Preparation and characterization of Bi2O3/XNBR flexible films for attenuating gamma rays
2018-06-27YiChuanLiaoDuiGongXuPengChengZhang
Yi-Chuan Liao•Dui-Gong Xu•Peng-Cheng Zhang
1 Introduction
X-rays and γ-rays have a wide range of applications in military,medical,health,scientific,and agricultural industries[1].However,these rays are also harmful to human health if they are not shielded properly,especially for the staff who handles nuclear materials or radioactive sources[2,3].Traditional material structures,such as metal[4,5],concrete[6],glass[7],and polymer[8,9],have been commonly used to attenuate γ-rays and other rays,after the addition of heavy metal elements.However,there are situations that require flexible materials for shielding lowenergy γ-rays,such as protective shades,clothing,gloves,and helmets.Therefore,many attempts have been made to such develop flexible materials[10–12].Chai et al.prepared X-ray-shielding materials using W and Bi2O3with methyl vinyl silicone rubber(VMQ)as the matrix[13].The VMQ composite was fabricated by a plate-vulcanizing machine using solid rubber.However,there are significant differences between solid rubber and rubber latex,such as the molding method,product properties,and applications.Rubber latex is widely used in our daily lives as a flexible material.Carboxylated nitrile butadiene rubber(XNBR)is a copolymer of butadiene,acrylonitrile,and acrylic or meth-acrylic acid.It can be vulcanized through a variety of methods to achieve an excellent performance[14–16].Furthermore,XNBRL is a latex that can be prepared in any arbitrary shape,possessing good plasticity,elasticity,and resistance to gamma radiation aging[17].
Lead(Pb)has been commonly used at an early stage[18].However,materials containing Pb are toxic,making post-treatment a large environmental problem.Thus,there is a tremendous need to develop materials free of Pb for γray shielding.The element Bi(Z=83,Ar=209)is located next to Pb(Z=82,Ar=207)in the periodic table of elements.However,Bi is not suitable due to its low melting point(271°C).The post-treatment of the flexible attenuation material is usually conducted using a burning method.The flexible matrix mainly degrades into CO2and H2O during the burning at a decomposition temperature higher than 500°C,and the attenuation material is left behind for collection and storage.Bi2O3(with a melting point of 825 °C)has been used in glass for γ-ray attenuation as it is odorless,non-toxic,inexpensive,abundant,and has stable mechanical and chemical properties[19,20].All these characteristics indicate that a Bi2O3/XNBR film can be used as a flexible material against low-energy γ-rays radiation.
In the present work,XNBRL and Bi2O3were selected as the matrix material and the attenuation material,respectively.Different concentrations of Bi2O3(30–70 wt%)were dispersed in the XNBRL via physical and chemical methods.The mechanical properties(tensile strength,elongation at break,and shore hardness)and low-energy(20–100 keV) γ-ray attenuation properties of the Bi2O3/XNBR flexible films were investigated.
2 Experimental
2.1 Preparation of the Bi2O3dispersion
The purity,density,and median diameter(D50)of the Bi2O3powder(Beijing Xing Rong Yuan Technology Co.,Ltd.)used in these experiments were 99.9%,8.9 g cm-3,and 13.7 μm,respectively.The Bi2O3powder was pretreated by ball milling for 12 h at 250 rpm.The Bi2O3dispersion consisted of Bi2O3,distilled water,and the dispersant(Nekal,BX,C18H24SO3Na)with a mass ratio of 1:0.5:0.003.
2.2 Preparation of the XNBRL mixture
The XNBRL mixture consisted of XNBRL,a compounding agentdispersion,KOH,casein,and polyoxyethylene alkyl ether(Levelling Agent O,Peregal-O).XNBRL(Nipol LX552,Zeon,Japan)is a type of aqueous latex.The solid content was approximately 44 wt%,and the remaining content of the mixture was distilled water.The mixture viscosity was approximately 30 mPa s and its density approximately 1.02 g cm-3.All chemicals and reagents were used as received.The compounding agent dispersion of the XNBRL included a vulcanizer(S,sulfur),active agent(ZnO,zinc oxide),accelerator(ZDC,zinc diethyl dithiocarbamate),reinforcing filler(C,carbon black),and BX.The composition of the XNBRL mixture is presented in Table 1.The compounding agent was ballmilled at 250 rpm for 12 h.The mass ratio of the compounding agent and distilled water was 1:1.5.The compounding agent dispersion was slowly added to the latex via stirring.Then,the KOH,the casein,and the Peregal-O solution were mixed into the latex.KOH was used to adjust the pH of the latex to 9–10 to enhance its chemical stability.The viscosity of the latex was controlled by adjusting the contents of the casein and the Peregal-O solution.
2.3 Preparation of the Bi2O3/XNBR flexible films
The Bi2O3/XNBR mixture consisted of the Bi2O3dispersion(30–70 wt%)andthe XNBRL mixture.For example,the mass fractions of Bi2O3and XNBR in a 30 wt%Bi2O3/XNBR film are 30 and 70 wt%,respectively,normalized by their dry weight,and so on.The Bi2O3dispersion was added to the XNBRL mixture via stirring.Letting the Bi2O3/XNBRL mixture equilibrate for 48 h,the desired latex was subsequently obtained after filtration and de-foaming.The Bi2O3/XNBR film was then prepared by dip-molding.The dip plate,or mold,was submerged in the latex mixture for several seconds and raised to allow the film to set.After drying,the film was vulcanized in hot air in an oven(100°C,60 min).Afterward,the Bi2O3/XNBR flexible film was ready for γ-ray attenuation.The thickness of the film was controlled by the number of dips.After a single dip,the thickness of the film was 0.1–0.3 mm.Thicker films were obtained via multiple dips.
