Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae)
2018-06-06
State Key Laboratory of Crop Stress Biology for Arid Areas/Key Laboratory of Applied Entomology, College of Plant Protection,Northwest A&F University, Yangling 712100, P.R.China
1. Introduction
The diamondback moth,Plutella xylostella(L.) (Lepidoptera:Plutellidae) is one of the most devastating pests worldwide,attacking various cruciferous plants, particularly cabbage and radish (Xia 2013). The control of this pest chiefly relies on the utility of chemical insecticides, which in turn causes many negative consequences, i.e.,P. xylostellahas become resistant to many insecticides. So far, the costs for managing andcontrolling the pest is estimated to be 4–5 billion USD annually, which leads many researchers to pay attention to the studies ofP. xylostellaacross many countries (Talekar and Shelton 1993; Zaluckiet al.2012).
In insects, the sensillum is a specialized structure of the epidermis and acts as a receptor for perception of a variety of environmental stimuli. Odorants, for instance,can be detected by olfactory sensilla and mediate insect behaviors (Schneider 1964). The cephalosome (head) of an insect, and especially the antennae and mouthparts, has various types of sensilla that play important roles in their behaviors, including host selection, feeding, mate attraction,oviposition, defense, and migration (Schoonhovenet al.1998; Antonet al.2003). In recent years, biological controls,including the application of sex pheromones, have become increasingly important (Stelinskiet al.2006).
The structure and function of sensilla in Lepidoptera have been well known for decades, and several studies have investigated sensilla on antennal or larval mouthparts ofP. xylostella(Yanget al.2001; Weiet al.2003; Yanet al.2014). To better understand their olfactory system and behavioral mechanisms that would be involved in the biological control of this pest, we comprehensively researched the type, size, and distribution of antennal and mouthpart sensillae on both larvae and adults ofP. xylostellaby using a scanning electron microscope (SEM).
2. Materials and methods
2.1. Source of insects
P. xylostellaspecimens usedin our experiments were provided by the Applied Entomology Lab in Northwest A&F University, China. Larvae were obtained from the vegetable orchards in Taibai County, Shaanxi, China in 2010, and then reared on the host plant (cabbage) seedlings in a climate chamber under controlled conditions ((25±1)°C; (60±10)%RH; 16 h light:8 h dark) (Yanget al.2004).
2.2. Scanning electron microscope
Heads from 20 larvae (in 3rd instar) and adults (10 males and 10 females) were placed in Carnoy’s fixative solution(95% ethanol and glacial acetic acid with ratio of 3:1) for 12 h and then kept overnight in 2% glutaraldehyde at 4°C.The following day, the samples were cleaned twice with a phosphate buffer (each for 10 min), and washed in 100%ethanol solution in an ultrasonic cleaner (three times, each for 2 min). Then, the antennae and mouthparts of larvae and adults were removed under a microscope using sharp blades. The antennae and mouthparts of larvae and adults were dehydrated through a graded ethanol series of 30, 50,70, 80, and 90% (for 15 min each), then fully dehydrated in 100% ethanol (three times for 30 min each), and lastly cleaned in isoamyl acetate (three times for 10 min each).After critical point drying with carbon dioxide, the specimens were attached to a holder using electric adhesive tape,sputter-coated with gold, examined and photographed with an S-4800 FE-SEM (at 10.0 kV). The sensilla were classified according to their morphology, shape and size,following Schneider (1964).
2.3. Statistical analysis
The morphology, number and distribution of the sensilla were examined from the antennae and mouthparts of larvae and adults. The images were compiled using Adobe Photoshop CS5. The length and diameter of sensilla (mean±SE) were measured on at least 30 sensilla of the same type by using the Sigma Scan Pro Measurement System 5.0 Software.Differences in size of antennal sensilla between males and females were statistically analyzed using an independent samplet-test (P<0.05) in the SPSS16.0 Statistical Software.
3. Results
3.1. Larval head capsule
CapsuleThe head ofP. xylostellalarva is a typical, rounded,sclerotized capsule composed of a chewing mouthpart, a pair of antennae and six pairs of stemmata on the anterior surface (Fig. 1-A). The mouthpart is hypognathous,consisting of a labrum, a mandible, a maxilla, a labium, and a hypopharynx (Fig. 1-B).
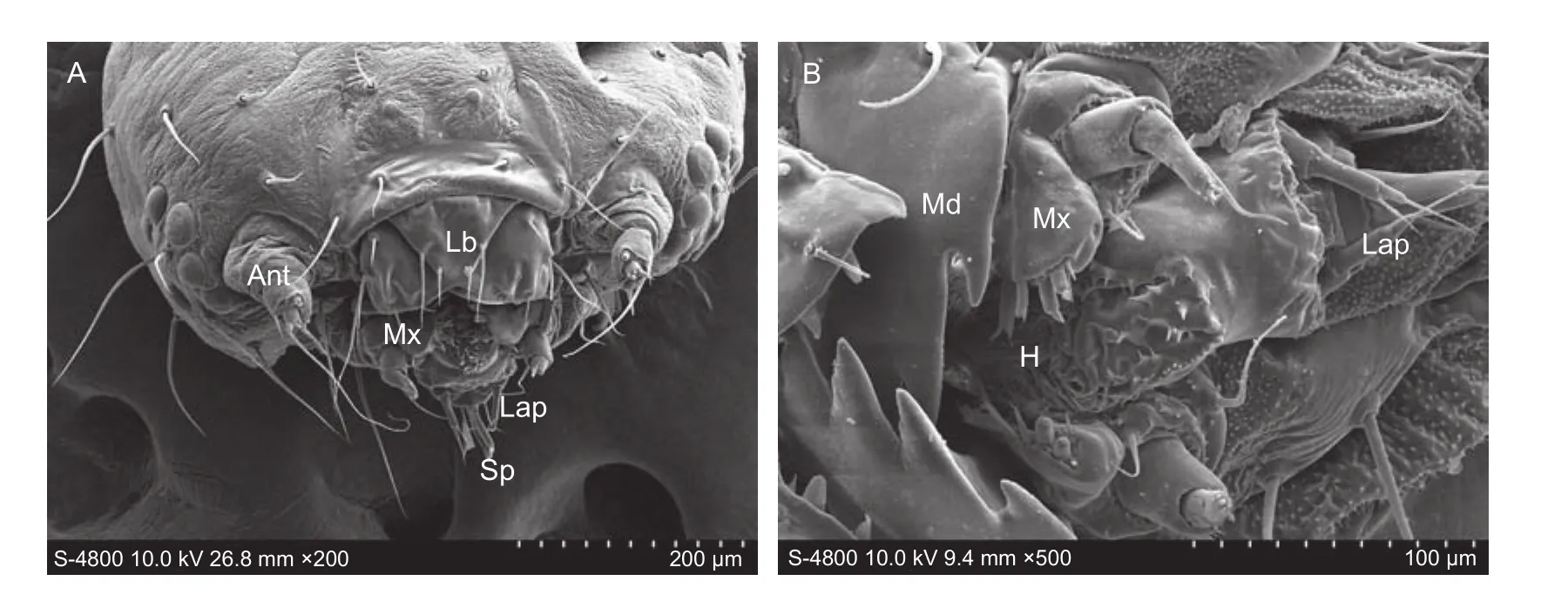
Fig. 1 Larval head capsule of Plutella xylostella. A, frontal view of the head. B, ventral view of the mouthparts. Ant, antenna; Lb,labrum; Mx, maxilla; Lap, labial palp; Sp, spinneret; Md, mandible; H, hypopharynx.
