Long-term grazing exclusion influences arbuscular mycorrhizal fungi and their association with vegetation in typical steppe of lnner Mongolia, China
2018-06-06CHENXuejiaoLINQimeiZHAOXiaorongCHENHaoWENJingLIYingLIGuitong
CHEN Xue-jiao, LIN Qi-mei, , ZHAO Xiao-rong, , CHEN Hao, WEN Jing, LI Ying, LI Gui-tong,
1 College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193, P.R.China
2 Key Laboratory of Arable Land Conservation (North China), Ministry of Agriculture/Key Laboratory of Plant-Soil Interactions,Ministry of Education, Beijing 100193, P.R.China
1. Introduction
The typical steppe in Xilinguole League, 1.91×107ha, about 25% of the total grassland in Inner Mongolia, China (SBXL 2008), is considered as one of the most popular sheep production regions in China and even in the whole Eurasian grassland. However, because of intensive livestock grazing over the last few decades, the steppe has suffered from severe deterioration, including soil erosion, sandification,and vegetation degradation (Liet al.2015; Miaoet al.2015;Liuet al.2017). In comparison with the 1950s, the forage yield in Inner Mongolia has declined by 30–50% and the carrying capacity decreased by 4.1×109sheep units (Zhanget al.2014).
Grazing exclusion is usually considered as an effective method for restoring the degraded grassland and increasing ecosystem sustainability, for its favorable influence on vegetation (Chenget al.2016; Zhuet al.2016), soil physicochemical and microbiological properties (Wanget al.2015; Liuet al.2016). Arbuscular mycorrhizal (AM) fungi,one of the most representative fungi in grassland soil, can form symbioses with most plants and further be a significant strategy for vegetation growing in poor environment (Tianet al.2009; Smith and Smith 2011). However, current data have shown that the responses of AM fungi to grazing exclusion are inconsistent. For example, Su and Guo (2007)measured higher spore density and species richness of AM fungi in the fenced typical steppe for more than 30 years,compared with over-grazed plot. Guoet al.(2016) found that grazing exclusion induced higher mycorrhizal frequency and colonization intensity in a meadow steppe whereas lower these values in typical steppe un-grazed for 12 years.Zhouet al.(2013) also determined much lower hyphal length density, Shannon diversity and Pielou evenness index of AM fungi in an 18-year grazing exclusion plot of desert grassland, than those in a 14-year un-grazed plot after slightly grazing. Moreover, Petipas and Brody (2014) did not detect any significant difference in AM fungal species richness and infectivity between the excluded and grazed plots in a semiarid savanna. Grigera and Oesterhelld (2004)even revealed a promotion of mycorrhizal colonization by animal grazing in the dominant grass species of a flooding pampa, Argentina.
It is obvious that the responses of AM fungi to grazing exclusion may depend on the heterogeneity of grassland types, exclusion durations, topography, climate, and even methodological approaches (Grigera and Oesterhelld 2004;Piippoet al.2011; Baiet al.2013). In essence, the growth of AM fungi is directly influenced by both of vegetation and soil conditions. Additionally, the activity of soil phosphatase which plays a major role in the mineralization process of organic phosphorus (P) could also indirectly influence AM fungiviaP dynamics, especially in arid grassland soil with low available P (McDowellet al.2002; Wanget al.2014).To date, there is limited information about the long-term effect of grazing exclusion on seasonal dynamic of AM fungi and its relationship with aboveground vegetation and P uptake in the typical steppe. In this study, two grazing exclusion sites since 1983 and 1996, respectively, and one free-grazing site, were chosen to investigate: (1) the root colonization, spore density, and hyphal length density of AM fungi, (2) the aboveground biomass and P uptake of plants, (3) soil physicochemical properties and the activity of alkaline phosphatase, within the growing season (May to September) in the typical steppe of Xilinguole League,Inner Mongolia, China. The purposes were aimed at understanding the responses and seasonal changes of AM fungi to long-term grazing exclusion and its association with aboveground vegetation. We hypothesized that both AM fungi and vegetation would be simultaneously promoted during long-term grazing exclusion period. The seasonality of the association might become an important factor to regulate the effect of grazing exclusion on both of AM fungi and vegetation in the typical steppe.
2. Materials and methods
2.1. Study site
The research site, Baiyinxile rangeland, is located in the Xilinguole League (44°3´57´´N, 116°29´28´´E; 800–1 800 m above sea level). This region has a temperate semiarid continental climate with annual mean temperature of 2.5°C, ranging from –20°C in January to 21°C in July.Annual mean precipitation is approximately 300 mm with more than 70% occurring between June and August, and annual mean evaporation is around 1 200–1 500 mm. The soil is Kastanozems (FAO soil classification),consisting of 7.78% clay, 12.22% silt, and 81.60% sand. The vegetation is classified as typical steppe type, dominated byStipa grandis,Leymus chinensis,Agropyron michnoi,Carex korshinskyi, andCleistogenes squarrosa.
2.2. Experimental design
The typical steppe was degraded before the experiment due to over-grazing (Liuet al.2016). Two sites, each approximately 9 000 m2(150 m×60 m), were fenced to exclude livestock grazing in 1983 and 1996, and named as E83 and E96, respectively. The fenced area is never mowed and all the litters are directly returned into the soil. Another area outside the fenced paddocks, approximately 2 800 m2(140 m×20 m), was remained free-grazing (named as FG) and had a grazing intensity about 9 sheep units ha–1yr–1. In order to avoid unpredictable trampling and ingesting of livestock,this site was fenced during the experimental period.These 3 sites were adjacent and thus similar in soil type and physiographic conditions (slope degree, altitude and terrains) before the experiment started. However, significant changes in soil and vegetation occurred during the exclusion periods. For example, both the two grazing exclusion sites had 13 plant species in 2014. E83 was dominated byS.grandis,A.michnoi,C.squarrosa,C.korshinskyi,Kochia prostrateandAchnatherum sibiricum, while E96 byS.grandis,L.chinensis,A.michnoi,K.prostrataandC.korshinskyi. Additionally, FG had 15 plant species dominated byS.grandis,C.korshinskyi, andC.squarrosa.