2.4 Property characterizations
A digital viscometer(NDJ-5S,Shanghai Ping Xuan Scientific Instrument Co.,Ltd.)was used to measure the viscosity of the latex at room temperature.The D50of the Bi2O3particles was measured by a laser particle size analyzer(Mastersizer 2000)using deionized water as the dispersing agent,with a scanning speed of 1000 times/second.The dispersion state of Bi2O3in the rubber latex was analyzed using a scanning electron microscope(SEM,JSM6390 LV,Japan JEOL)and an energy-dispersive spectrometer(EDS).Fourier transform infrared(FTIR)spectra were recorded at room temperature using a Nicolet FTIR Nexus with a 4 cm-1resolution in the range of 4000–400 cm-1.The test mode was set to total reflection.X-ray diffraction(XRD)was performed on a TD3500 X-ray diffractometer(China DanDong TongDa)under the following conditions:Cu Kα radiation(λ =0.15406 nm)at a voltage of 35 kV.The scanning rate was 10°/min over a range of 10–70°.Thermogravimetric analyses(TGA)were conducted by utilizing an SDT Q600 instrument(TA,USA)from 30 to 700 °C at 20 °C/min.The tensile properties(tensile strength and elongation at break)of the film were tested using a tensile strength tester(AI-3000,Gotech Testing Machines Inc.).The hardness of the films was measured using a shore durometer(LX-A,China Jun Ping Machinery Factory).

Table 1 Dry weight composition of the XNBRL mixture
2.5 Gamma ray attenuation measurements
Canberra’s portable In Situ Object Counting System(ISOCS)passive efficiency calibration machine for lowbackground high-purity germanium(HPGe)gamma spectrometry was used to measure the γ-ray spectra.The probe was a coaxial germanium detector.Its sensitive area was ø 80 mm×30 mm,and its energy resolution was 474 eV.The instrument was cooled with liquid nitrogen for more than six hours before operation.The radiation sources were241Am(59.5 keV)and133Ba(30.7 keV and 81.0 keV).The doses of the sources were in the millicurie range.The distance between the radiation source and the HPGe probe surface was over 25 cm,which reduced the probability of coincidence and cascading.Each sample was tested under each energy for 360 s.The peak intensity of the γ-rays was calculated as the peak area.
The γ-rays attenuation equation can be calculated using Beer and Lambert’s law[21]:

where I(d)and I0are the peak intensity of the γ-rays with and without the Bi2O3/XNBR film between the radiation source and the HPGe probe,respectively.d is the thickness of the Bi2O3/XNBR film.μ is the linear attenuation coefficient of the Bi2O3/XNBR film,which can be obtained by measuring I0,I(d),and d.
For a given material,if μ is obtained by Eq.(1),its attenuation efficiency(AE)with an arbitrary thickness of d can be evaluated by Eq.(2)[22]:

For a given material,if an attenuation efficiency for the actual working conditions is required,the requisite d of the material can be determined by Eq.(2).
3 Results and discussion
3.1 Dispersion state of Bi2O3in the XNBR
Obtaining a uniform and stable dispersion of Bi2O3in the XNBRL is a challenging because of the density inhomogeneity.Adding Bi2O3powder directly into XNBRL leads to uneven dispersion,particle agglomeration,and sedimentation problems.The Bi2O3powders must be well dispersed before being added into the XNBRL.The welldispersed Bi2O3was realized by milling.The granular shape of the Bi2O3particles does not change after being milled.As shown in Fig.1,after being milled for 12 h,the D50of the Bi2O3particles decreased from 13.7 to 8.1 μm,and their specific surface area increased from 0.488 to 0.763 m2g-1.A larger specific surface area corresponded to a better dispersity.This revealed that milling was beneficial to the dispersal of Bi2O3particles in water.
Without BX,the Bi2O3powder settled within 1 h(upper left in Fig.2a).With BX,the sedimentation of the Bi2O3powder was negligible within 1 h(upper right in Fig.2a).The dispersion state of Bi2O3in the water directly in fluenced the dispersion state of Bi2O3in the XNBRL.A welldispersed Bi2O3dispersion may not lead to a well-dispersed state of Bi2O3in the XNBRL.However,an underdispersed Bi2O3dispersion led to an under-dispersed state of Bi2O3in the XNBRL(lower left in Fig.2a).
The molecular formulas of Bi2O3,BX,and XNBR are shown in Fig.2b.The FTIR spectra of Bi2O3and BX are shown in Fig.2c.The broad peak at 3455 cm-1(peak 1)was attributed to the associate hydrogen bond O-H stretching.The O-H was formed by the interaction of O in the Bi2O3with H in the H2O.The peak at 400-1200 cm-1was attributed to the Bi-O deformation(Bi2O3)[23].The peak at 3068 cm-1(peak 2)was attributed to the C-H stretching(naphthalene).The peak at 1680 cm-1(peak 3)was attributed to the C=C stretching(naphthalene).The peak at 1380 cm-1(peak 4)was attributed to the C-H deformation(-C4H9).The peak at 845 cm-1(peak 5)was attributed to C-C stretching.The peak at 650 cm-1(peak 6)was attributed to the-SO3Na deformation.After adding 0.3%BX into Bi2O3,the FTIR spectra of Bi2O3were not clear,as they were obscured by the FTIR spectra of BX.This suggests that the affinity interaction between BX and Bi2O3was significant(Fig.2c).The BX contains a hydrophilic(-SO3Na)and a lipophilic group(-C18H24).As a commonly used surfactant,the hydrophilic property of the BX can increase the dispersal of the filler particles in water[24].
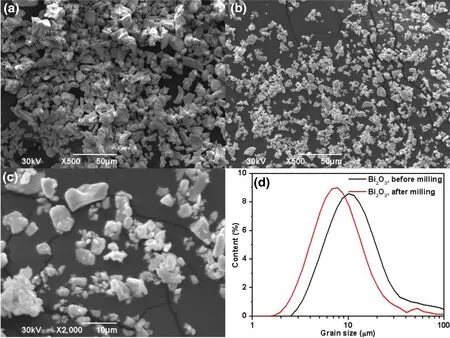
Fig.1(Color online)SEM images of the Bi2O3particles before milling(a)and after milling for 12 h(b,c);grain size distribution of the Bi2O3 particles before and after milling for 12 h(d)
After the Bi2O3dispersion and XNBRL were mixed together,casein and Peregal-O solution were added as a thickener and stabilizer,respectively,to prevent the sedimentation of the Bi2O3particles in the XNBRL.According to Stokes law,the gravity settling velocity is proportional to the square of the particle radius and inversely proportional to the viscosity of the dispersal medium[25].The viscosity of the latex was controlled by adjusting its casein and Peregal-O contents.The proper viscosity of an XNBRL mixture was 60–80 mPa s.When the Bi2O3content was less than 50 wt%,the sedimentation in the Bi2O3/XNBRL mixture was not obvious even after 5 days.When the Bi2O3content was 50–70 wt%,sedimentation occurred over time.After stirring,the sedimentation was re-dispersed.For a Bi2O3/XNBRL mixture containing more than 70 wt%Bi2O3,the sedimentation problem was considerable,which needs to be solved.The preparation of a Bi2O3/XNBR film with a higher filler content with good stability is one of the possible directions in the future.