AntennaThe antennae ofP. xylostellalarva (113.9 μm long) are located between the ocelli and the mandible.Each consists of three components, a scape, pedicel, and flagellum (Fig. 2-A). The basal scape is (56.47±2.64) μm wide and (71.45±5.50) μm long, without any sensilla. The pedicel is (50.22±1.63) μm wide and (23.21±1.58) μm long, with three sensilla basiconica (B1, (23.24±1.27) μm long and (7.10±0.24) μm wide; B2, (36.69±1.93) μm long and (9.82±0.58) μm wide; B3, (7.26±0.45) μm long and(1.64±0.21) μm wide) and two sensilla chaetica (C1, short and slender, (21.14±1.46) μm long, (2.24±0.04) μm wide; C2,longer, (141.59±6.21) μm long, (3.91±0.24) μm wide) on distal surface (Fig. 2-B–D). The flagellum is shorter, (14.50±1.03) μm wide and (19.31±1.15) μm long, with three sensilla basiconica (B4–B6) and one sensillum styloconicum (St). B6((19.70±0.87) μm long and (6.62±0.23) μm wide) is similar to B1 and B2, significantly longer than B4 ((3.84±0.14) μm long and (2.82±0.03) μm wide) and B5 ((3.57±0.06) μm long and (2.27±0.03) μm wide). The sensillum styloconicum is cylinder-shaped with a large socket at base, (17.23±3.05) μm long (Fig. 2-B–D).
3.2. Larval mouthpart
LabrumThe labrum ((105.41±5.24) μm long and (183.47±6.29) μm wide) is trapezoidal profiling a V-shape notch, with six pairs of zygomorphic sensilla chaetica distributed on dorsal surface (C1, (23.44±2.17) μm long, (2.70±0.02) μm wide; C2, (68.34±4.13) μm long, (4.28±0.32) μm wide; C3, (260.70±1.87) μm long, (2.78±0.10) μm wide;C4, (24.51±1.45) μm long, (2.73±0.12) μm wide; C5,(49.90±3.11) μm long, (4.52±0.27) μm wide; C6,(22.67±1.70) μm long, (3.51±0.12) μm wide) (Fig. 3-A).The epipharynx has three types of sensilla, a pair of flattened sensilla digitiformia ((55.74±5.33) μm long and(17.20±2.11) μm wide), located near the lateral margin,covered by microtrichia. Three pairs of flattened and thick sensilla chaetica (S1, (26.20±0.91) μm long, (6.24±0.45) μm wide; S2, (34.59±1.70) μm long, (7.34±0.56) μm wide; S3,(31.63±1.24) μm long, (4.31±0.21) μm wide) are near the anterior margin. Three pairs of epipharynx sensilla (Es)are near the median area. In addition, the base of the epopharynx is covered with many microtrichia (Fig. 3-B).MandibleThe mandible ((144.71±5.54) μm long and(130.47±4.42) μm wide) is strongly sclerotized, forming six dentitions (T1–T6) on the apical margin of each mandible.Two sensilla chaetica are located on the proximal margin(C1, (34.45±3.51) μm long, (3.44±1.45) μm wide; C2,(81.52±3.58) μm long, (4.83±1.27) μm wide) (Fig. 3-C and D).
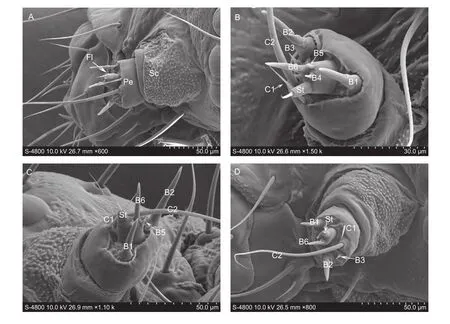
Fig. 2 Sensilla on the larval antenna of Plutella xylostella. A, dorsal view of the three segmented antennae. B, ventral magnifying view of the antenna. C, dorsal magnifying view of antenna. D, distal magnifying view of the antenna. Fl, flagellum; Sc, scape;Pe, pedicel; C1–C2, sensilla chaetica; B1–B6, sensilla basiconica; St, sensillum styloconicum.