2.3. Sampling procedure
Soil samples were collected from each site along three transects (150 m×20 m for grazing exclusion sites and 47 m×20 m for grazing site) every month from May to September in 2014. In each transect, 15 soil cores (7 cm in diameter) were collected uniformly at a depth of 0–20 cm and then mixed thoroughlyin situas one composite sample(about 500 g). A total of nine soil samples (three for each site, considered as three replicates) were collected each month. The samples were stored in an ice chamber and transported to the laboratory as soon as possible. Root samples were isolated from the soil by nylon net for assaying AM fungal colonization, and the remaining soils were sieved through 2-mm mesh. A small amount of each sieved soil sample was stored at 4°C for determining soil enzymes.The rest was air-dried for measuring soil physicochemical properties, spore density, and hyphal length density of AM fungi. The background parameters of soil properties were given in Table 1.
Three quadrats (1 m×1 m) were randomly selected in each sampling plot (totally 9 quadrats in each site). The aboveground plant biomass was cut at the ground level and then determined from May to September. In order to investigate the contribution of dominant plant in total aboveground biomass and P content, three well-grown and intact dominant grass ofS.grandiswas randomly selected in July during the flowering stage from each sampling site and the roots were analyzed for AM fungal colonization.
2.4. Measurement of soil properties
Soil pH was measured at a ratio of 1:2.5 (w/v) soil to deionized water with a pH meter (UB-7, Denver Instrument,USA). Soil bulk density and particle size distribution were determined by the core method (Blake and Hartge 1986)and pipette method (Gee and Or 2002), respectively. Soil organic matter (SOM) content was determined by potassium dichromate volumetric method. Soil available P was extracted by Olsen method with a spectrometer (UV2300,Techcomp, China). Total nitrogen (N) was digested by H2SO4and then determined by a KDY-9820 automatic Kieldahl apparatus (Bao 2000). The activity of soil alkaline phosphatase was measured following the method from Tabatabai (1994) and expressed as μgp-nitrophenol h–1g–1soil.
2.5. Determination of AM fungal parameters
Root samples were washed in a 1-mm sieve with tap water to remove the adhered soil and then were cut into 1-cm segments. The root segments were dipped in 10% KOH(w/v) for 60 min in a 90°C water bath, and then rinsed with tap water for several times after cooling to room temperature.Following acidified with 2% HCl (v/v) for 5 min, the roots were stained with a trypan blue solution (0.05%) at 90°C for 30 min and then cleaned with the lactoglycerol at room temperature.Each sample was prepared for two slides containing 30 root segments. The roots with AM fungal structure were counted under a light microscope (CX21, OLYMPUS, Japan)at 100 magnifications based on Mycorrhizal Manual which can be found online (https://www2.dijon.inra.fr/mychintec/Protocole/protoframe.html). The AM fungal root colonization was expressed as intensity of mycorrhizal colonization in roots according to Trouvelotet al.(1986) and calculated in computer program of Mycocalc (http://www.dijon.inra.fr/mychintec/Mycocalc-prg/download.html).
Air-dried and sieved soil was used for measuring spore density and hyphal length density of AM fungi. The spores were extracted by wet sieving, followed by sucrose densitygradient centrifugation and decanting according to the method described by Anet al.(1990), and then observed under a stereomicroscope (SZX16, OLYMPUS, Japan) at 16–25 magnification. Hyphal length density was determined by suction filtration and gridline-intersect (Jakobsenet al.1992).
2.6. Analysis of vegetation biomass and P uptake
Following washed with deionized water, the collected plants without litter were heated at 105°C for 10 min todeactivate enzyme activity and then oven-dried at 80°C overnight for weighting. The P content was determined using molybdenum blue colorimetry (UV2300, Techcomp,China) following digestion with H2SO4-H2O2(Bao 2000),and P uptake by aboveground vegetation (g m–2) was then calculated using P content and plant biomass.
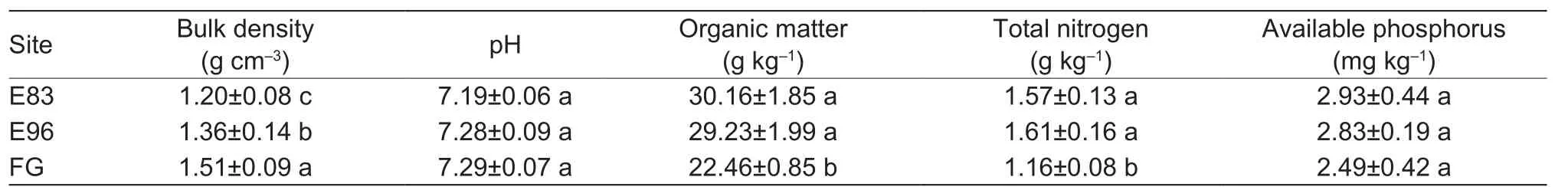
Table 1 Background parameters of the soils collected from grazing exclusion sites since 1983 (E83) and 1996 (E96) and free grazing site (FG) in the typical steppe of Xilinguole League, Inner Mongolia, China
2.7. Statistical analysis
All the data were expressed as the means of three replicates and oven-dried base unless specifically illustrated. The variance of the detected parameters were analyzed by two-way analysis of variance (ANOVA) with a software of SPSS (PASW Statistic 18). The significant differences for each variable among different treatments or seasons were revealed by the least significant difference (LSD) test at theP<0.05 level. Pearson’s correlation analysis was used to examine relationships among aboveground vegetation, AM fungi, and soil alkaline phosphatase.