In summary,physical and chemical methods were used to tackle the sedimentation problem of Bi2O3.Physical methods were used to reduce the particle radius and to increase the latex viscosity.The chemical method consisted of adding a dispersant.
SEM images and EDS images of the 50 wt%Bi2O3/XNBR flexible films are shown in Fig.3.There were no pores or cracks observed in the films.The EDS images of Bi,C,N,O,Zn,S,and K are presented in Fig.3b–h,respectively.The elements Bi,C,N,O,Zn,S,and K were well distributed in the Bi2O3/XNBR flexible films,indicating that the Bi2O3particles and other agents were well distributed in the XNBR.
3.2 XRD of the Bi2O3/XNBR films

Fig.2(Color online)a Diagrammatic sketch of the dispersion state of Bi2O3in the XNBRL;b molecular formula of Bi2O3,BX and XNBR;c FTIR spectra of Bi2O3and BX
The XRD pattern of the XNBR and Bi2O3/XNBR flexible films is shown in Fig.4.The raw XNBR denotes the raw material purchased without an added compounding agent.The sole peak at 19°revealed the amorphous structure of the XNBR[26].After vulcanization,the peak amplitude weakened,and the peak position shifted to the right by 1°–20°.The three main peaks of Bi2O3were observed at 27°,33°,and 46°.The XRD pattern of Bi2O3was identical to the standard card(JCPDS:41-1449)[27,28].The used Bi2O3was an alpha-type monoclinic system(a=0.585 nm,b=0.817 nm,c=0.751 nm).The relative peak amplitude at 20°weakened,and the relative peak amplitudes of the three main peaks of Bi2O3increased with the increasing Bi2O3content in the XNBR.After the added Bi2O3exceeded 70 wt%,the XRD pattern of the Bi2O3/XNBR flexible films was close to that of Bi2O3,and the peak from XNBR was not clearly resolved.
3.3 FTIR spectroscopy of the Bi2O3/XNBR films
The FTIR spectra of the Bi2O3/XNBR films are shown in Fig.5.The characteristic IR bands of the XNBR agreed with the literature data,which is illustrated as follows[29–31]:The peak at 3457 cm-1was attributed to the O-H stretching.The peak at 2926–2920 cm-1was attributed to the CH2stretching(butadiene).The peak at 2851–2846 cm-1was attributed to the CH2stretching(acrylonitrile).The peak at 2237 cm-1was attributed to the C≡N stretching(acrylonitrile).The positions of these three peaks did not change before and after curing.The peak at 1737–1731 cm-1for XNBR was attributed to the C=O stretching(carboxyl).After vulcanization,the C=O stretching vibration peak was no longer detected,which demonstrated that the unsaturated C=O bond transformed into a saturated bond during the process.Hence,the curing reaction of the C=O bond was complete.The peak at 1698 cm-1was attributed to the C=C stretching(isoprene).This peak appeared in the raw XNBR spectrum but not in the XNBR and Bi2O3/XNBR spectra,which demonstrated that the C=C bond(isoprene)became a saturated bond after the vulcanization.Hence,the curing reaction of the C=C bond(isoprene)was complete.The peak at 1596 cm-1was attributed to the stretching of the zinc carboxylate salt[32].This peak did not appear in the XNBR and Bi2O3/XNBR,but appeared in the raw XNBR.This result also suggested that a reaction occurred between the C=O and ZnO.The peak at 1440–1437 cm-1was attributed to C-H deformation.The peak at 1188–1183 cm-1was attributed to C-C stretching(carboxyl).The peak at 1044–1035 cm-1was attributed to C-C stretching.The peak at 968–966 cm-1was attributed to H-C=C deformation(main chain,carboxyl group).The peak at 919–909 cm-1was attributed to H-C=C deformation(side chain).
3.4 TGA of the Bi2O3/XNBR films
The TGA results for the XNBR and Bi2O3/XNBR films are shown in Fig.6.The vertical axis is the mass loss of the sample at different temperatures.The XNBR contained 10 wt%of a non-melting substance.The sum of the nonmelting substance and the mass loss was 100%.The Bi2O3did not melt below 700 degrees due to its high melting point(825°C).Suppose the mass fraction of Bi2O3in the Bi2O3/XNBR is x,and the mass loss of the Bi2O3/XNBR is ml,then,the Bi2O3content was calculated as follows:

Fig.3(Color online)a SEM images and b–h EDS images of 50 wt%Bi2O3/XNBR film

The mass loss of the nominal 30 wt%Bi2O3/XNBR was 63 wt%.The Bi2O3content was calculated as follows:100-63%/0.9=30%,indicating that the Bi2O3content was about 30 wt%and so on.The results indicate that the designed contents of Bi2O3in the Bi2O3/XNBR films are in line with the actual contents.