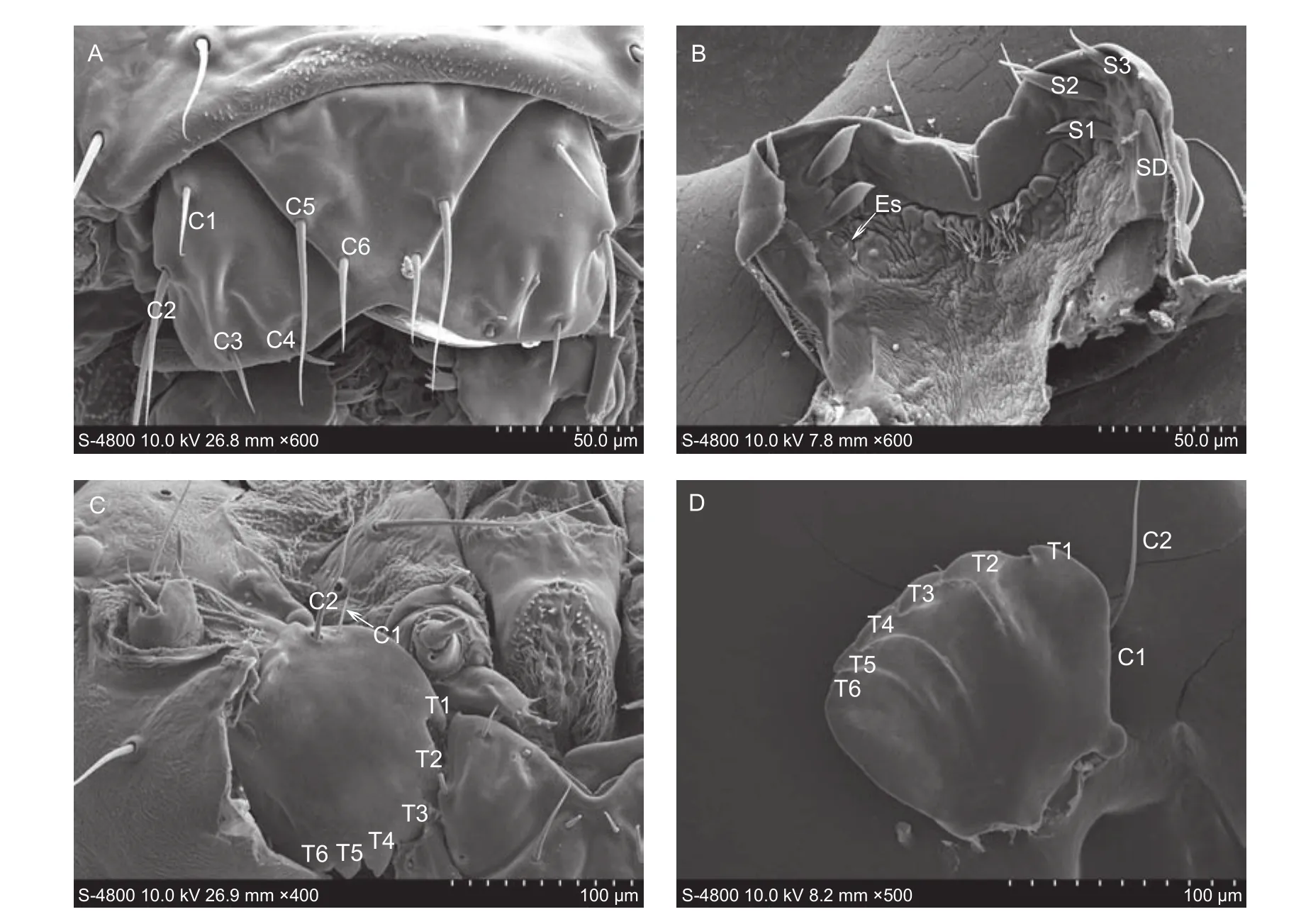
Fig. 3 The labrum and mandible of Plutella xylostella larvae. A, frontal view of the labrum. B, inner view of the epipharynx. C,inner view of the mandible. D, outer view of mandible. C1–C6, sensilla chaetica; S1–S3, flattened and thick sensilla chaetica; Es,epipharyngeal sensilla; SD, sensilla digitiformium; T1–T6, distal teeth.
HypopharynxThe hypopharynx is located in the central mouthpart and bears numerous microtrichia in rows on the margin of the tongue (Fig. 1-B).
MaxillaeEach maxilla consists of cardo ((28.84±3.20) μm long and (51.24±3.71) μm wide), stipes ((20.56±2.28) μm long and (46.14±4.02) μm wide), galea and a maxillary palp (Fig. 4-A). The cardo connects to stipes, and bears a sensillum chaeticum (C1, (78.60±4.42) μm long and(4.10±0.21) μm wide). The stipes equip a sensillum chaeticum (C2, (51.33±4.12) μm long and (3.20±0.89) μm wide) (Fig. 4-A). The galea has eight sensilla on its distal surface,two sensilla styloconica (St1, (14.10±2.01) μm long and (6.24±1.32) μm wide; St2, (17.21±2.05) μm long and (7.32±1.18) μm wide) in the medial of the galea terminal, three sensilla basiconica (B1, (8.19±0.41) μm long and (2.13±0.08) μm wide; B2, (10.38±0.92) μm long and (2.31±0.07) μm wide; B3, (6.12±0.43) μm long and(2.33±0.05) μm wide), and three flattened and long sensilla chaetica in a row (C3, (20.25±2.21) μm long and (3.34±0.29) μm; C4, (22.48±2.14) μm long and (3.00±0.12) μm wide; C5, (26.62±2.23) μm long and (3.14±0.25) μm wide)(Fig. 4-B and C).
The two-segmented maxillary palp is (25.45±1.14) μm long and (21.89±2.34) μm wide. The terminal segment of the maxillary palp bears an elongate and broad sensillum digitiformium (SD, (13.72±1.47) μm long and (3.92±0.33) μm wide) and two sensilla placodea (P) on the lateral side of the distal segment. The base part bears a sensillum styloconicum ((5.11±0.22) μm long and (2.50±0.02) μm wide) and seven sensilla basiconica (B1–B7) (Fig. 4-D).
LabiumThe labium has a pair of rounded labial palps and an elongated tube-like spinneret ((62.24±4.47) μm long) (Fig. 5-A). The labial palp is (34.59±2.71) μm long,bearing a sensillum basiconicum (B, (5.20±1.12) μm long and (1.43±0.13) μm wide) at the base, and a sensillum styloconicum (St, (31.51±3.91) μm long and (4.42±0.22) μm wide). The sensillum chaeticum (C1, (20.24±3.01) μm long and (1.77±0.11) μm wide) is situated on the dorsal side of palp. A pair of sensilla chaetica (C2, (52.4±4.32) μm long and (3.51±0.72) μm wide) is found at the ventral of the labium (Fig. 5-A and B).
3.3. Adult mouthparts
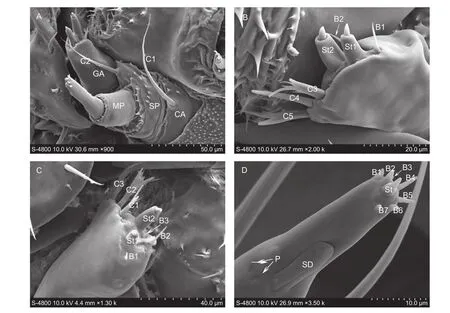
Fig. 4 The hypopharynx and maxilla of Plutella xylostella larva. A, the inner view of maxilla. B and C, the distal view of the galea.D, the distal segment of the maxillary palp. CA, cardo; SP, stipes; GA, galea; Mp, maxillary palp; C1–C5, sensilla chaetica; B1–B7,sensilla basiconica; St, sensilla styloconicum; SD, sensillum digitiformium; P, sensillun placodeum.
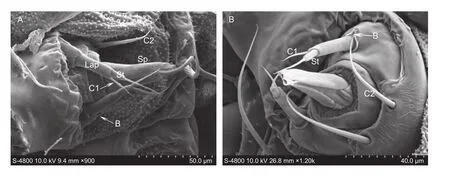
Fig. 5 Labium sensilla of Plutella xylostella larva. A, the ventral view of the labium. B, the magnifying view of palp. Lap, labial palps; Sp, spinneret; C1–C2, sensillum chaeticum; St, sensillum styloconicum; B, sensillum basiconicum.