3. Results
3.1. AM fungal spore density and hyphal length density
The spore densities in E83, E96 and FG were 12–28, 11–19,and 5–14 spores g–1soil, respectively. Consequently, both the grazing exclusion sites of E83 and E96 had 71–300%and 36–180%, respectively, higher spore density than that in FG (P<0.05) (Fig. 1-A). The hyphal length density in E83, 9–15 m g–1soil, was 34–168% as high as that in FG(P<0.05) from May to September. As for E96, its hyphal length density was 6–14 m g–1soil and 42–94% higher than that in FG in May, June, and July. Generally, the longer the grazing exclusion duration, the higher densities of both spore and hyphal length were detected in most months (P<0.05).In addition, a larger difference in either spore or hyphal length densities among the three sites was observed from May to June, but less in September.
Both spore and hyphal length densities of AM fungi in the three sites had remarkable seasonal changes, increasing from May to August and then decreasing in September(Fig. 1). These changes were much more drastic in FG than in E83 and E96, e.g., the spore and hyphal length densities in FG site increased by 180 and 248%, respectively, from May to August, whereas only 20–36% and 74–130% in both fenced sites. Exclusion treatments and seasons showed interactions in both spore and hyphal length densities of AM fungi. These interactions were more significant for spore density (P<0.001) than for hyphal length density (P<0.05).The highest spore density occurred in June for E83, but in July and August for E96 and FG (Fig. 1-A), while the highest hyphal length density in all sites was detected in August (Fig. 1-B).
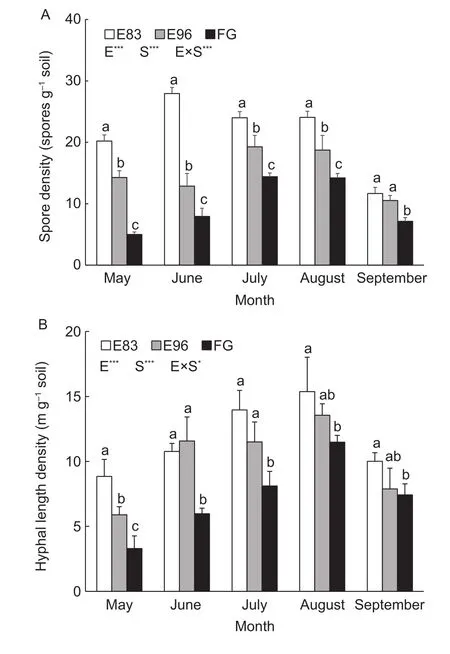
Fig. 1 Seasonal changes of spore (A) and hyphal length density(B) of arbuscular mycorrhizal fungi in the grazing exclusion sites since 1983 (E83) and 1996 (E96), and free grazing site (FG).E, S and E× S represent the effect of exclusion, season, and their interactions on spore and hyphal length densities. The bars in each column represent SD of the means. The different lowercase letters reveal the significant difference among the three treatments in each month at P<0.05 (*, P<0.05; **, P<0.01;***, P<0.001).
3.2. AM fungal colonization
The colonization intensities of AM fungi in the three sites ranged from 14 to 74%, and showed obvious seasonal changes, increasing from May (<30%) to July (>65%) but decreasing in September (<65%) (Fig. 2). In particular, FG had more drastic seasonal change than both E83 and E96,in which the colonization intensity increased greatly by 350%from May to July and decreased by 54% in September. But for E83 and E96, the colonization intensities increased by 175–212% from May to July and decreased by 11–43% in August or September. All these three sites had similar AM fungal colonization intensities during June and July, whereas both E83 and E96 had significantly higher colonization intensities than FG at the initial and final growth periods(Fig. 2). Obviously, mycorrhizal colonization intensity was also influenced by the interactions between exclusion treatment and season (P<0.05). As for the dominant plantS.grandis, the colonization intensity in E83 was significantly higher than E96 and FG during the flowering stage of July(Table 2).
3.3. Soil alkaline phosphatase activity
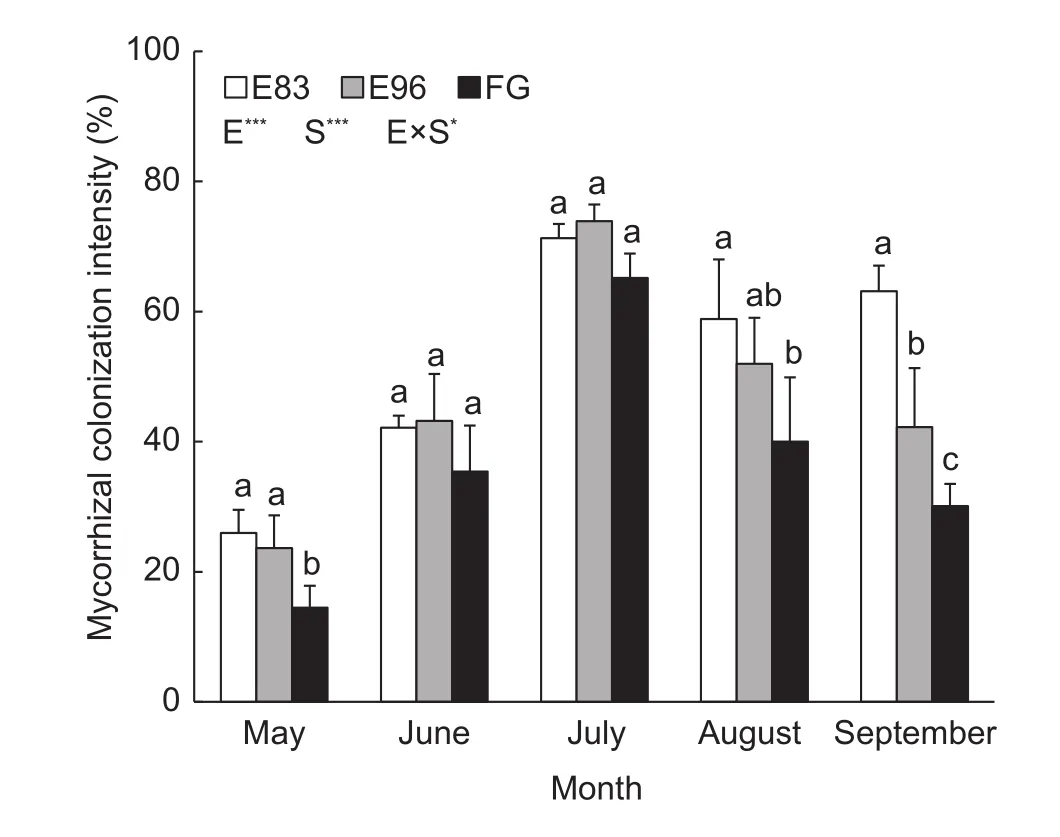
Fig. 2 Colonization intensity of arbuscular mycorrhizal fungi in the roots collected from the grazing exclusion sites since 1983(E83) and 1996 (E96) and free grazing site (FG). E, S and E× S represent the effect of exclusion, season, and their interactions,respectively. The bars in each column represent standard deviation of the means. The different lowercase letters reveal the significant difference among the three treatments in each month at P<0.05 (*, P<0.05; **, P<0.01; ***, P<0.001).