Fig.4(Color online)XRD pattern of the Bi2O3,XNBR,and Bi2O3/XNBR films
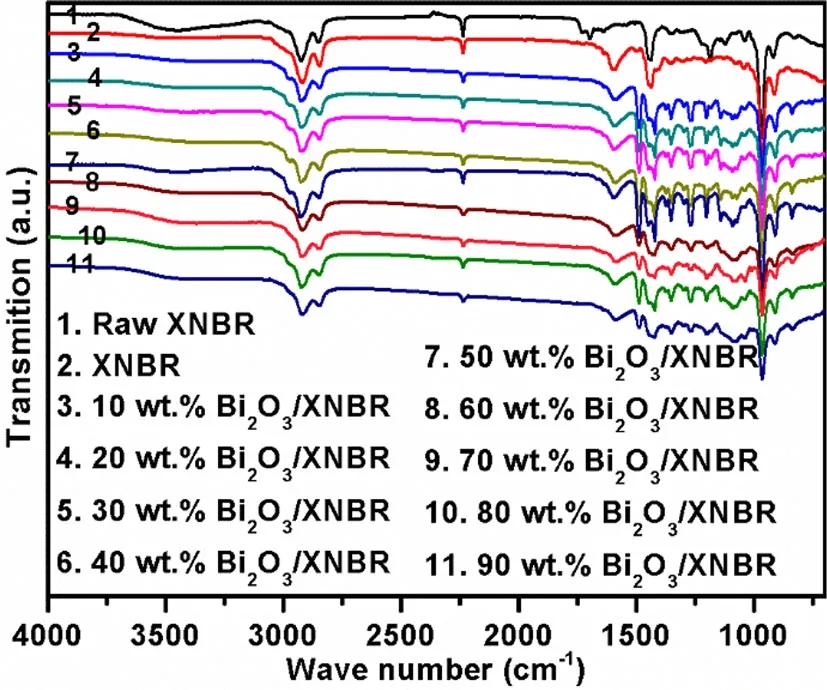
Fig.5(Color online)FTIR spectra of the XNBR and Bi2O3/XNBR films
The decomposition temperature obtained from TGA is a measure of thermal stability[33,34].The T10%is the temperature at which 10%of the initial mass is lost.The decomposition temperatures of the XNBR and Bi2O3/XNBR films are specified in Table 2.With the increase in the Bi2O3content,the decomposition temperature of the Bi2O3/XNBR films increased.This result indicates that Bi2O3improves the thermal stability of XNBR.
3.5 Mechanical properties of the Bi2O3/XNBR films
The tensile strength of the un-vulcanized XNBR was 0.3 MPa(1 MPa=10 kgf cm-2),and its elongation at break was 3000%.XNBR must be vulcanized for practical applications.The compounding agent is indispensable,as illustrated in Fig.7.The XNBR film was not strong enough without the added compounding agent.The vertical ordinate in Fig.7 shows the use of only ZDC,ZnO,C,and S as the compounding agents and the absence of C,ZnO,ZDC,and S.The tensile strength of the vulcanized XNBR was 27 MPa with the appropriate compounding agents and vulcanization conditions(100°C,60 min),with an elongation at break of 1283%.These values were higher than the previously reported ones[35].
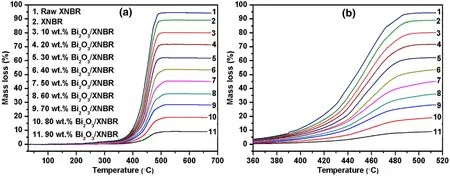
Fig.6(Color online)TGA of the XNBR and Bi2O3/XNBR films:a total,b segment
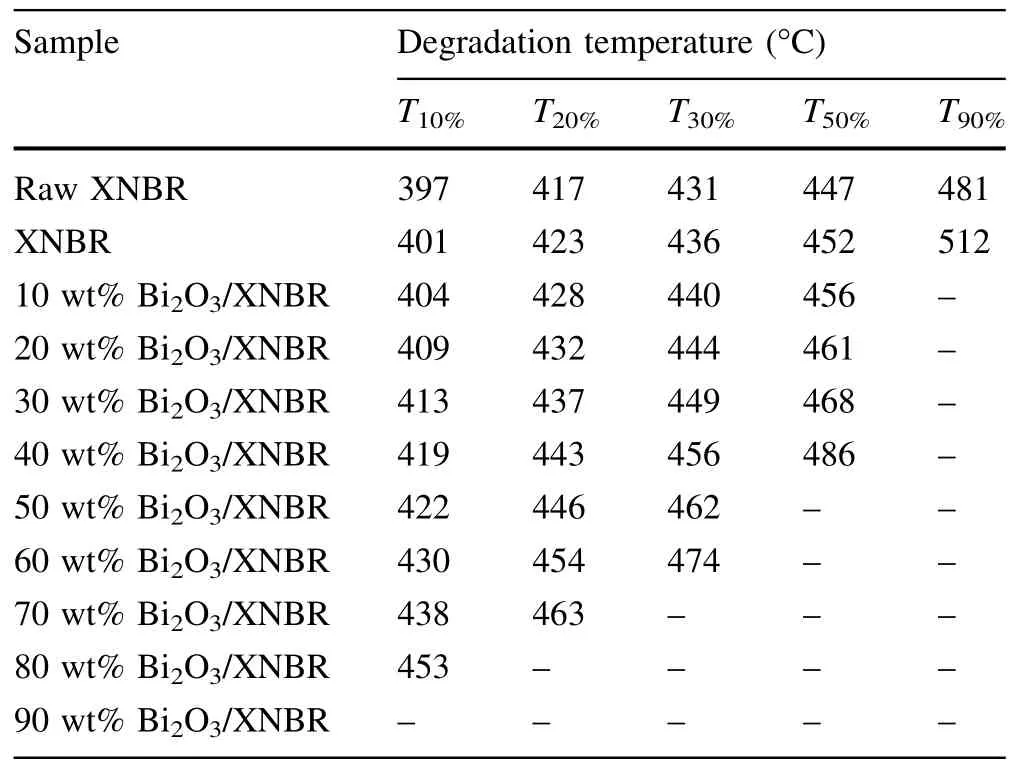
Table 2 Degradation temperatures of the XNBR and Bi2O3/XNBR films

Fig.7 Tensile strength of the XNBR films without appropriate compounding agents
The tensile properties of the flexible films with different Bi2O3contents are characterized in Fig.8.As the Bi2O3content increased,the tensile strength and elongation at break of the Bi2O3/XNBR films decreased.This is caused by the filler-particle concentration dependence of the composite mechanical properties[36].When the Bi2O3/XNBR system contained a sufficient amount of the reinforcement agent,the additional increase in the Bi2O3concentration did not help further enhancing the composite strength.When the Bi2O3content was below 70 wt%,the minimum tensile strength of the Bi2O3/XNBR film was greater than 5 MPa,and the minimum elongation at break was above 500%,which were higher values than those reported for rubber materials used for γ-ray shielding[13].As the Bi2O3content increased,the tensile properties of the film degraded.The tensile properties of the Bi2O3/XNBR films containing more than 70 wt%Bi2O3were not suitable for applications.The preparation of a Bi2O3/XNBR film with a higher filler content possessing good tensile properties is one of the research directions in the future.