The mouthparts in the adult ofP. xylostellaconsist of the labrum, mandibles, maxillae, labial palpi and proboscis.The labrum ((101.26±6.41) μm wide and (25.42±2.44) μm long) is degenerated to a small triangle, entirely covered with numerous microtrichia (Fig. 6-B). The mandible is also rudimentary, equipped with approximately 20 sensilla chaetica (length: 28.2–60.1 μm) (Fig. 6-B). The maxilla is reduced to the large three-segmented maxillary palps((127.80±5.44) μm long). Sensilla trichodea are found at the terminal of the second segment (Fig. 6-A). The huge labial palp is (905.86±13.24) μm long, consists of three segments, and is entirely covered by scales (Fig. 6-C).Sensilla squamiformium ((40.72±6.24) μm long) are found on the second segment (Fig. 6-D). The proboscis is coiled by two interlocked galeae on their dorsal and ventral sides(Fig. 7-A). Four types of proboscis sensilla are present:sensilla trichodea, sensilla chaetica, sensilla basiconica,and sensilla styloconica.
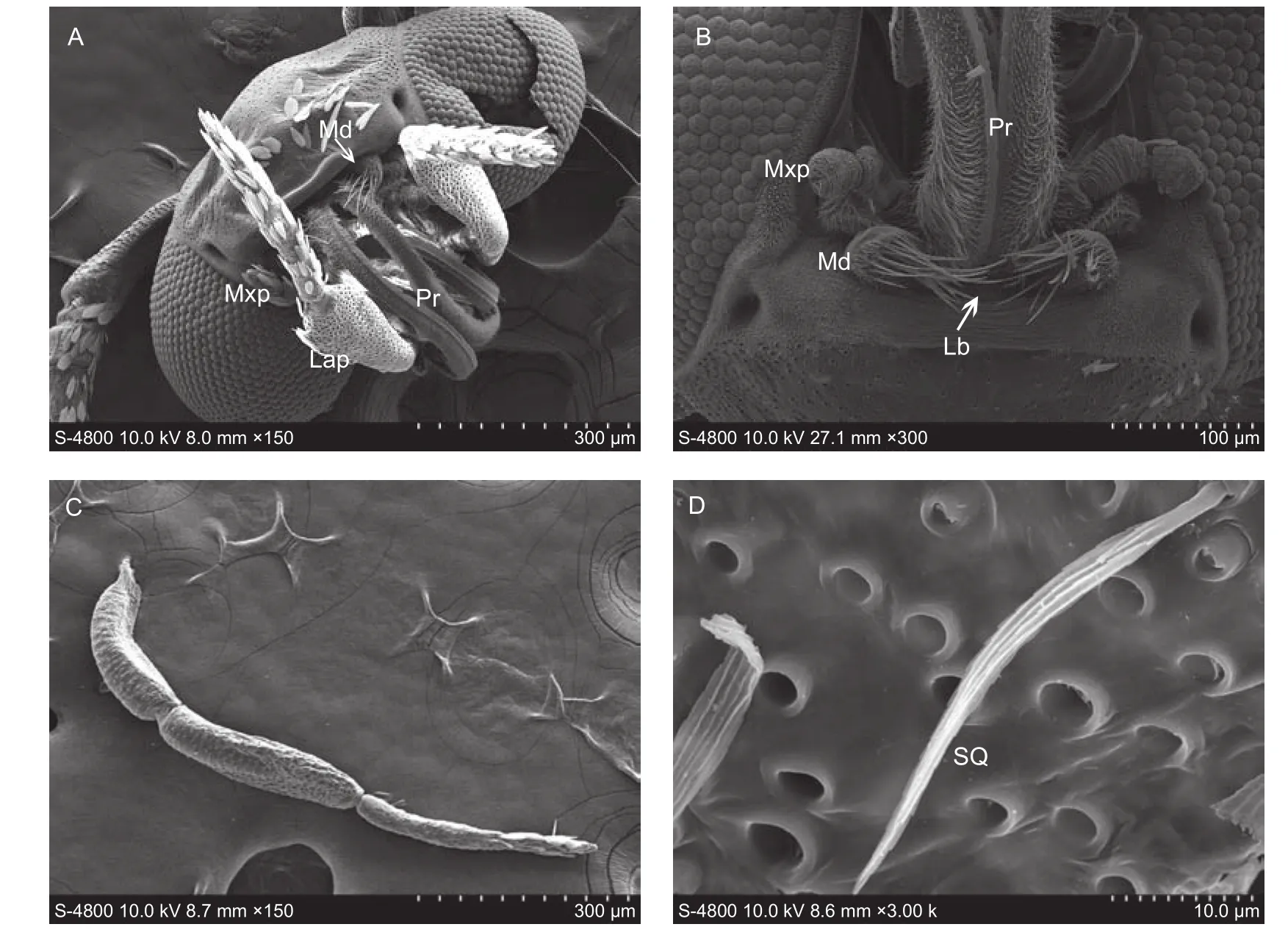
Fig. 6 The head and mouthparts of Plutella xylostella adult. A, frontal view of the adult head. B, ventral view of the adult mouthparts.C, labial palp. D, sensilla squamiformia. Md, mandibles; Mxp, maxillary palpi; Pr, proboscis; Lap, labial palpi; Lb, labrum; SQ,sensilla squamiformia.
Sensilla trichodea ((8.51±0.67) μm long and (1.10±0.02) μm wide) are mainly distributed at the base of the proboscis (Fig. 7-C). Sensilla chaetica ((16.36±1.67) μm long and (3.21±0.74) μm wide) with a grooved surface number less than 10 on the galea (Fig. 7-D). Sensilla basiconica((2.84±0.7) μm long and (1.29±0.02) μm wide) are present on the dorsal surface of the proboscis (Fig. 7-E). Sensilla styloconica ((14.54±2.05) μm long and (2.70±0.71) μm wide) are mainly distributed on the terminal surface of the proboscis. These sensilla possess a socket with four pikes surrounding it (Fig. 7-F).
3.4. Adult antennae
In total, seven types of sensilla were observed on the antennae ofP. xylostella, sensilla trichodea, sensilla chaetica, sensilla coeloconica, sensilla styloconica, sensilla squamiformia, Böhm sensilla, and sensilla basiconica. The length and basal width of these sensilla are presented for the antennae of both sexes in Table 1. More specifically, sexual dimorphism occurs in the size of antennal sensilla, i.e., the length of sensilla trichodea and sensilla coeloconica and the width of sensilla chaetica and sensilla styloconica. Notably,sensilla basiconica are only found on the antennae of males.
The sensilla trichodea are hair-like and mainly distributed on each sub-segment of the flagellum. These sensilla are more abundant on the antennae of males than females, and the length is significantly longer in the male flagellum than in the female flagellum (Fig. 8-B).