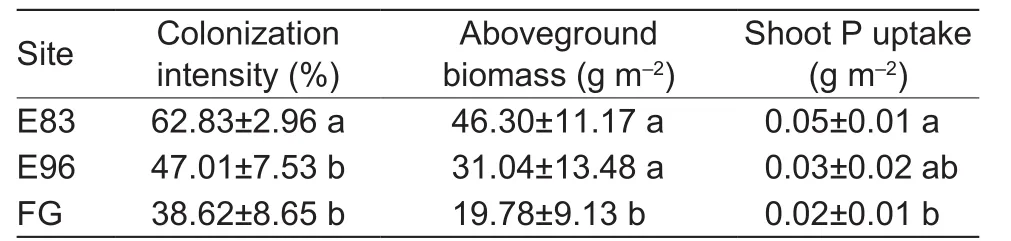
Table 2 Mycorrhizal colonization intensity, aboveground biomass, and shoot P uptake of Stipa grandis under grazing exclusion sites since 1983 (E83) and 1996 (E96) and free grazing site (FG) in July
Soil alkaline phosphatase activity, ranging from 160.45 to 675.06 μgp-nitrophenol h–1g–1soil, showed remarkable seasonal changes, with the highest in June but the lowest in September in all three sites (Table 3). During the grass growth period, soil alkaline phosphatase activities in both grazing exclusion sites of E83 and E96 were similar, but 20–102% higher than that in FG. In particular, soil alkaline phosphatase activity in FG site in September was the lowest and only accounted for 50–60% of those in grazing exclusion sites.
3.4. Plant aboveground biomass and P uptake
The aboveground biomass showed seasonal changes in all these three sites, the largest biomass was detected in August, 94.07, 92.50, and 51.86 g m–2for E83, E96, and FG, respectively. The grazing exclusion sites always had 75–992% higher aboveground biomass than that of FG in the whole season (Fig. 3-A). Nearly half of the biomass in the three sites was contributed by the dominant plantS.grandisthough its aboveground biomass was significantly different between the excluded and grazed sites (Table 2).
Similarly, the three sites showed different seasonal changes in P uptake of the plant shoots (Fig. 3-B). The shoot P uptake in both E83 and E96 increased to the highest level,more than 0.11 g m–2in June and then quickly decreased to lower than 0.02 g m–2in September, while the peak(0.04 g m–2) in FG site delayed to July. The plant shoots in E96 tended to absorb more P than that in E83 during May and June. Moreover, the largest difference in P uptake among the three sites occurred in June. The dominant grass ofS.grandiscontributed to the whole P uptake by 35–50%.The grass in both grazing exclusion sites tended to have higher P uptake than that of FG (Table 2).
3.5. Correlations among the plants and AM fungal parameters
Plant aboveground biomass was closely correlated with shoot P uptake. In addition, both of them were highly significant correlated with root colonization intensity, spore density, hyphal length density and soil alkaline phosphatase activity, with one exception in which no obvious correlation was found between shoot P uptake and root colonization intensity (Table 4).
4. Discussion
4.1. Effect of long-term grazing exclusion on AM fungi
It is widely known that over-grazing induces soil degradation and may thereby hinder AM fungal development (Su and Guo 2007; Baiet al.2013). However, the grazing exclusion can improve soil physical, chemical and even biological properties (Liuet al.2016, 2017; Xionget al.2016), and thus produces better conditions for the survival and development of AM fungi, and their infection in plant roots as well (Eschenet al.2009; Kamraniet al.2014; Guoet al.2016). Consequently, the grazing exclusion paddocks usually showed much higher AM fungal parameters, such as spore and hyphal length densities, root colonization intensity,compared with free grazing paddock (Figs. 1 and 2, Table 2),which agrees with a previous report from Wanget al.(2014).The improvement of soil properties like low bulk density, high soil organic matter and total nitrogen after grazing exclusion might be one of the probable reasons (Table 1; Baiet al.2013; Liuet al.2016). It is notable that soil fertility did not continue to increase when the grazing exclusion durations increased from 18 years (E96) to 31 years (E83) (Table 1),which suggested that the soil nutritional status might be stable after a certain exclusion period. Jinget al.(2014)also reported that grazing exclusion for 10–15 years could achieve satisfactory recovery of the degraded typical steppe in the Loess Plateau, China. Moreover, the different grazing exclusion durations showed stronger influence on spore density but much weaker on other AM fungal parameters. It is thus presumed that spore density may sensitively reflect the impacts of long-term grazing exclusion on AM fungi since the survival strategy of spores in soil.