Fig.8(Color online)a Tensile strength and b elongation at break for Bi2O3/XNBR flexible films under harsh environments
As for the XNBR flexible films after harsh treatment,the minimum tensile strength was above 20 MPa,and the minimum elongation at break above 900%.After successive submersion in gasoline,1.5 M sulfamic acid,and 10 M NaOH for 72 h at room temperature,the tensile strength and the elongation at break of the Bi2O3/XNBR films decreased.However,the reductions were not considerable.They were close to that of the original state without the harsh treatment.This suggested that the Bi2O3/XNBR films were resistant to oil,acid,and alkali.For the samples with Bi2O3less than 70 wt%,after the harsh treatment,the minimum tensile strength was above 5 MPa,and the minimum elongation at break above 500%,which were suitable values for general applications.The tensile properties of the Bi2O3/XNBR films containing more than 70 wt%Bi2O3were not suitable for applications.
The mass variation ratio of the Bi2O3/XNBR films under harsh environments also demonstrated their resistivity to oil,acid,and alkali.As shown in Fig.9,after successive submersion in gasoline,1.5 M sulfamic acid,and 10 M NaOH for 72 h at room temperature,the mass ratio of the Bi2O3/XNBR flexible films varied within a very small range.Figures 8 and 9 show that the Bi2O3/XNBR flexible films were also resistant to hot air.

Fig.9(Color online)Mass variations in the Bi2O3/XNBR films under harsh environments
As shown in Fig.10,the raw XNBR film was very soft.After vulcanization,it was still a soft material with an enhanced shore hardness.As the Bi2O3content increased,the shore hardness of the Bi2O3/XNBR filmsalso increased.The films with less than 70 wt%Bi2O3were still sufficiently flexible,in comparison with latex gloves,for applications.The Bi2O3/XNBR films containing more than 70 wt%Bi2O3were too hard for applications.
A Bi2O3/XNBR film with an appropriate Bi2O3content can beselected to satisfy the mechanical property requirements for practical applications.
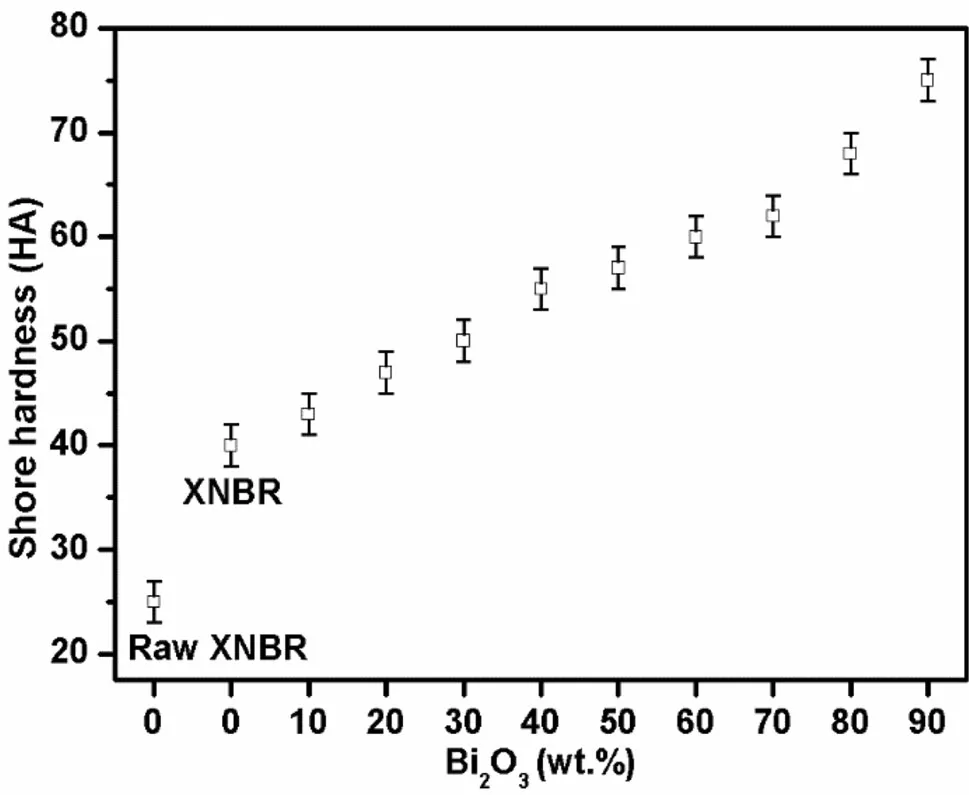
Fig.10 Shore hardness of the Bi2O3/XNBR flexible films
3.6 Attenuation properties of the Bi2O3/XNBR films
The density of the Bi2O3/XNBR composite was calculated by Eq.(5):
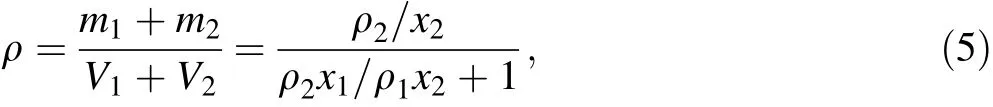
where the variables m,v,ρ,and x represent the mass,volume,density,and mass fraction,respectively.Subscripts 1 and 2 represent Bi2O3and XNBR,respectively.Thus,m1/m2=x1/x2= ρ1v1/ρ2v2,and x1+x2=1.The experimentalvalues of ρ1and ρ2were 8.9 and 1.07 g cm-3,respectively,which were measured by Archimedes’principle.The density of the Bi2O3/XNBR flexible film is shown in Fig.11a.The experimental and calculated values were in good agreement.
