expected.Moreover,after being treated with FeCl3solution,the PEG-PDAC/P(AAm-co-AAc)DN hydrogel is endowed with ascorbic acid activated shape memory properties.Importantly,we find a new way to prepare DN gels both with high mechanical strength and ascorbic acid activated shape memory properties.

FIG.6 (a)Photographs of ascorbic acid-responsive shape memory performance,(b)stress-strain curves of the representative sample with and without Fe3+,and(c)the shape fixity ratio Rfand shape recovery ratio Rrin shape memory behaviours for 3 cycles.
V.ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China(No.51273189),the National Science and Technology Major Project of the Ministry of Science and Technology of China(No.2016ZX05016),and the National Science and Technology Major Project of the Ministry of Science and Technology of China(No.2016ZX05046).
[1]Y.M.Chen,M.Tanaka,J.P.Gong,K.Yasuda,S.Yamamoto,M.Shimomura,and Y.Osada,Biomaterials 28,1752(2007).
[2]Z.Wei,J.H.Yang,J.X.Zhou,F.Xu,M.Zrínyi,P.H.Dussault,Y.Osada,and Y.M.Chen,Chem.Soc.Rev.43,8114(2014).
[3]Y.Qiu and K.Park,Adv.Drug.Deliver.Rev.53,321(2001).
[4]I.Roy and M.N.Gupta,Chem.Biol.10,1161(2003).
[5]W.J.Zheng,Z.Q.Liu,F.Xu,J.Gao,Y.M.Chen,J.P.Gong,and Y.Osada,Macromol.Chem.Phys.216,641(2015).
[6]Y.F.Li,N.Khuu,A.Gevorkian,S.Sarjinsky,H.Therien-Aubin,Y.H.Wang,S.Cho,and E.Kumacheva,Angew.Chem.Int.Ed.Engl.56,6083(2017).
[7]H.Thérien-Aubin,Y.H.Wang,K.Nothdurft,E.Prince,S.Cho,and E.Kumacheva,Biomacromolecules 17,3244(2016).
[8]Z.W.Li,W.Lu,T.Ngai,X.X.Le,J.Zheng,N.Zhao,Y.J.Huang,X.F.Wen,J.W.Zhang,and T.Chen,Polym.Chem.7,5343(2016).
[9]M.R.Islam,A.Ahiabu,X.Li,and M.J.Serpe,Sensors 14,8984(2014).
[10]H.Xiao,W.Lu,X.X.Le,C.X.Ma,Z.W.Li,J.Zheng,J.W.Zhang,Y.J.Huang,and T.Chen,Chem.Commun.52,13292(2016).
[11]C.Maldonado-Codina and N.Efron,Ophthal.Physl.Opt.24,551(2004).
[12]Y.Okumura and K.Ito,Adv.Mater.13,485(2001).
[13]T.Sakai,T.Matsunaga,Y.Yamamoto,C.Ito,R.Yoshida,S.Suzuki,N.Sasaki,M.Shibayama,and U.I.Chung,Macromolecules 41,5379(2008).
[14]K.Haraguchi and T.Takehisa,Adv.Mater.14,1120(2002).
[15]J.P.Gong,Y.Katsuyama,T.Kurokawa,and Y.Osada,Adv.Mater.15,1155(2003).
[16]T.Nakajima,H.Furukawa,Y.Tanaka,T.Kurokawa,Y.Osada,and J.P.Gong,Macromolecules 42,2184(2009).
[17]S.Ahmed,T.Nakajima,T.Kurokawa,M.Anamul Haque,and J.P.Gong,Polymer 55,914(2014).
[18]J.P.Gong,Soft Matter 6,2583(2010).
[19]T.Nakajima,H.Sato,Y.Zhao,S.Kawahara,T.Kurokawa,K.Sugahara,and J.P.Gong,Adv.Funct.Mater.22,4426(2012).
[20]Y.Zhao,T.Nakajima,J.J.Yang,T.Kurokawa,J.Liu,J.S.Lu,S.J.Mizumoto,K.Sugahara,N.Kitamura,K.Yasuda,A.U.D.Daniels,and J.P.Gong,Adv.Mater.26,436(2014).
