Improvement of cortical granules migration and in vitro embryo production of vitrified bovine oocyte by 9-cis retinoic acid
2018-06-04MojtabaRashediAliRoozBatavaniRezaGolamNajafi
Mojtaba Rashedi, Ali Rooz Batavani, Reza Golam Najafi
1Department of Clinical Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
2Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
1. Introduction
Cryopreservation is an important tool of assisted reproductive technologies, but access to higher efficiency of oocyte cryopreservation is still a challenge. The ability to cryopreserve oocytes effectively would significantly improve animal breeding programs and biodiversity preservation[1]. However, the cryopreservation of bovine oocytes remains inefficient, as oocytes have been shown to be highly sensitive to both chilling and exposure to cryoprotectant agents[2]. Following cryopreservation, the displayed compromised developmental competence of oocytes may be due to damages to the meiotic apparatus including disorganization of spindle and disappearance of microtubules as well as other ultrastructural changes[3,4]. Oocyte vitrification caused major ultrastructural alternations in microvilli,vesicle formation, mitochondria, and ooplasm of germinal vesicle oocytes, which were preserved better in mature oocytes than immature ones[5]. The ultrastructural consequences of vitrifying meiosisⅡ (MⅡ) bovine oocytes cause alterations in the oocyte after thawing [e.g. clustered cortical granules (CGs) rather than solitary CGs aligned along the oolemma] which were apparently reflected in subsequent fertilization and embryonic development[6]. The major biological change in the oocytes following vitrification, as noted by Hyttel et al[6], was loss of peripheral distribution of CGs, typical of in vivo matured oocytes and, to some degree, in vitro. Instead, CGs clusters were found, and furthermore various degrees of degeneration of granules were observed, rendering the oocytes highly penetrable susceptible to polyspermy. CGs are the intermediaries to block the polyspermy occurring during fertilization[6]. Moreover, it was found that exposure to cryoprotectant was able to augment intracellular Ca++and this may operate premature exocytosis of material of cortical granular cause to enzymatic changes of the zona pellucida and make it harder to bind spermatozoa (zona hardening)[7], which inhibited in vitro fertilization (IVF). The timing of the polyspermy block was disrupted by premature release of CGs the and the rates of polyspermy and subsequently polyploidy increased[5].
Retinoic acid (RA) is derived from the vitamin A. It is the most important retinoid during embryogenesis of vertebrate[8] and in mammals, maintenance of pregnancy is strongly dependent on RA.It has an important biological function including differentiation,cell growth regulation, maturation of oocyte, and development of embryo[9-11]. In cells, all-trans-RA is reversibly converted to 9-cis-RA and other isomers. Both RAs enter the nucleus and bind to specific receptors[10]. The presence of both nuclear RA receptors(retinoic acid receptor and retinoid X receptor) in the cumulus cells and in oocyte till the hatched blastocyst stages[12] indicates that RA could play important roles during early embryonic development. In cumulus-oocyte complexes (COCs), RAs may adjust transcriptional activity and may affect on in vitro maturation (IVM) and latter development by direct effect on the oocyte and surrounding granulosa cells[10]. Lucrative effects of RAs and retinoids have been indicated in bovine oocytes, chiefly on maturation of oocyte and embryonic development following IVM[13]. Adding 9-cis-RA to the IVM medium within pre-maturation of bovine COCs promoted cytoplasmic maturation and embryonic development following IVF, such as blastocyst development and hatching rates[10]. It has also been expressed that RA may enhance embryonic development by oxidative stress prevention[14], and by adjusting the expression of some growth factors within maturation doe to its effects on mRNA processing and quality[15]. The presence of 9-cis-RA within maturation of oocyte allowed perfect CGs migration and the cluster formation lining the oocyte cytoplasmic membrane together during nucleus progression to the MⅡ stage in bovine[16], and canine[17].
As RA affects on cells to regulate cytoplasmic maturation through effects on CGs migration in oocytes during IVM and also, according to clustering of CGs after thawing of vitrified oocytes. The goals of this study were to evaluate the effects of 9-cis-RA on IVM medium,CGs migration and embryonic development (the third day of culture)of vitrified matured bovine oocytes.