The mass attenuation coefficient of the Bi2O3/XNBR was calculated by Eq.(6):[37,38]

where μmis the mass attenuation coefficient of the Bi2O3/XNBR.The variables μ and ρ are the line attenuation coefficient and density of the Bi2O3/XNBR,respectively.The variables μm,1and μm,2are the mass attenuation coefficient of Bi2O3and XNBR,respectively.The mass attenuation coefficients of Bi2O3for γ-rays can be obtained through a Monte Carlo simulation or literature database research.The mass attenuation coefficient of Bi2O3for 59.5 keV γ-rays was 0.42736 m2kg-1as simulated by the Monte Carlo method,which was consistent with the literature value[39].Because XNBR is mainly composed of carbon,the mass attenuation coefficient of XNBR was approximately equal to that of carbon.According to the literature,the mass attenuation coefficient of carbon for 59.5 keV γ-rays is 0.017 m2kg-1[40].
The linear attenuation coefficient of Bi2O3for low-energy γ-rays was slightly lower than that of PbO(Fig.11a).However,Bi2O3is non-toxic,which is an advantage.
The linear attenuation coefficient of the Bi2O3/XNBR flexible film was calculated by Eq.(7):

The linear γ-ray attenuation coefficients of the Bi2O3/XNBR flexible film for energies 30.7,59.5,and 81.0 keV are shown in Fig.11b,c.The experimental values of μ obtained from Eq.(1)and the calculated values from Eq.(7)were in good agreement and consistent with the literature[27].Ideally,the higher the Bi2O3content,the greater the γ-ray attenuation.Since each sample was exposed to each energy only for a short time,there no obvious change was observed through SEM after the lowenergy γ-ray attenuation test.

Fig.11(Color online)a Linear attenuation coefficients of Bi2O3and PbO,inset image:densities of the Bi2O3/XNBR films;b,c linear γray attenuation coefficients of the Bi2O3/XNBR films
The attenuation efficiencies of the Bi2O3/XNBR flexible films,with different thicknesses and different Bi2O3contents,for selected γ-ray energies,were calculated by Eq.(2).Figure 12 presents the γ-ray attenuation efficiencies of the Bi2O3/XNBR flexible films for energies 30.7,59.5,and 81.0 keV,respectively.The attenuation ef ficiency of the Bi2O3/XNBR flexible films for low-energy(20–100 keV) γ-rays can be obtained using the same method.This investigation aids the engineering design.If an attenuation efficiency requirement is proposed,a suitable thickness and Bi2O3content can be used to satisfy it.For a Bi2O3/XNBR flexible film,the Bi2O3content can be calculated by measuring its density and the thickness of the film,and then,its γ-ray attenuation efficiency can be assessed.
4 Conclusion
The design,development,and investigation of Bi2O3/XNBR flexible films were successfully conducted.The important conclusions are summarized below:
The Bi2O3sedimentation problem in the XNBRL was solved by reducing the Bi2O3particle radius,increasing the viscosity of the latex,and adding a dispersant.The microscopy results confirmed that Bi2O3was well dispersed in the XNBRL.
When the Bi2O3content was below 70 wt%,the tensile strength of the Bi2O3/XNBR film was greater than 5 MPa,and the minimum elongation at break was above 500%.The prepared Bi2O3/XNBR flexible films prepared by dipmolding were resistant to oil,acid,alkali,and hot air.As the Bi2O3content increased,the mechanical properties of the films degraded.The mechanical properties of the Bi2O3/XNBR films containing more than 70 wt%Bi2O3were not suitable for applications.The preparation of a Bi2O3/XNBR film with a higher filler content and good mechanical properties is one of the research directions in the future.
The linear attenuation coefficients of the Bi2O3/XNBR films experimentally obtained were in good agreement with the calculated values.The attenuation efficiencies of the Bi2O3/XNBR films with different thicknesses and different Bi2O3contents were obtained.The results demonstrated that the Bi2O3/XNBR flexible films have a good attenuation effect for low-energy(20–100 keV)γ-rays.
This work aids the engineering and design of the films.A Bi2O3/XNBR film with an appropriate Bi2O3content can be selected to meet the requirements on mechanical properties,thickness,and attenuation efficiency in practical applications.
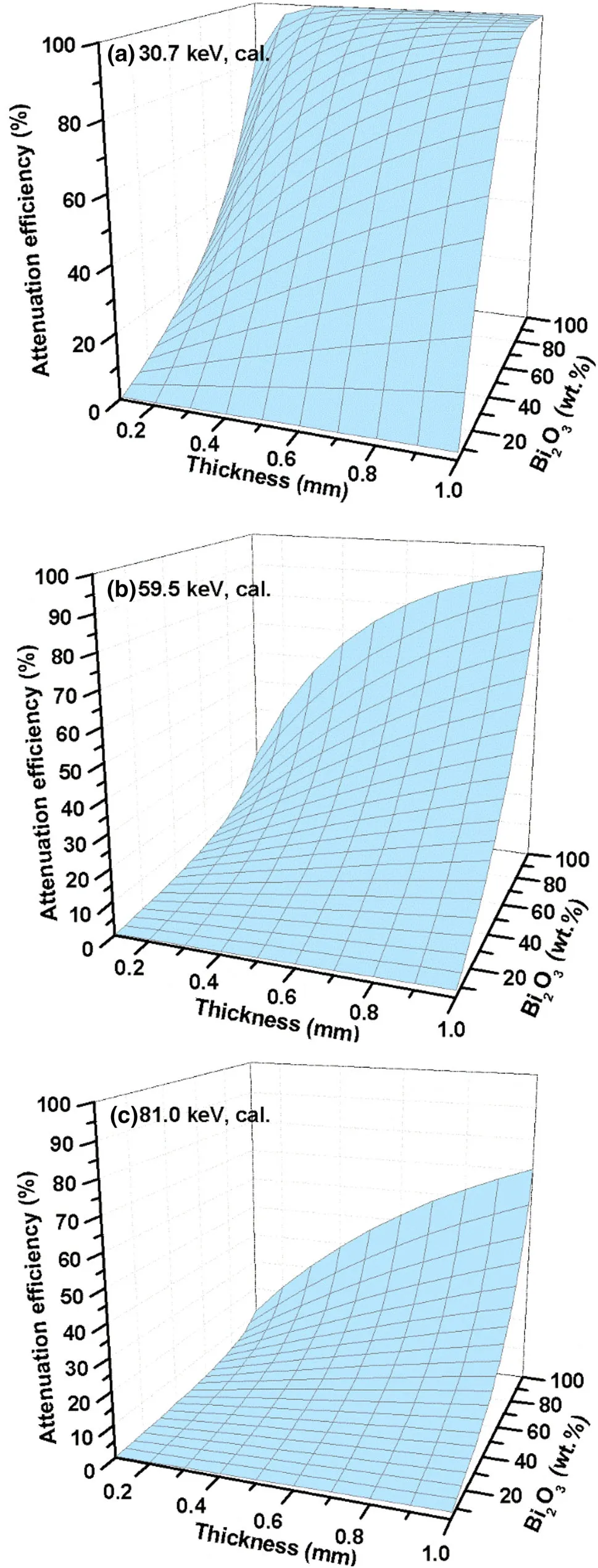
Fig.12 Attenuation efficiencies of the Bi2O3/XNBR films for γ-ray energies:a 30.7 keV,b 59.5 keV,and c 81.0 keV
1.S.Fu,Y.Sun,Y.Lu,Current status of radiation therapy for prostate cancer.Nucl.Sci.Tech.18,65–72(2007).https://doi.org/10.1016/S1001-8042(07)60021-9