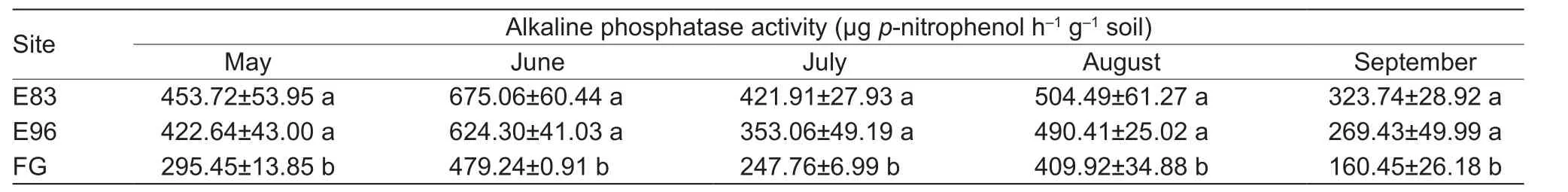
Table 3 Alkaline phosphatase activity in the soils collected from grazing exclusion sites since 1983 (E83) and 1996 (E96) and free grazing site (FG) in the typical steppe of Xilinguole League, Inner Mongolia, China
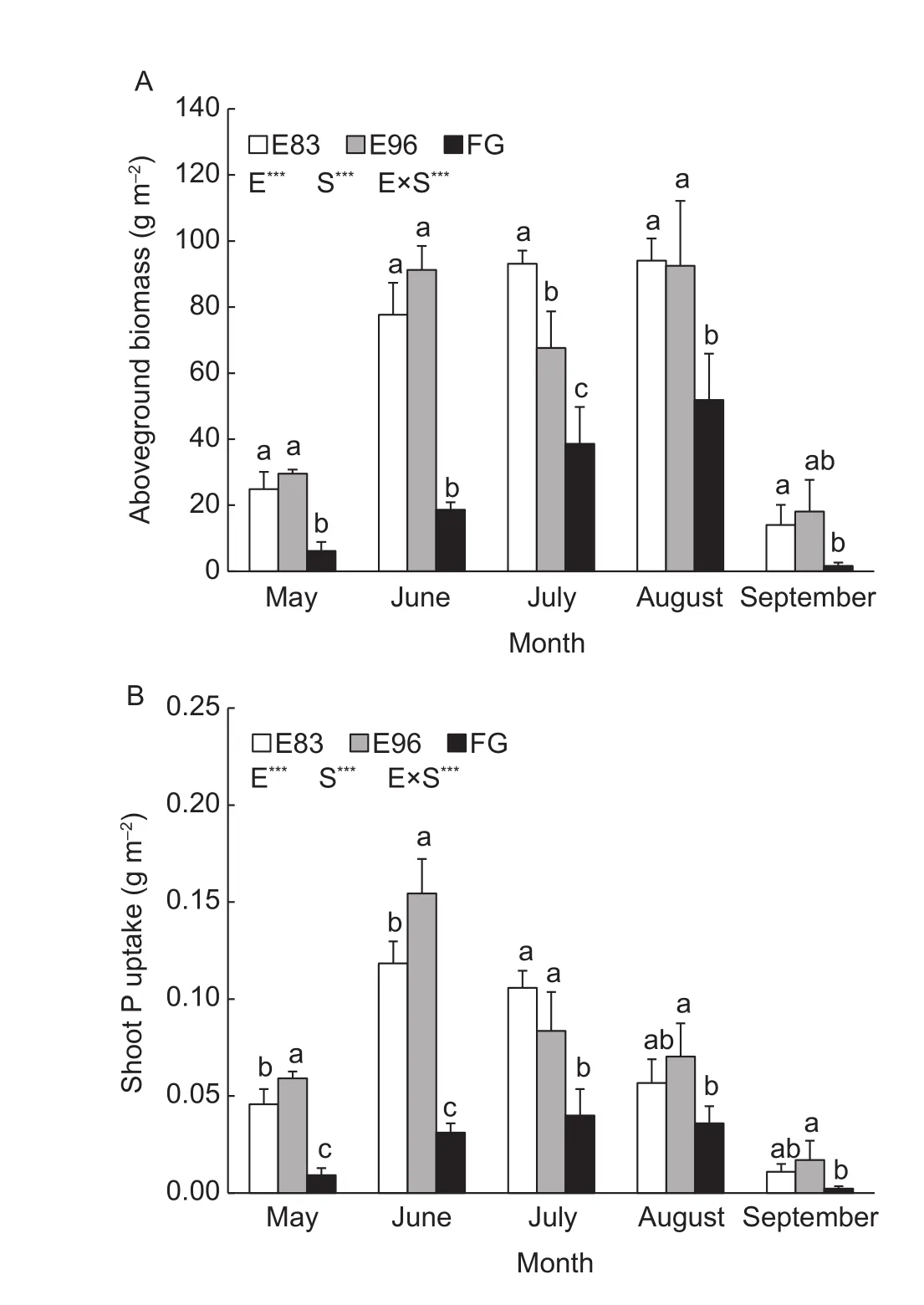
Fig. 3 The aboveground biomass (A) and shoot P uptake (B)of plants collected from the grazing exclusion sites since 1983(E83) and 1996 (E96) and free grazing site (FG). E, S and E× S represent the effect of exclusion, season, and their interactions,respectively. The bars in each column represent standard deviation of the means. The different lowercase letters reveal the significant difference among the three treatments in each month at P<0.05 (*, P<0.05; **, P<0.01; ***, P<0.001).
The remarkable seasonal changes in spore density,hyphal length density, and root colonization intensity of AM fungi were presumably attributed to the variations of temperature and precipitation (Hoffmannet al.2016). All these parameters of AM fungi reached the peak levels in summer time (Figs. 1 and 2). Zangaroet al.(2013)observed similar results in the grassland of southern Brazil.However, the less intense seasonal changes of these fungal parameters in the fenced sites, compared with the free grazing site, could be explained as high resistance and resilience of AM fungi to the environmental changes.It is notable that the spore density showed much stronger responses to grazing management and seasonal changes,compared with hyphal length density and colonization intensity. That means spore density may be more sensitive as to reflecting the status of the typical steppe. In addition,no obvious difference on colonization intensity was detected during fast growth season (June and July) of the plant among these three sites (Fig. 2), which indicated that the seasonality could regulate the effect of grazing management on AM fungi colonization. In other words, the growth and condition of AM fungi in the typical steppe were significantly affected by the interactions between exclusion treatment and season.