2. Materials and methods
2.1. Oocyte recovery
The ovaries of slaughtered Holstein cows were put in normal saline solution (9 mg/mL NaCl) comprising antibiotics (100 UI/mL penicillin,and 100 µg/mL streptomycin sulfate) and maintained at 30 ℃-35 ℃till COCs were recovered. Washing of ovaries were done twice with distilled water and once with freshly prepared normal saline solution.The COCs were aspirated from visible 2 to 8 mm follicles through an 18-gauge needle which was connected to a 10 cc syringe and were recovered into a 50 mL conical tube. Follicular fluid containing COCs were put in a collecting medium [tissue culture medium-199 (TCM-199) + 25 mM 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) + bovine serum albumin(BSA) 0.4 g/L supplemented with 2 UI/mL of heparin].
2.2. IVM
COCs, whose oocytes were enclosed in a compact cumulus associated with evenly granulated cytoplasm, were selected to mature in vitro. Every COC was washed three times in a washing medium[HEPES-TCM-199, NaHCO3(2.5 mg/mL), glutamax (5 µL/mL), fatal bovine serum (FBS) (10%), penicillin/streptomycin (1 µg/mL)] and twice in a maturation medium, which consisted of TCM199, FBS(10%), NaHCO3(2.5 mg/ mL), glutamax (5 µL/mL), gentamicin (50µg/mL), human follicle-stimulating hormone (Folltropin) (1 µg/mL),human chorionic gonadotropin (5 IU/mL), 17b-estradiol (1 µg/mL),and sodium pyruvate (0.22 mM/mL). Maturation was carried out by culturing nearly 50 COCs in 500 µL of the maturation medium in 4-well dishes at 38.5 ℃ in 5% CO2in the air and high humidity for 22-24 h. For using 9-cis-RA in IVM, it was solved in ethanol, then aliquoted, and finally stored at -80 ℃ in the darkness.
2.3. Labeling of oocytes with fluorescein isothiocyanate(FITC)-lectins for CGs and chromosomal staining
Following eliminating of the surrounding expanded cumulus cells with a narrowed glass pipette, the zona pellucida was removed by 0.1% pronase. The zona-free oocytes were washed three times, then were fixed by 2% paraformaldehyde in a phosphate buffer solution(PBS) for at least 12 h at 5 ℃ in a 35 mm dish, and finally were washed four times in a blocking solution (PBS containing sodium azide, and 100 mM glycine). Oocytes in the blocking medium were incubated with 10 µg/mL FITC-labeled lens culinaris agglutinin(LCA, FL-1041, Vector Labs, Inc., Burlingame, CA, USA) for 15 min in the darkness. Propidium iodide was used to stain chromatin, so 10 µg/mL propidium iodide was added to LCA-stained zona-free oocytes for 5 min. Following staining, the oocytes were washed, then 5-10 oocytes were mounted between a coverslip and a glass slide that were supported by silicone with an antifade mounting medium, and the nail polish was used to seal coverslip. Samples were evaluated using a laser-scanning confocal microscopy, which was carried out by a Bio-Rad MRC 1024 ES associated with a Krypton ion laser for the simultaneous excitation of fluorescein for CGs and propidium iodide for DNA (488, laser line and 680 DF 32, respectively). The digital images were recorded and archived.
2.4. IVF
Sperm preparation was performed using a swim-up process. Briefly,one frozen semen straw of a specific Holstein bull was thawed in a pre-warmed water bath and was added to a conical tube including 1 mL of pre-equilibrated swim-up medium [Alfa MEM, BSA (0.6%),epinephrine + hypotaurine + penicillamine (2 µL)]. One hour after incubation, almost 700 µL of the supernatant including the motile sperm was transferred. Then, the sperms were centrifuged for 7 min at 200 g and the upper layer was aspirated to leave a pellet of almost 100 µL in volume. A hemocytometer was used to determine sperm concentration. The COCs were washed in the collecting medium twice and then were put in 4-well culture dishes including a preequilibrated fertilization medium [TCM-199, heparin (2 µg/mL),BSA (0.6%), gentamycin (50 µg/mL), epinephrine + hypotaurine+ penicillamine (2 µL), sodium pyruvate (0.2 mM)] with heparin(10 µg/mL). Then, spermatozoa were added at a concentration of 1×106 sperms/mL in 500 µL of the fertilization medium per each well including maximum 20 COCs. IVF had been performed by incubating sperms and oocytes together for 18-20 h at 38.5 ℃ in 5%CO2and high humidity.