2.Q.Liu,B.Jiang,L.P.Jiang et al.,Clinical report of three cases of acute radiation sickness from a 60Co radiation accident in Henan province in China.J.Radiat.Res.49,63–69(2008).https://doi.org/10.1269/jrr.07071
3.S.Zhao,S.Huang,S.Liu et al.,Measurements of 134Cs and 137Cs in urine and estimation of the internal dose of an adult exposed to the Chernobyl Accident.Nucl.Sci.Tech.18,115–117(2007).https://doi.org/10.1016/S1001-8042(07)60030-X
4.I.Akkurt,H.Akyıldırım,B.Mavi et al.,Photon attenuation coefficients of concrete includes barite in different rate.Ann.Nucl.Energy 37,910–914(2010).https://doi.org/10.1016/j.anu cene.2010.04.001
5.M.E.Medhat,Y.Wang,Investigation on radiation shielding parameters of oxide dispersion strengthened steels used in high temperature nuclear reactor applications.Ann.Nucl.Energy 80,365–370(2015).https://doi.org/10.1016/j.anucene.2015.01.044
6.M.H.Kharita,M.Takeyeddin,M.Alnassar et al.,Development of special radiation shielding concretes using natural local materials and evaluation of their shielding characteristics.Prog.Nucl.Energy 50,33–36(2008).https://doi.org/10.1016/j.pnu cene.2007.10.004
7.N.Singh,K.J.Singh,K.Singh et al.,Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials.Nucl.Instrum.Methods Phys.Res.,Sect.B 225,305–309(2004).https://doi.org/10.1016/j.nimb.2004.05.016
8.J.Kim,D.Seo,B.C.Lee et al.,Nano-W dispersed gamma radiation shielding materials.Adv.Eng.Mater.16,1083–1089(2014).https://doi.org/10.1002/adem.201400127
9.W.Qin,D.Peng,X.Wu,Study on the damage effects of electron and proton combined irradiation on T700/cyanate composites.Nucl.Instrum.Methods Phys.Res.,Sect.B 312,126–130(2013).https://doi.org/10.1016/j.nimb.2013.07.017
10.J.P.Mccaffrey,H.Shen,B.Downton et al.,Radiation attenuation by lead and nonlead materials used in radiation shielding garments.Med.Phys.34,530–537(2007).https://doi.org/10.1118/1.2426404
11.D.N.A.Dodoo-Amoo,S.Landsberger,J.M.MacDonald et al.,Development of composite materials for non-leaded gloves for use in radiological hand protection.Health Phys.84,737–746(2003).https://doi.org/10.1097/00004032-200306000-00006
12.M.E.Cournoyer,G.L.George,R.L.Dodge et al.Replacement of lead-loaded glovebox glove with attenuation medium that are not RCRA-hazardous metals.2010,17th Pacific Basin Nuclear Conference Cancun mexico
13.H.Chai,X.B.Tang,M.X.Ni et al.,Preparation and properties of novel, flexible,lead-free X-ray-shielding materials containing tungsten and bismuth(III)oxide.J.Appl.Polym.Sci.133,43012–43018(2016).https://doi.org/10.1002/app.43012
14.M.Mirzaei Aliabadi,G.Naderi,S.J.Shahtaheri et al.,Transport properties of carboxylated nitrile butadiene rubber(XNBR)-nanoclay composites;a promising material for protective gloves in occupational exposures.J.Environ.Health Sci.Eng.12,51–58(2014).https://doi.org/10.1186/2052-336x-12-51
15.M.A.Misman,A.R.Azura,Z.A.A.Hamid,The physical and degradation properties of starch-graft-acrylonitrile/carboxylated nitrile butadiene rubber latex films.Carbohydr.Polym.128,1–10(2015).https://doi.org/10.1016/j.carbpol.2015.04.004
16.K.P.Nair,A.B.Nair,R.Joseph,Carboxylated acrylo nitrile butadiene rubber latex/kaolin nanocomposites:preparation and properties.Compos.Interfaces 21,571–583(2014).https://doi.org/10.1080/15685543.2014.899198
17.G.Marković,M.S.Marinović-Cincović,V.Jovanovićet al.,Gamma irradiation aging of NBR/CSM rubber nanocomposites.Compos.B Eng.43,609–615(2012).https://doi.org/10.1016/j.compositesb.2011.11.056
18.S.E.Gwaily,M.Madani,H.H.Hassan,Lead—Natural rubber composites as gamma radiation shields.II:high concentration.Polym.Compos.23,495–499(2002).https://doi.org/10.1002/pc.10450
19.S.A.M.Issa,A.M.A.Mostafa,Effect of Bi2O3in borate-telluritesilicate glass system for development of gamma-rays shielding materials.J.Alloys Compd.695,302–310(2017).https://doi.org/10.1016/j.jallcom.2016.10.207
20.W.Hao,Y.Gao,X.Jing et al.,Visible light photocatalytic properties of metastable γ-Bi2O3with different morphologies.J.Mater.Sci.Technol.30,192–196(2014).https://doi.org/10.1016/j.jmst.2013.09.023
21.B.A.Benetskii,M.V.Plotnikova,Gamma-radiation accumulation factors for composite materials and radiation shields.Bull.Lebedev.Phys.Inst.39,113–117(2012).https://doi.org/10.3103/S1068335612040045
22.V.P.Singh,S.P.Shirmardi,M.E.Medhat et al.,Determination of mass attenuation coefficient for some polymers using Monte Carlo simulation.Vacuum 119,284–288(2015).https://doi.org/10.1016/j.vacuum.2015.06.006