The sensilla chaetica are needle-like, and straight with a grooved surface. These sensilla are composed of a round socket, are wide at the base and sharp toward the tip. These sensilla are mainly present among the flagellomeres, and there are many more on the terminal segment of flagellum(Fig. 8-C). This type of sensilla is significantly wider on female antennae.
The sensilla coeloconica are composed of an upright central peg with a grooved surface and surrounded by a ring of 12–16 cuticular spines. These sensilla are found distributed on the center and terminal segments of a flagellum (Fig. 8-D). The length of the sensilla in females is significantly longer than that in males.
The sensilla styloconica is thumb-like with a cone-shaped tip. The slightly cuticular ridges are found on the surface of these sensilla. They occur at the distal margin of flagellum,and only one sensillum is present on each sub-segment of the flagellum (Fig. 8-E and F). Sexual dimorphism is observed in the length of these sensilla.
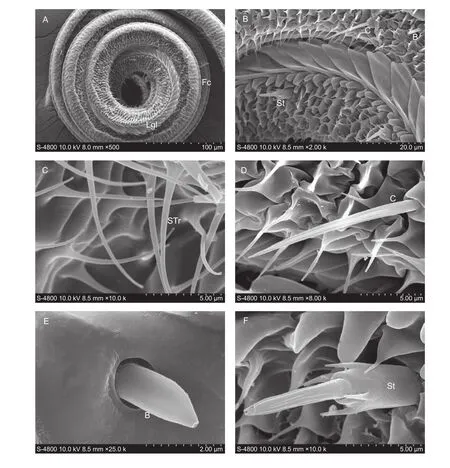
Fig. 7 The proboscis of Plutella xylostella adult. A, lateral view of theproboscis. B, interior view of the proboscis. C, sensilla trichodea. D, sensillum chaeticum. E, sensillum basiconicum. F, sensillum styloconicum. Fc, food canal; Lgl, ligulae of the galea linkage; STr, sensilla trichodea; B, sensillum basiconicum; St, sensillum styloconicum; C, sensillum chaeticum.
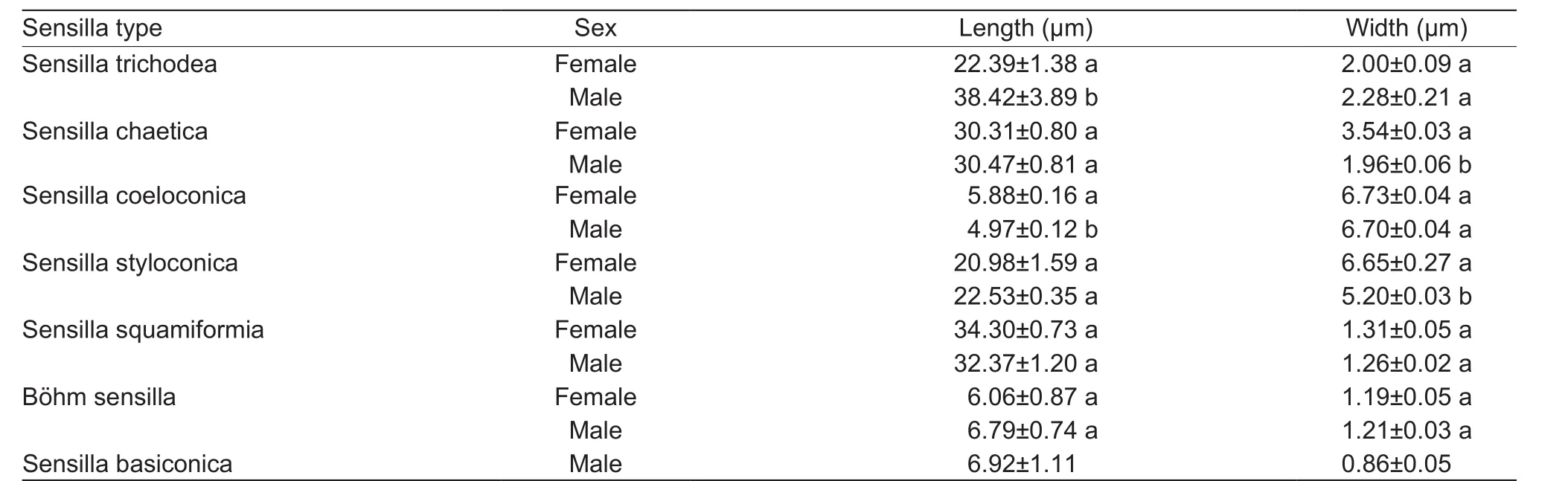
Table 1 The size of the sensilla on the antennae of adult of Plutella xylostella
The sensilla squamiformia are scale-like and elongated with a distal tapering. These sensilla are mainly distributed on the scape and pedicel segments (Fig. 8-G). They are statistically similar in their length and basal width in male and female antennae.
Böhm sensilla are short and sharp with smooth surface cuticles. These sensilla are inserted as clusters to the base of the scape and pedicel segments (Fig. 8-H). No sexual dimorphism was found in the size of these sensilla.
The sensilla basiconica are short and have a blunt seta.These sensilla are distributed on each sub-segment of the flagellum. Sensilla basiconica are only found on the antennae of males (Fig. 8-F).
4. Discussion
In this study, sensilla both on the larval and adult antennae and mouthparts of the diamondback mothP. xylostellawere examined using a scanning electron microscope. Generally,on larval antennae, three sensilla basiconica and two sensilla chaetica were observed on the pedicel segment and three sensilla basiconica and one sensillum styloconicum were found on the flagellum. Their structures, types and distribution were similar to other lepidopteran caterpillars,viz,Pentateucha inouei,Spodoptera exigua,Carposina sasakii, andCarposina coreana(Lin 2002; Liet al.2008;Liuet al.2011; Chen and Hua 2014). The structure and function of the sensilla basiconica, sensilla chaetica and sensilla styloconicum have been well studied in the past few decades. The multiparous sensilla basiconica play an important role in sensing olfactory functions (Schoonhoven and Dethier 1964; Hanson and Dethier 1973; Dethier 1980).The uniporous sensilla chaetica are suggested to function as mechanoreceptors (Zacharuk 1980; Bakeret al.1986;Faucheux 1995). The uniporous sensilla styloconica is characteristic of cold-sensitive receptors (Schoonhoven 1967).