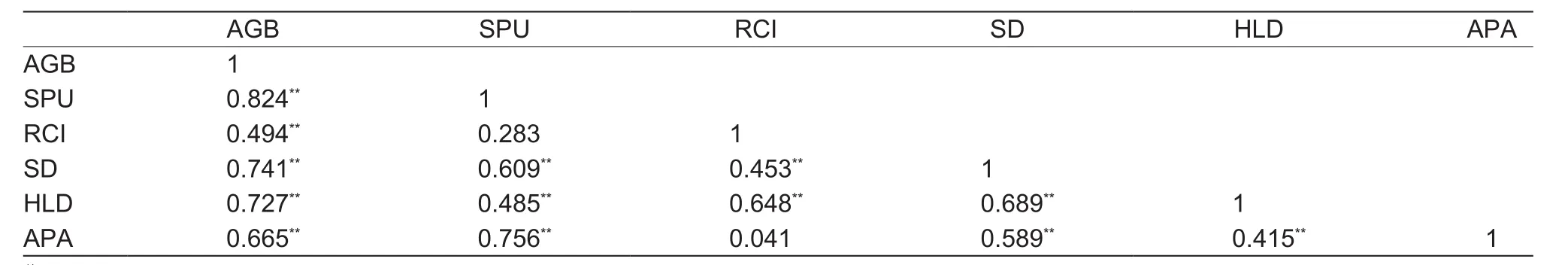
Table 4 Matrix of Pearson’s correlation coefficients among the parameters belonged to plants, arbuscular mycorrhizal fungi, and soil alkaline phosphatase1)
4.2. Association of AM fungi with plant aboveground biomass and P uptake
A significant number of studies have shown that most AM fungi can form a mutual association with most plants in terrestrial ecosystems (Khidiret al.2010; Porras-Alfaroet al.2011). Around 56% of the perennial plants and 65%of monocotyledonous forage species in the typical steppe of Inner Mongolia were reported to be infected by AM fungi and form mycorrhiza (Tianet al.2009). The host plants provide a sufficient quantity of carbohydrates, up to 20–30% of the total, for AM fungal growth and function(Walderet al.2012). Meanwhile, AM fungi can promote plant growth, nutrient uptake and even ameliorate some abiotic stresses (Jayne and Quigley 2014; Baumet al.2015;Dhawiet al.2016). The extra-radical hyphae of AM fungi are believed to extend root hairs into the surrounding soil,and thus increase the absorption and transport speed of nutrients or water by plant roots (Smith and Smith 2011).Heijdenet al.(2008) measured that around 75% of the P requirement of plants could be supplied by AM fungi in natural ecosystems. Consequently, the relevant parameters of AM fungi were usually positively correlated with vegetative parameters (Eschenet al.2009; Yanget al.2010; Baet al.2012). We obtained similar results in which spore density,hyphal length density and root colonization intensity of AM fungi were positively correlated with the plant aboveground biomass and even shoot P uptake (Table 4), which might imply the importance of AM fungi in the steppe vegetation.However, it should be notable that a few researchers obtained the negative relationships between AM fungi and plants (Antoninkaet al.2011; Veigaet al.2011; Reidingeret al.2012; Hiiesaluet al.2014). They explained as the differences in the types of AM fungi and plants involved in their studied samples. We also did not find a significant relationship between root colonization intensity and shoot P uptake. A decreased dependence of plants in the excluded sites on AM fungi might account for this phenomenon.Kahiluotoet al.(2012) also posited that the N supply in soil played a determining role in the benefit derived from AM fungi on plant P uptake and growth. Moreover, AM fungal taxa differ in their contributions to nutrient uptake(Smith and Smith 2011), as do the responses of different functional plants (Yanget al.2016). That means the overall contribution of AM fungi to plant P uptake is a result of the interactions among host plants, AM fungi, and environmental factors. However, as we did not measure the species or community composition of the AM fungi or vegetation in the present study, a more detailed examination is needed in the future.
Alkaline phosphatase is believed to play an important role in the conversion of organic P into the forms that are easily used by plants (Nagyet al.2009). A few reports have shown that the activity of alkaline phosphatase is correlated with AM fungal parameters such as spore density and hyphal length density (Heet al.2010; Wanget al.2014), and vegetative parameters as well (Pilar-izquierdoet al.2012).Similar results were determined in this study (Table 4), which might imply that alkaline phosphatase was involved in soil P bioavailability and promoted the growth of both AM fungi and steppe vegetation.
5. Conclusion
Long-term grazing exclusion largely enhanced AM fungal spore and hyphal length density, root colonization intensity and even soil alkaline phosphatase activity due to soil improvement. All the fungal parameters showed remarkable seasonal changes due to their quick responses to the changes in temperature and precipitation. However, the less intense seasonal changes in the fenced sites could be attributed to high resistance and resilience of AM fungi to the environmental changes. The AM fungi played important roles in typical steppe since AM fungal spore density,hyphal length density and root colonization intensity were all positively correlated with the plant aboveground biomass and even shoot P uptake. The spore density, compared with other AM fungal parameters, could sensitively reflect the impacts of long-term grazing exclusion on AM fungi since survival strategy of spores in soil.
Acknowledgements
We thank Prof. Gu Feng from China Agricultural University for his assistance in experimental design and technical support. This work was supported by the National Basic Research Program of China (2014CB138801) and the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT0412).
An Z Q, Hendrix J W, Hershman D E, Henson G T. 1990.Evaluation of the ‘most probable number’ (MPN) and wetsieving methods for determining soil-borne populations of endogonaceous mycorrhizal fungi.Mycologia,82, 516–581.