[21]Y.Tanaka,R.Kuwabara,Y.H.Na,T.Kurokawa,J.P.Gong,and Y.Osada,J.Phys.Chem.B 109,11559(2005).
[22]Q.M.Yu,Y.Tanaka,H.Furukawa,T.Kurokawa,and J.P.Gong,Macromolecules 42,3852(2009).
[23]A.Nakayama,A.Kakugo,J.P.Gong,Y.Osada,M.Takai,T.Erata,and S.Kawano,Adv.Funct.Mater.14,1124(2004).
[24]T.C.Suekama,J.Hu,T.Kurokawa,J.P.Gong,and S.H.Gehrke,ACS Macro.Lett.2,137(2013).
[25]L.H.Weng,A.Gouldstone,Y.H.Wu,and W.L.Chen,Biomaterials 29,2153(2008).
[26]Z.Tao,K.Peng,Y.J.Fan,Y.F.Liu,and H.Y.Yang,Polym.Chem.7,1405(2016).
[27]F.Peng,G.Z.Li,X.X.Liu,S.Z.Wu,and Z.Tong,J.Am.Chem.Soc.130,16166(2008).
[28]Y.J.Fan,W.F.Zhou,A.Yasin,H.Z.Li,and H.Y.Yang,Soft Matter 11,4218(2015).
[29]R.M.Fuoss,Discuss.Faraday.Soc.11,125(1951).
[30]B.D.Fairbanks,M.P.Schwartz,A.E.Halevi,C.R.Nuttelman,C.N.Bowman,and K.S.Anseth,Adv.Mater.21,5005(2009).
[31]J.Cui,M.A.Lackey,A.E.Madkour,E.M.Saffer,D.M.Griffin,S.R.Bhatia,A.J.Crosby,and G.N.Tew,Biomacromolecules 13,584(2012).
[32]A.Yasin,H.Z.Li,Z.Lu,S.U.Rehman,M.Siddiq,and H.Y.Yang,Soft Matter 10,972(2014).
[33]P.J.Flory,Principles of Polymer Chemistry,Ithaca,New York:Cornell University Press,(1953).
[34]T.Tanaka,Phys.Rev.Lett.40,820(1978).
[35]T.Matsuda,T.Nakajima,Y.Fukuda,W.Hong,T.Sakai,T.Kurokawa,U.I.Chung,and J.P.Gong,Macromolecules 49,1865(2016).
[36]Y.H.Na,Y.Tanaka,Y.Kawauchi,H.Furukawa,T.Sumiyoshi,J.P.Gong,and Y.Osada,Macromolecules 39,4641(2006).
[37]H.R.Brown,Macromolecules 40,3815(2007).
[38]Y.Tanaka,EPL(Eur.Lett.)78,56005(2007).
[39]S.M.Liang,Z.L.Wu,J.Hu,T.Kurokawa,Q.M.Yu,and J.P.Gong,Macromolecules 44,3016(2011).
[40]T.Nakajima,T.Kurokawa,S.Ahmed,W.I.Wu,and J.P.Gong,Soft Matter 9,1955(2013).
[41]F.Rived,M.Rosés,and E.Bosch,Anal.Chim.Acta 374,309(1998).
[42]S.Y.Zheng,H.Y.Ding,J.Qian,J.Yin,Z.L.Wu,Y.H.Song,and Q.Zheng,Macromolecules 49,9637(2016).
[43]P.Lin,S.H.Ma,X.L.Wang,and F.Zhou,Adv.Mater.27,2054(2015).
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Nucleation of Boron-Nitrogen on Transition Metal Surface:A First-Principles Investigation
- Electronic Structures and Optical Properties of Ga Doped Single-Layer Indium Nitride
- Electronic Structure and Optical Properties of K2Ti6O13Doped with Transition Metal Fe or Ag
- Maximum Thermodynamic Electrical Efficiency of Fuel Cell System and Results for Hydrogen,Methane,and Propane Fuels
- Non-Adiabatic Molecular Dynamics Simulations of Non-Charge-Transfer and Charge-Transfer Scattering in H++CO2at ELab=30 eV
- A High-Performance and Flexible Chemical Structure&Data Search Engine Built on CouchDB&ElasticSearch