Six types of sensilla on the larval mouthparts were identified: sensilla chaetica, sensilla digitiformia, sensilla epipharyngeal, sensilla basiconica, sensilla styloconica and sensilla placodea. Although these sensilla have been reported in previously studies of other lepidopteran larvae,their morphology, distribution and size vary among different species, e.g.,Manduca sexta,Choristoneura fumiferana,Spodoptera exigua, andC. coreana(De Boeret al.1977;Albert 1980; Liet al.2008; Chen and Hua 2014). There are six sensilla chaetica on the labrum, as described in other moth species, i.e.,C. sasakii(Liuet al.2011). Several studies noted that these aporous sensilla are associated with olfactory reception function (Albert 1980; Kent and Hildebrand 1987). Three pairs of epipharynx sensilla on the labrum were found in our study; however, studies of other moth species found different numbers of these sensilla,e.g.,C. sasakii,C. coreana,C. fumiferana,M. sexta, andEuxoa messoria(Albert 1980; Devitt and Smith 1982; Kent and Hildebrand 1987; Liuet al.2011; Chen and Hua 2014).Epipharynx sensilla are inferred to have an olfactory function for detecting plant volatiles (Albert 1980; Faucheux 1995).
Two sensilla chaetica on the mandible of the larvae ofP. xylostellaare inferred to have a mechanoreceptive function (Albert 1980; Kent and Hildebrand 1987); however,the five sensilla chaetica on the maxilla could be considered as olfactory receptors involved in odor recognition (Hanson 1970). Two sensilla styloconica on the galea may contribute to finding sources of food (Ishikawaet al.1969). Seven sensilla basiconica and one sensillum styloconicum on the maxillary palp are similar to the descriptions forAcrolepiopsis assectella,C. sasakiiandC. coreana(Al-Rouz and Thibout 1989; Liuet al.2011; Chen and Hua 2014);while Faucheux (1995) reported eight sensilla basiconica and no sensilla styloconica on the maxillary palp of the larvae ofHomoeosoma nebulella. Sensilla basiconica may play a role as chemoreceptors (Ishikawaet al.1969).Sensilla digitiformia on the maxillary palp are considered to have a humidity and temperature sensitive function (Keil 1996; Devitt and Smith 1982). In general, variation in the structure and quantity of sensilla on the maxillary palp can be regarded as defining characteristics for identification of larvae.
We identified seven types of sensilla on the antennae ofP. xylostellaadults: sensilla trichodea, sensilla chaetica,sensilla coeloconica, sensilla styloconica, sensilla squamiformia, Böhm sensilla and sensilla basiconica.These sensilla closely resemble those observed in many other Lepidoptera (Wanget al.2002). Sensilla trichodea are the most abundant and widespread sensilla on the antennae ofP. xylostella. This type of multiparous sensilla is olfactory receptors of host plant volatiles and sex pheromones (Zacharuk 1980; Chintaet al.1997;Shields and Hildebrand 2001; Maidaet al.2005). These multiparous sensilla basiconica were only found on the antennae of males, and their function could be related to responding to sex pheromone components (Meriveeet al.1999; Zhanget al.2011). Sensilla styloconica may be sensitive to temperature and humidity (Hallberget al.1994)or air (Olson and Andow 1993). Besides, these uniporous sensilla are more abundant in females than in males ofP. xylostella. This could explain the fact that females are more sensitive to the environment and more easily find host plants. The aporous sensilla chaetica are considered to be chemoreceptors (Tarumingkenget al.1976; Liet al.2006). We find only one type of sensilla coeloconica;however, studies of other moth species have shown these sensilla can be divided into more subtypes,viz,Sitotroga cerealella,andM. sextaandMythimna separate(Maet al.2017). These multiparous sensilla are sensitive to plant volatiles and CO2(Bruce and Cork 2001; Park and Hardie 2002). Böhm sensilla are similar in most insect species, as mechanoreceptors with a proprioceptive function (Li and Bai 2004). Sensilla squamiformia are commonly present in Lepidoptera insects, but their function is not yet clear and it remains to be demonstrated whether these aporous sensilla have a mechanoreceptive function (Lavoie-Dornik and McNeil 1987; Faucheux 1990; Yanget al.2012).
The sensilla on the mouthparts are described for the first time in the adult ofP. xylostella. In total, we found five types of sensilla, especially on the proboscis ofP. xylostella: sensilla chaetica, sensilla trichodea, sensilla squamiformia, sensilla basiconica and sensilla styloconica.The morphology of these mouthparts is similar to other Lepidoptera insects,Laspeyresia pomonella,Agrotis ypsilonandSynempora andesaeetc. (Goldware and Barnes 1973; Faucheux 2008; Xue and Hua 2014). Sensilla trichodea are the most abundant type of sensilla, occurring throughout the entire proboscis. The moth can utilize them as mechanosensilla to process information during nectar feeding and pollenation (Gilbert 1972; Krenn 1998, 2010;Mollemanet al.2005; Zenker 2011). Sensilla chaetica could be involved in detecting the position and movement of the proboscis (George and Nagy 1984; Krennet al.2005).Sensilla basiconica occur on the outside of the proboscis,have a gustatory function (Walterset al.1998) and detect flow rate when feeding (Krenn 1998; Inoueet al.2009).Sensilla styloconica are involved in responding to chemical substances, especially saccharides (Blaney and Simmonds 1988; Krenn 1998). Besides, they can also provide information such as the depth of insertion into the flower during nectar feeding (Krenn 1998; Krenn and Penz 1998).
5. Conclusion
In summary, we identified six types of sensilla on the larval mouthparts and three types of sensilla on the larval antennae ofP. xylostella. In our study, we found sensilla chaetica on the larval antennae, and sensilla chaetica,sensilla digitiformia, and sensilla epipharyngeal on the larval mouthparts. For adults, seven types of sensilla are found on the antennae in males while six types of sensilla(sensilla basiconica absent) occur in females. This study is basically consistent with previous reports on the external morphology and distribution of these sensilla (Yanget al.2001). Sexual dimorphism is also found in their number and in the size of these sensilla (Yanet al.2014). In addition, we also describe for the first time the five types of sensilla on the mouthparts. In general, our study provides useful information for the taxonomy of Lepidoptera, for further electrophysiological studies into the function of these sensilla, and better understanding of the behavioral mechanisms related to pest control.
Acknowledgements
We thank the anonymous reviewers for their valuable comments on this manuscript. This research was supported by the China Postdoctoral Science Foundation(2013M542388), the Postdoctoral Scientific Research Project in Shaanxi Province, China, the Fundamental Research Funds for the Central Universities of China(2014YB087) and the Agricultural Science and Technology Innovation in Shaanxi Province, China (2016NY-058). We thank Prof. John Richard Schrock from Emporia State University, Kansas, USA for advice and editing of this manuscript.
Albert P J. 1980. Morphology and innervation of mouthpart sensilla in larvae of the spruce budworm,Choristoneura fumiferana(Clem.) (Lepidoptera: Tortricidae).Canadian Journal of Zoology,58, 842–851.