23.S.Hariharan,R.Udayabhaskar,T.R.Ravindran et al.,Surfactant assisted control on optical, fluorescence and phonon lifetime in α-Bi2O3microrods.Spectrochim.Acta,Part A 163,13–19(2016).https://doi.org/10.1016/j.saa.2016.02.045
24.S.Chakraborty,I.Sarkar,D.K.Behera et al.,Experimental investigation on the effect of dispersant addition on thermal and rheological characteristics of TiO2nano fluid.Powder Technol.307,10–24(2017).https://doi.org/10.1016/j.powtec.2016.11.016
25.P.C.Hiemenz,R.Rajagopalan,Principles of Colloid and Surface Chemistry(Marcel Dekker,New York,1997)
26.Z.N.Ain,A.R.Azura,Effect of different types of filler and filler loadings on the properties of carboxylated acrylonitrile–butadiene rubber latex films.J.Appl.Polym.Sci.119,2815–2823(2011).https://doi.org/10.1002/app.32984
27.M.R.Ambika,N.Nagaiah,V.Harish et al.,Preparation and characterisation of Isophthalic-Bi2O3polymer composite gamma radiation shields.Radiat.Phys.Chem.130,351–358(2017).https://doi.org/10.1016/j.radphyschem.2016.09.022
28.R.Sharma,M.Khanuja,S.N.Sharma et al.,Reduced band gap&charge recombination rate in Se doped α-Bi2O3leads to enhanced photoelectrochemical and photocatalytic performance:theoretical&experimental insight.Int.J.Hydrog.Energy 42,20638–20648(2017).https://doi.org/10.1016/j.ijhydene.2017.07.011
29.L.Wang,W.Wang,Y.Fu et al.,Enhanced electrical and mechanical properties of rubber/graphene film through layer-bylayer electrostatic assembly.Compos.Part B Eng.90,457–464(2016).https://doi.org/10.1016/j.compositesb.2015.12.048
30.X.He,T.Li,Z.Shi et al.,Thermal-oxidative aging behavior of nitrile-butadiene rubber/functional LDHs composites.Polym.Degrad.Stab.133,219–226(2016).https://doi.org/10.1016/j.polymdegradstab.2016.08.018
31.A.Laskowska,M.Zaborski,G.Boiteux et al.,Effects of unmodified layered double hydroxides MgAl-LDHs with various structures on the properties of filled carboxylated acrylonitrile–butadiene rubber XNBR.Eur.Polym.J.60,172–185(2014).https://doi.org/10.1016/j.eurpolymj.2014.09.013
32.R.L.Sala,T.M.Arantes,E.Longo et al.,Evaluation of modified silica nanoparticles in carboxylated nitrile rubber nanocomposites.Colloids Surf.A 462,45–51(2014).https://doi.org/10.1016/j.colsurfa.2014.08.012
33.Y.Han,L.X.Mao,H.W.Meng et al.,Novel self-crosslinking film from hydrogenated carboxylated nitrile rubber latex.J.Appl.Polym.Sci.131,39865–39871(2014).https://doi.org/10.1002/app.39865
34.G.Janowska,A.Kucharska-Jastrzabek,A.Kasiczak et al.,Thermal properties and combustibility of cross-linked XNBR/CSM blends.J.Therm.Anal.Calorim.104,1107–1115(2011).https://doi.org/10.1007/s10973-011-1328-9
35.T.Biswas,D.K.Basu,Cure synergism in XNBR vulcanization in presence of thiophosphoryl disul fides and amine disul fide/thiazole accelerators.J.Appl.Polym.Sci.60,1349–1359(1996).https://doi.org/10.1002/(sici)1097-4628(19960531)60:9<1349::aid-app10>3.0.co;2-y
36.W.Qin,D.Peng,X.Wu et al.,Study on the resistance performance of TiO2/cyanate ester nano-composites exposed to electron radiation.Nucl.Instrum.Methods Phys.Res.,Sect.B 325,115–119(2014).https://doi.org/10.1016/j.nimb.2014.01.022
37.I.Akkurt,A.M.El-Khayatt,The effect of barite proportion on neutron and gamma-ray shielding.Ann.Nucl.Energy 51,5–9(2013).https://doi.org/10.1016/j.anucene.2012.08.026
38.S.A.M.Issa,Effective atomic number and mass attenuation coefficient of PbO–BaO–B2O3 glass system.Radiat.Phys.Chem.120,33–37(2016).https://doi.org/10.1016/j.radphyschem.2015.11.025
39.K.Kirdsiri,J.Kaewkhao,N.Chanthima et al.,Comparative study of silicate glasses containing Bi2O3,PbO and BaO:radiation shielding and optical properties.Ann.Nucl.Energy 38,1438–1441(2011).https://doi.org/10.1016/j.anucene.2011.01.031
40.S.M.Vahabi,M.Bahreinipour,M.S.Zafarghandi,Determining the mass attenuation coefficients for some polymers using MCNP code:a comparison study.Vacuum 136,73–76(2017).https://doi.org/10.1016/j.vacuum.2016.11.011
杂志排行
Nuclear Science and Techniques的其它文章
- Gamma irradiation-induced effects on the properties of TiO2 on fluorine-doped tin oxide prepared by atomic layer deposition
- Preliminary analysis of tritium fuel cycle in Z-pinch-driven fusion– fission hybrid reactor
- Investigation of high-temperature-resistant rhenium–boron neutron shields by experimental studies and Monte Carlo simulations
- Monte Carlo simulation of incident electrons passing through thin metal layer
- Annual effective dose values from137Cs activity concentrations in soils of Manisa,Turkey
- Investigation of SPECT/CT cardiac imaging using Geant4