2.5. In vitro culture (IVC)
To separate cumulus cells, presumptive zygotes were pipetted and were washed three times in the collecting medium, and before being transferred to droplets, they were washed once in synthetic oviductal fluid medium. Culture of embryos were performed in synthetic oviductal fluid supplemented with BSA (0.8%), sodium pyruvate(0.33 mM), 1××MEM nonessential amino acids (10 µg/mL), 1×MEM essential amino acids (20 µg/mL), glutamine (5 µL/mL), myoinositol (2.8 mg/mL), sodium citrate (0.15 mg/mL), and gentamicin(25 µg/mL) at 24 h post-fertilization. These IVC droplets (1-2 µL per embryo) were provided in 4-well dishes with mineral oil and had been equilibrated for 2 h in the incubator before zygotes were added. Culturing was performed at 38.5 ℃ with 5% CO2in the air.Evaluation of embryonic development was carried out on day 3.
2.6. Oocyte vitrification and thawing
To vitrification, the matured oocytes were equilibrated at 37 ℃ for 5 min in vitrification solution 1 including TCM-199, ethylene glycol 7.5% (v/v), dimethyl sulfoxide (DMSO) 7.5% (v/v), and newborn calf serum (CS) 20%. Following equilibration, matured oocytes were placed into three 20 µL-drops of vitrification solution 2 including TCM-199, ethylene glycol 15% (v/v), DMSO 15% (v/v), CS 20%(v/v), and sucrose 17.1% (w/v) for 45-60 s at 37 ℃. Finally, five oocytes were located on every cryotop (Kitazato Co., Japan) with a minimum surrounding of vitrification solution 2 using a narrow glass pipette under a stereomicroscope; and then immediately the cryotop was immersed into liquid nitrogen. The oocytes were thawed by plunging the cryotop into a 2 mL droplet of the thawing solution including TCM-199, + CS 20% (v/v) and sucrose 17.1% (w/v) for 1 min at 37 ℃ in a 35 mm petri dish. Then the oocytes were washed 3 times at 37 ℃ in Dulbecco’s phosphate-buffered saline which was supplemented with CS 5% (v/v).
2.7. Experiment design
A total of 120 COCs were randomly selected and divided into two groups: control group and treatment group. 60 randomly selected COCs of the control group were matured in IVM medium, and then their oocytes were vitrified and thawed, among which 30 randomly selected thawed oocytes were stained by FITC-LCA to evaluate CGs migration and 30 remaining thawed mature oocyte were used to IVF and IVC protocols. Other 60 randomly selected COCs of treatment group were matured in IVM medium containing 9-cis-RA, and then their oocytes were vitrified and thawed, among which 30 randomly selected thawed oocytes were stained by FITC-LCA to evaluate CGs migration and 30 remaining thawed mature oocyte were used to IVF and IVC protocols.
2.8. Statistical analysis
The data on CGs migration and development rate (%) were submitted to one way analysis of variance (ANOVA) and Duncan’s test.
3. Results
The results of IVC in the control group on day 3 showed that 30%oocytes had been developed to 4-8 cells embryos. Oocytes that had received RA in IVM medium in treatment group showed 40% of in vitro embryo production. Statistically, the difference was significant(P<0.05).
After evaluating CGs migration in vitrified mature oocyte in 60 oocytes (30 oocytes in control group and 30 oocytes in treatment group), CGs distribution were found in four pattern: pattern 1:distribution of CGs in the central medullary zone as clusters or among the oocyte cytoplasmic membrane and the central zone(Figure 1a); pattern 2: complete migration of CGs and formation of clusters lining the oocyte cytoplasmic membrane (Figure 1b); pattern 3: formation of a monolayer CGs lining the oocyte cytoplasmic membrane (Figure 1c); and pattern 4: showing exocytosis of CGs in which no CGs or some small clusters were seen (Figure 1d).
Pattern 1 was found only in immature oocytes which were not matured during IVM, and the rate of this pattern was 10% in both groups. Pattern 2 was often seen in oocytes which were matured (MⅠ),but two oocytes in this pattern were completely matured (MⅡ) which belonged to both groups (each group had one oocyte); in the control group, this pattern had a 40% occurrence but in the treatment group,it was only 20%; the difference was statistically significant. Pattern 3 was found in oocytes which were completely matured; the presence of this pattern was 47% and 70% in the control and treatment groups,respectively; the difference was significant. Pattern 4 was observed only in one oocyte in the control group; it has a 1.5% occurrence in all 60 oocytes which were vitrified (in the control group it was 3%).
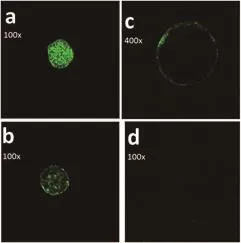
Figure 1. Patterns of CGs distribution in vitrified bovine oocytes.