Al-Rouz H, Thibout E. 1989. Morphologie des sensilles cephaliques larvaires d’Acrolepiopsis assectella Zell.(Lepidoptera: Hyponomeutoidea).Annales de la Société Entomologique de France,25, 163–170. (in French)
Anton S, van Loon J J, Meijerink J, Smid H M, Takken W,Rospars J P. 2003. Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes.Arthropod Structure & Development,32,319–327.
Baker G T, Parrott W L, Jenkins J N. 1986. Sensory receptors on the larval maxillae and labia ofHeliothis zea(Boddie)andHeliothis virescens(F.) (Lepidoptera: Noctuidae).International Journal of Insect Morphology and Embryology,15, 227–232.
Blaney W M, Simmonds M S J. 1988. Food selection in adults and larvae of three species of Lepidoptera: A behavioural and electro physiological study.Entomologia Experimentalis et Applicata,49, 111–121.
De Boer G, Dethier V G, Schoonhoven L M. 1977.Chemoreceptors in the preoral cavity of the tobacco hornworm,Manduca sexta, and their possible function in feeding behaviour.Entomologia Experimentalis et Applicata,21, 287–298.
Bruce T J, Cork A. 2001. Electrophysiological and behavioral responses of femaleHelicoverpa armigerato compounds identified in flowers of African marigold,Tagetes erecta.Journal of Chemical Ecology,27, 1119–1131.
Chen J, Hua B Z. 2014. Ultramorphology of sensilla on the larval antennae and mouthparts ofCarposina coreanaKim(Lepidoptera: Carposinidae).Acta Entomologica Sinica,57,133–140. (in Chinese)
Chinta S, Dickens J C, Baker G T. 1997. Morphology and distribution of antennal sensilla of the tarnished plant bug,Lygus lineolaris(Palisot de Beauvois) (Hemiptera: Miridae).International Journal of Insect Morphology and Embryology,26, 21–26.
Dethier V G. 1980. Responses of some olfactory receptors of the eastern tent caterpillar (Malacosoma americanum) to leaves.Journal of Chemical Ecology,6, 213–220.
Devitt B D, Smith J J B. 1982. Morphology and fine structure of mouthpart sensilla in the dark-sided cutwormEuxoa messoria(Harris) (Lepidoptera: Noctuidae).International Journal of Insect Morphology and Embryology,11, 255–270.
Faucheux M J. 1990. Antennal sensilla in adultAgathiphaga vitiensisDumbl. andA. queenslandensisDumbl.(Lepidoptera: Agathiphagidae).International Journal of Insect Morphology and Embryology,19, 257–268.
Faucheux M J. 1995. Sensilla on the larval antennae and mouthparts of the European sunflower moth,Homoeosoma nebulellaDen. and Schiff. (Lepidoptera: Pyralidae).International Journal of Insect Morphology and Embryology,24, 391–403.
Faucheux M J. 2008. Mouthparts and associated sensilla of a South American moth,Synempora andesae(Lepidoptera:Neopseustidae).Revista de la Sociedad Entomologica Agentina,67, 21–33. (in French)
George J A, Nagy B A. 1984. Morphology, distribution,and ultrastructural differences of sensilla trichodea and basiconica on the antennae of the oriental fruit moth,Grapholitha molesta(Busck) (Lepidoptera: Tortricidae).International Journal of Insect Morphology and Embryology,13, 157–170.
Gilbert L E. 1972. Pollen feeding and reproductive biology ofHeliconiusbutterflies.Proceedings of the National Academy of Sciences of the United States of America,69, 1403.
Goldware M A, Barnes M M. 1973. Mouthparts of the adult codling moth,Laspeyresia pomonella(Lepidoptera:Olethreutidae).Annals of the Entomological Society of America,66, 349–351.
Hallberg E, Hansson B S, Steinbrecht R A. 1994. Morphological characteristics of antennal sensilla in the European cornborerOstrinia nubilalis(Lepidoptera: Pyralidae).Tissue and Cell,26, 489–502.
Hanson F E. 1970. Sensory responses of phytophagous Lepidoptera to chemical and tactile stimuli. In:Seminar on Control of Insect Behavior by Natural Products(Pap).Academic Press, New York. pp. 81–91.
Hanson F E, Dethier V G. 1973. Role of gustation and olfaction in food plant discrimination in the tobacco hornworm,Manduca sexta.Journal of Insect Physiology,19, 1019–1031.
Inoue T A, Asaoka K, Seta K, Imaeda D, Ozaki M. 2009.Sugar receptor response of the food-canal taste sensilla in a nectar-feeding swallowtail butterfly,Papilio xuthus.Naturwissenschaften,96, 355–363.
Ishikawa S, Hirao T, Arai N. 1969. Chemosensory basis of host plant selection in the silkworm.Entomologia Experimentalis et Applicata,12, 544–554.
Keil T A. 1996. Sensilla on the maxillary palps ofHelicoverpa armigeracaterpillars: In search of the CO2-receptor.Tissue and Cell,28, 703–717.
Kent K S, Hildebrand J G. 1987. Cephalic sensory pathways in the central nervous system of larvalManduca sexta(Lepidoptera: Sphingidae).Philosophical Transactions of the Royal Society of London(B: Biological Sciences),315, 1–36.
Krenn H W. 1998. Proboscis sensilla inVanessa cardui(Nymphalidae, Lepidoptera): Functional morphology and significance in flower-probing.Zoomorphology,118, 23–30.
Krenn H W. 2010. Feeding mechanisms of adult Lepidoptera:Structure, function, and evolution of the mouthparts.Annual Review of Entomology,55, 307–327.
Krenn H W, Penz C M. 1998. Mouthparts ofHeliconiusbutterflies(Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen-feeding behavior.International Journal of Insect Morphology and Embryology,27, 301–309.
Krenn H W, Plant J D, Szucsich N U. 2005. Mouthparts of flower-visiting insects.Arthropod Structure & Development,34, 1–40.
Lavoie-Dornik J, McNeil J N. 1987. Sensilla of the antennal flagellum inPseudaletia unipuncta(Haw.) (Lepidoptera:Noctuidae).International Journal of Insect Morphology and Embryology,16, 153–167.
Li J X, Wang J J, Deng W, Yang B, Li J, Liu H. 2008. Description of sensilla on the larval antennae and mouthparts ofSpodoptera exigua(Hübner) (Lepidoptera, Noctuidae).Acta Zootaxonomica Sinica,33, 443–448. (in Chinese)
Li K, Luo M H, Zhao G Q, Liu X G. 2006. Observation on the ultrastructures of antennal sensilla inHelicoverpa assulta.Journal of Henan Agricultural University,40, 250–253. (in Chinese)
Li X, Bai S. 2004. Ultrastructural studies on the antennal sensilla ofDiadegma semiclausumHellen (Hym, Ichneumonidae).Journal of Henan Agricultural University,38, 45–48. (in Chinese)
Lin C S. 2002. Sensilla on the larval antennae and mouthparts ofPentateucha inoueiOwada et Brechlin (Lepidoptera:Sphingidae).Formosan Entomology,22, 115–124.