Antoninka A, Reich P B, Johnson N C. 2011. Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem.New Phytologist,192, 200–214.
Ba L, Ning J X, Wang D L, Facelli E, Facelli J M, Yang Y N,Zhang L C. 2012. The relationship between the diversity of arbuscular mycorrhizal fungi and grazing in a meadow steppe.Plant and Soil,352, 143–156.
Bai G, Bao Y Y, Du G X, Qi Y L. 2013. Arbuscular mycorrhizal fungi associated with vegetation and soil parameters under rest grazing management in a desert steppe ecosystem.Mycorrhiza,23, 289–301.
Bao S D. 2000.Methods of Soil Agricultural Chemical Analysis.China Agriculture Press, Beijing. pp. 25–97, 268–270. (in Chinese)
Baum C, El-Tohamy W, Gruda N. 2015. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review.Scientia Horticulturae,187, 131–141.
Blake G R, Hartge K H. 1986. Bulk density. In: Klute A, ed.,Methods of Soil Analysis. American Society of Agronomy,Madison, WI, USA. pp. 363–375.
Cheng J M, Jing G H, Wei L, Jing Z B. 2016. Long-term grazing exclusion effects on vegetation characteristics,soil properties and bacterial communities in the semi-arid grasslands of China.Ecological Engineering,97, 170–178.
Dhawi F, Datta R, Ramakrishna W. 2016. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil.Chemosphere,157, 33–41.
Eschen R, Müller-Schärer H, Schaffner U. 2009. Aboveground environment type, soil nutrient content and arbuscular mycorrhizal fungi explain establishment success ofCentaurea jaceaon ex-arable land and in late-successional grasslands.Plant and Soil,322, 115–123.
Gee G W, Or D. 2002. Particle-size analysis. In: Dane J H, Topp G C, eds.,Methods of Soil Analysis. American Society of Agronomy, Madison, WI, USA. pp. 255–293.
Grigera G, Oesterheld M. 2004. Mycorrhizal colonization patterns under contrasting grazing and topographic conditions in the flooding pampa (Argentina).Rangeland Ecology and Management,57, 601–605.
Guo Y J, Du Q F, Li G D, Ni Y, Zhang Z, Ren W B, Hou X Y.2016. Soil phosphorus fractions and arbuscular mycorrhizal fungi diversity following long-term grazing exclusion on semi-arid steppes in Inner Mongolia.Geoderma,269,79–90.
He X L, Li Y P, Zhao L L. 2010. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere ofArtemisia ordosica, Krasch. in Mu Us sandland, China.Soil Biology and Biochemistry,42, 1313–1319.
Heijden M G A V D, Bardgett R D, Straalen N M V. 2008. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems.Ecology Letters,11, 296–310.
Hiiesalu I, Pärtel M, Davison J, Gerhold P, Metsis M, Moora M, Öpik M, Vasar M, Zobel M, Wilson S D. 2014. Species richness of arbuscular mycorrhizal fungi: Associations with grassland plant richness and biomass.New Phytologist,203, 233–244.
Hoffmann C, Giese M, Dickhoefer U, Wan H W, Bai Y F, Steffens M, Liu C Y, Butterbach-Bahl K, Han X G. 2016. Effects of grazing and climate variability on grassland ecosystem functions in Inner Mongolia: Synthesis of a 6-year grazing experiment.Journal of Arid Environments,135, 50–63.
Jakobsen I, Abbott L K, Robson A D. 1992. External hyphae of vesicular-arbuscular mycorrhizal fungi associated withTrifolium subterraneumL.New Phytologist,120, 509–516.
Jayne B, Quigley M. 2014. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: A meta-analysis.Mycorrhiza,24, 109–119.
Jing Z B, Cheng J M, Su J S, Bai Y, Jin J W. 2014. Changes in plant community composition and soil properties under 3-decade grazing exclusion in semiarid grassland.Ecological Engineering,64, 171–178.
Kahiluoto H, Ketoja E, Vestberg M. 2012. Plant-available P supply is not the main factor determining the benefit from arbuscular mycorrhiza to crop P nutrition and growth in contrasting cropping systems.Plant and Soil,350, 85–98.
Kamrani V, Alamdari P, Amanifar S. 2014. The relationships between some soil characteristics and abundance of arbuscular mycorrhizal fungi spores.International Journal of Biosciences,5, 263–268.
Khidir H H, Eudy D M, Porras-Alfaro A, Herrera J, Natvig D O,Sinsabaugh R L. 2010. A general suite of fungal endophytes dominate the roots of two dominant grasses in a semiarid grassland.Journal of Arid Environment,74, 35–42.
Li X L, Liu Z Y, Wang Z, Wu X H, Li X L, Hu J, Shi H X, Guo F H, Zhang Y, Hou X Y. 2015. Pathways ofLeymus chinensisindividual aboveground biomass decline in natural semiarid grassland induced by overgrazing: A study at the plant functional trait scale.PLoS ONE,10, e0124443.
Liu J, Zhang Q C, Li Y, Di H J, Xu J M, Li J Y, Guan X M, Xu X Y,Pan H. 2016. Effects of pasture management on soil fertility and microbial communities in the semi-arid grasslands of Inner Mongolia.Journal of Soils Sediments,16, 235–242.
Liu J H, Wu J J, Su H B, Gao Z H, Wu Z T. 2017. Effects of grazing exclusion in Xilin Gol grassland differ between regions.Ecological Engineering,99, 271–281.
McDowell R W, Brookes P C, Mahieu N, Poulton P R, Johnston A E, Sharpley A N. 2002. The effect of soil acidity on potentially mobile phosphorus in a grassland soil.Journal of Agricultural Science,139, 27–36.