4. Discussion
RA has been suggested as an important ingredient of IVM medium to improve cytoplasmic and nuclear maturation[16,18]. Retinoids may adjust transcriptional activity and may affect on IVM and further development by direct effects on the oocyte and granulosa cells[10]. In this study, the effects of 5 nm of 9-cis-RA on IVM of vitrified bovine oocytes were evaluated. CGs migration and 4-8 cells embryo development were significantly improved by adding 5 nM 9-cis-RA. It seems that RA improves developmental competence of oocytes by different pathways which enhance oocyte maturation, including mitogen-activated protein kinase phosphatases[19], antioxidant activity[20], decrease in apoptosis[17],increase in midkine which suppresses apoptosis[16], inhibition of tumor necrosis factor-α production[21], and effect on folliclestimulating hormone or luteinizing hormone receptor expression[22].In vitro 9-cis-RA was not able to improve the vitrified blastocysts ability to survive, while it enhanced CGs migration (complete CGs migration); therefore, the complete cytoplasmic CGs migration of oocytes matured in vitro with 9-cis-RA foretastes the obtained enhancement in oocyte developmental competence[16]. RA induced CGs migration before maturation; moreover, the CGs migration following RA disposal formed a uniform monolayer just beneath the cytoplasmic membrane of oocyte with less-clustering, so RA has a role in the enhancement of oocyte developmental competence[13].Adding this information together suggests that the effects of RA on oocyte maturation and developmental competence obtained in this study and previous studies is due to the enhancement of cytoplasmic maturation especially CGs distribution. Nevertheless, RA potentially can improve nuclear maturation by inducing positive effects on microfilaments and cytoskeleton, which affect cellular organelle displacement.
The potential effects of cryodamage on the oocyte cytoplasmic function will be inherently related to the stage of the cell cycle since the ultrastructural configuration in the cytoplasm is continually changing based on the stage of meiosis. This is especially true with respect to cytoskeleton distribution and/or function, which is essential for normal segregation of mitochondria, chromosomes, spindle rotation, cytokinesis, and pronuclei/nuclei formation. Moreover,premature CGs release can occur by or during cryopreservation[23].Translocation and release of CGs involves proper cytoskeleton function and plasma membrane organization. It has been reported that DMSO and 1,2-propanediol exposure during the cooling process in oocyte cryopreservation cause premature CGs release and zona hardening, compromising sperm penetration and fertilization; also,ethylene glycol causes transient calcium increases in mouse MⅡoocytes, which were able to afford hardening of zona placida, likely via operating the exocytosis of CGs, which per se is a calciumdependent incident[23]. Vitrifying MⅡ bovine oocytes results in alterations in the oocyte after thawing such as clustering of CGs(rather than the solitary alignment of CGs along the oolemma); in vitrified oocytes at 24 h post insemination, polyspermic penetrations were seen when vacuoles had degraded CGs content. It is believed that this event presents the oocytes highly sensitive to polyspermy.And 72 h post insemination, losing cleavage, blastomere vacuolization and degeneration were observed in vitrified oocytes[6].Evidently, MⅡoocytes had greater resistance to cryopreservation[24].Perfect CGs migration was obtained in canine matured oocytes with 5 nM 9-cis-RA, and was consistent with improvement in developmental competence. It has been demonstrated that retinoids have had lucrative effects on bovine oocytes maturation and embryonic development following to IVM[13]. The data in the study are similar to those mentioned above, for instance, complete CGs migration just beneath the oolemma was observed in vitrified oocyte which had received RA during IVM, while vitrified oocytes which had not received RA showed little complete CGs migration and much clustering. This lack of complete CGs distribution in absence of RA was associated with lower in vitro embryo production rate, and it reinforces the hypophysis that vitrification increases polyspermy.In the study, one oocyte which was free of CGs in the control group was found. Probably, this oocyte showed premature CGs exocytosis which causes hardening of zona placida and lack of fertilization.
In conclusion, in the IVM medium 5 nM 9-cis-RA was beneficial to IVC and cytoplasmic maturation of vitrified bovine oocytes. It seems that oocytes developmental competence is associated with the relative CGs migration. However, much more studies are required to clarify the mechanisms by which 9-cis-RA affects on the CGs migration of oocytes.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Zhou GB, Li N. Bovine oocytes cryoinjury and how to improve their development following cryopreservation. Anim Biotechnol 2013; 24: 94-106.