Liu Z, Hua B Z, Liu L. 2011. Ultrastructure of the sensilla on larval antennae and mouthparts in the peach fruit moth,Carposina sasakiiMatsumura (Lepidoptera: Carposinidae).Micron,42, 478–483.
Ma M, Chang M M, Lu Y, Lei C L, Yang F L. 2017. Ultrastructure of sensilla of antennae and ovipositor ofSitotroga cerealella(Lepidoptera: Gelechiidae), and location of female sex pheromone gland.Scientific Reports,7, 40637.
Maida R, Mameli M, Müller B, Krieger J, Steinbrecht R A. 2005.The expression pattern of four odorant-binding proteins in male and female silk moths,Bombyx mori.Journal of Neurocytology,34, 149–163.
Merivee E, Rahi M, Luik A. 1999. Antennal sensilla of the click beetle,Melanotus villosus(Geoffroy) (Coleoptera:Elateridae).International Journal of Insect Morphology and Embryology,28, 41–51.
Molleman F, Krenn H W, Van Alphen M E, Brakefield P M,Devries P J, Zwaan B J. 2005. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology.Biological Journal of the Linnean Society,86, 333–343.
Olson D M, Andow D A. 1993. Antennal sensilla of femaleTrichogramma nubilale(Ertle and Davis) (Hymenoptera:Trichogrammatidae) and comparisons with other parasitic Hymenoptera.International Journal of Insect Morphology and Embryology,22, 507–520.
Park K C, Hardie J. 2002. Functional specialisation and polyphenism in aphid olfactory sensilla.Journal of Insect Physiology,48, 527–535.
Schneider D. 1964. Insect antennae.Annual Review of Entomology,9, 103–122.
Schoonhoven L, Dethier V G. 1964. Sensory aspects of hostplant discrimination by lepidopterous larvae.Archives Néerlandaises de Zoologie,16, 497–530.
Schoonhoven L M. 1967. Some cold receptors in larvae of three Lepidoptera species.Journal of Insect Physiology,13, 821–826.
Schoonhoven L M, Jermy T, Van Loon J J A. 1998.Plant-Insect Biology from Physiology to Evolution. Chapman Hall, London.
Shields V D C, Hildebrand J G. 2001. Responses of a population of antennal olfactory receptor cells in the female mothManduca sextato plant-associated volatile organic compounds.Journal of Comparative Physiology(A),186,1135–1151.
Stelinski L L, Miller J R, Ledebuhr R, Gut L J. 2006. Mechanized applicator for large-scale field deployment of paraffin-wax dispensers of pheromone for mating disruption in tree fruit.Journal of Economic Entomology,99, 1705–1710.
Talekar N S, Shelton A M. 1993. Biology, ecology, and management of the diamondback moth.Annual Review of Entomology,38, 275–301.
Tarumingkeng R C, Coppel H C, Matsumura F. 1976. Morphology and ultrastructure of the antennal chemoreceptors and mechanoreceptors of workerCoptotermes formosanusShiraki.Cell and Tissue Research,173, 173–178.
Walters B D, Albert P J, Zacharuk R Y. 1998. Morphology and ultrastructure of sensilla on the proboscis of the adult spruce budworm,Choristoneura fumiferana(Clem.) (Lepidoptera:Tortricidae).Canadian Journal of Zoology,76, 466–479.
Wang G R, Guo Y Y, Wu K M. 2002. Study on the ultrastructures of antennal sensilla inHelicoverpa armigera.Journal of Integrative Agriculture,1, 896–899.
Wei H, Yang G, Wang Q, Hou Y, You M. 2003. Electron microscope scanning of diamondback moth,Plutella xylostellaL.Journal of Fujian Agriculture and Forestry University(Natural Science Edition),32, 434–437. (in Chinese)
Xia X F, Zheng D D, Lin H L, You M S. 2013. Isolation and identification of bacteria from the larval midgut of diamondback moth,Plutella xylostella(L.).Chinese Journal of Applied Entomology,50, 770–776. (in Chinese)
Xue S, Hua B Z. 2014. Proboscis sensilla of the black cutwormAgrotis ypsilon(Rottemberg) (Lepidoptera: Noctuidae).Journal of Asia-Pacific Entomology,17, 295–301.
Yan X Z, Deng C P, Sun X J, Hao C. 2014. Effects of various degrees of antennal ablation on mating and oviposition preferences of the diamondback moth,Plutella xylostellaL.Journal of Integrative Agriculture,13, 1311–1319.
Yang F, Zhang Y, Zhang W, Xu B, Wu Q. 2004. An artificial rearing method of diamondback moth byBrassica napus.Entomological Knowledge,41, 483–486.
Yang G, Huang G C, You M S. 2001. The ultrastructure and function of antennae of diamondback moth.Journal of Fujian Agricultural University,30, 75–79. (in Chinese)
Yang Q, Zhao K J, Wang K Q, Han L L, Yang S. 2012.Observation on antennal sensilla ofLeguminivora glycinivorellawith scanning electron microscope.Chinese Journal of Applied Entomology,49, 1321–1326. (in Chinese)
Zacharuk R Y. 1980. Ultrastructure and function of insect chemosensilla.Annual Review of Entomology,25, 27–47.
Zalucki M P, Shabbir A, Silva R, Adamson D, Liu S S, Furlong M J. 2012. Estimating the economic cost of one of the world’s major insect pests,Plutella xylostella(Lepidoptera:Plutellidae): Just how long is a piece of string?Journal of Economic Entomology,105, 1115–1129.
Zenker M Í M, Penz C, De Paris M, Specht A. 2011. Proboscis morphology and its relationship to feeding habits in noctuid moths.Journal of Insect Science,11, 42.
Zhang J, Guan L, Ren B. 2011. Fine structure and distribution of antennal sensilla of longicorn beetlesLeptura arcuataandLeptura aethiops(Coleoptera: Cerambycidae).Annals of the Entomological Society of America,104, 778–787.
杂志排行
Journal of Integrative Agriculture的其它文章
- Improve access to the EU market by identifying French consumer preference for fresh fruit from China
- Management and prevention of mastitis: A multifactorial approach with a focus on milking, bedding and data-management
- Elimination of ceftiofur hydrochloride residue in postpartum cows’milk after intramammary infusing at dry-off
- Evaluation of a new qPCR test to identify the organisms causing high total bacterial count in bulk tank milk
- Prevalence and characteristics of extended spectrum β-lactamaseproducing Escherichia coli from bovine mastitis cases in China
- Evolutionary analysis of plant jacalin-related lectins (JRLs) family and expression of rice JRLs in response to Magnaporthe oryzae