Miao R H, Jiang D M, Musa A, Zhou Q L, Guo M X. 2015.Effectiveness of shrub planting and grazing exclusion on degraded sandy grassland restoration in Horqin sandy land in Inner Mongolia.Ecological Engineering,74, 164–173.
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M.2009. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated.New Phytologist,181, 950–959.
Petipas R H, Brody A K. 2014. Termites and ungulates affect arbuscular mycorrhizal richness and infectivity in a semiarid savanna.Botany,92, 233–240.
Piippo S, Markkola A, Härmä E, Tuomi J. 2011. Do compensatory shoot growth and mycorrhizal symbionts act as competing above- and below-ground sinks after simulated grazing?Plant Ecology,212, 33–42.
Pilar-izquierdo M C, Ortega N, Perez-mateos M, Busto M D.2012. Barley seed coating with free and immobilized alkaline phosphatase to improve P uptake and plant growth.Journal of Agricultural Science,150, 691–701.
Porras-Alfaro A, Herrera J, Natvig D O, Lipinski K, Sinsabaugh R L. 2011. Diversity and distribution of soil fungal communities in a semiarid grassland.Mycologia,103, 10–21.
Reidinger S, Eschen R, Gange A C, Finch P, Bezemer T M.2012. Arbuscular mycorrhizal colonization, plant chemistry,and aboveground herbivory onSenecio jacobaea.Acta Oecologica,38, 8–16.
SBXL (Statistics Bureau of Xilinguole League). 2008.Statistical Yearbook of Xilinguole League. China Statistics Press,Beijing. (in Chinese)
Smith S E, Smith F A. 2011. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales.Annual Review of Plant Biology,62,227–250.
Su Y Y, Guo L D. 2007. Arbuscular mycorrhizal fungi in nongrazed, restored and over-grazed grassland in the Inner Mongolia Steppe.Mycorrhiza,17, 689–693.
Tabatabai M A. 1994. Soil enzymes. In: Weaver R W, Angle J S, Bottomly P S, eds.,Methods of Soil Analyses,Part 2,Microbiological and Biochemical Properties. Soil Science Society of America, Madison. pp. 775–833.
Tian H, Gai J P, Zhang J L, Christie P, Li X L. 2009. Arbuscular mycorrhizal fungi associated with wild forage plants in typical steppe of eastern Inner Mongolia.European Journal of Soil Biology,45, 321–327.
Trouvelot A, Kough J L, Gianinazzi-Pearson V. 1986. Mesure du taux de mycorhization VA d’un système radiculaire.Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Physiological and Genetical Aspects of Mycorrhizae, Proceedings of the 1st European Symposium on mycorrhizae, Gianinazzi-Pearson V, Gianinazzi S, eds.,Institut National de la Recherche Agronomique, Paris. pp.217–221. (in French)
Veiga R S L, Jansa J, Frossard E, Heijden M G A V D. 2011.Can arbuscular mycorrhizal fungi reduce the growth of agricultural weeds?PLoS ONE,6, 1–10.
Walder F, Niemann H, Natarajan M, Lehmann M F, Boller T,Wiemken A. 2012. Mycorrhizal networks: Common goods of plants shared under unequal terms of trade.Plant Physioloogy,159, 789–797.
Wang C Y, He N P, Zhang J J, Lv Y L, Wang L. 2015. Long-term grazing exclusion improves the composition and stability of soil organic matter in Inner Mongolian grasslands.PLoS ONE,10, e0128837.
Wang Q, Bao Y Y, Liu X W, Du G X. 2014. Spatio-temporal dynamics of arbuscular mycorrhizal fungi associated with glomalin-related soil protein and soil enzymes in different managed semiarid steppes.Mycorrhiza,24, 525–538.
Xiong D P, Shi P L, Zhang X Z, Zou C B. 2016. Effects of grazing exclusion on carbon sequestration and plant diversity in grasslands of China - A meta-analysis.Ecological Engineering,94, 647–655.
Yang C, Hamel C, Schellenberg M P, Perez J C, Berbara R L.2010. Diversity and functionality of arbuscular mycorrhizal fungi in three plant communities in semiarid grasslands national park, Canada.Microbial Ecology,59, 724–733.
Yang H S, Xu J L, Guo Y, Koide R T, Dai Y J, Xu M M, Bian L P, Bian X M, Zhang Q. 2016. Predicting plant response to arbuscular mycorrhizas: The role of host functional traits.Fungal Ecology,20, 79–83.
Zangaro W, Rostirola L V, Souza P B D, Alves R D A, Lescano L E A M, Rondina A B L, Nogueira M A, Carrenho R. 2013.Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil.Mycorrhiza,23, 221–233.
Zhang Y J, Zhang X Q, Wang X Y, Liu N, Kan H M. 2014.Establishing the carrying capacity of the grasslands of China: A review.The Rangeland Journal,36, 1–9.
Zhou W P, Xiang D, Hu Y J, Li Z F, Chen B D. 2013. Influences of long-term enclosure on the restoration of plant and AM fungal communities on grassland under different grazing intensities.Acta Ecologica Sinica,33, 3383–3393. (in Chinese)
Zhu G Y, Deng L, Zhang X B, Shangguan Z P. 2016.Effects of grazing exclusion on plant community and soil physicochemical properties in a desert steppe on the Loess Plateau, China.Ecological Engineering,90, 372–381.
杂志排行
Journal of Integrative Agriculture的其它文章
- Improve access to the EU market by identifying French consumer preference for fresh fruit from China
- Management and prevention of mastitis: A multifactorial approach with a focus on milking, bedding and data-management
- Elimination of ceftiofur hydrochloride residue in postpartum cows’milk after intramammary infusing at dry-off
- Evaluation of a new qPCR test to identify the organisms causing high total bacterial count in bulk tank milk
- Prevalence and characteristics of extended spectrum β-lactamaseproducing Escherichia coli from bovine mastitis cases in China
- Evolutionary analysis of plant jacalin-related lectins (JRLs) family and expression of rice JRLs in response to Magnaporthe oryzae