[2] Diez C, Munoz M, Caamano JN, Gomez E. Cryopreservation of the bovine oocyte: Current status and perspectives. Reprod Domest Anim 2012;47(suppl 3): 76-83.
[3] Albarracin JL, Morató R, Rojas C, Mogas T. Effects of vitrification in open pulled straws on the cytology of in vitro matured prepubertal and adult bovine oocytes. Theriogenology 2005; 63: 890-901.
[4] Morató R, Izquierdo D, Albarracín JL, Anguita B, Palomo MJ, Jiménez-Macedo AR, et al. Effects of pre-treating in vitro-matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Mol Reprod Dev 2008; 75: 191-201.
[5] Fuku E, Xia L, Downey BR. Ultrastructural changes in bovine oocytes cryopreserved by vitrification. Cryobiology 1995; 32: 139-156.
[6] Hyttel P, Vajta G, Callesen H. Vitrification of bovine oocytes with the open pulled straw method: Ultrastructural consequences. Mol Reprod Dev 2000; 56: 80-88.
[7] Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction (Cambridge, England)2006; 131: 53-61.
[8] Rhinn M, Dollé P. Retinoic acid signalling during development.Development 2012; 139: 843-858.
[9] Fujiwara S, Kawamura K. Acquisition of retinoic acid signaling pathway and innovation of the chordate body plan. Zoolog Sci 2003; 20: 809-818.
[10] Hidalgo CO, Diez C, Duque P, Facal N, Gomez E. Pregnancies and improved early embryonic development with bovine oocytes matured in vitro with 9-cis-retinoic acid. Reproduction (Cambridge, England) 2003;125: 409-416.
[11] Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 2009; 7: e002.
[12] Mohan M, Malayer JR, Geisert RD, Morgan GL. Expression patterns of retinoid X receptors, retinaldehyde dehydrogenase, and peroxisome proliferator activated receptor gamma in bovine preattachment embryos.Biol Reprod 2002; 66: 692-700.
[13] Duque P, Diez C, Royo L, Lorenzo PL, Carneiro G, Hidalgo CO, et al.Enhancement of developmental capacity of meiotically inhibited bovine oocytes by retinoic acid. Hum Reprod 2002; 17: 2706-2714.
[14] Ahlemeyer B, Bauerbach E, Plath M, Steuber M, Heers C, Tegtmeier F,et al. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med 2001; 30: 1067-1077.
[15] Gomez E, Rodríguez A, Goyache F, Díez C, José-Royo L, Moreira PN,et al. Retinoid-dependent mRNA expression and poly-(A) contents in bovine oocytes meiotically arrested and/or matured in vitro. Mol Reprod Dev 2004; 69: 101-108.
[16] Gomez E, Royo LJ, Duque P, Carneiro G, Hidalgo CO, Goyache F, et al. 9-cis retinoic acid during in vitro maturation improves development of the bovine oocyte and increases midkine but not IGF-I expression in cumulus-granulosa cells. Mol Reprod Dev 2003; 66: 247-255.
[17] Liang S, Kang J, Jin H, Liu X, Li J, Li S, et al. The influence of 9-cisretinoic acid on nuclear and cytoplasmic maturation and gene expression in canine oocytes during in vitro maturation. Theriogenology 2012; 77:1198-1205.
[18] Almiñana C, Gil MA, Cuello C, Caballero I, Roca J, Vazquez JM. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod Fertil Dev 2008; 20: 483-489.
[19] Old RW, Smith DP, Mason CS, Marklew S, Jones EA. Effects of retinoic acid on Xenopus embryos. Biochem Soc Symp 1996; 62: 157-174.
[20] Imam A, Hoyos B, Swenson C, Levi E, Chua R, Viriya E, et al. Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J 2001; 15:28-30.
[21] Deb GK, Dey SR, Bang JI, Cho SJ, Park HC, Lee JG, et al. 9-cis retinoic acid improves developmental competence and embryo quality during in vitro maturation of bovine oocytes through the inhibition of oocyte tumor necrosis factor-α gene expression. J Anim Sci 2011; 89: 2759-2767.
[22] Hattori M, Takesue K, Nishida N, Kato Y, Fujihara N. Inhibitory effect of retinoic acid on the development of immature porcine granulosa cells to mature cells. J Mol Endocrinol 2000; 25: 53-61.
[23] Smith GD, Villa-Diaz LG. Potential developmental consequences of cryopreservation of mammalian oocytes and embryos. In: Tucker MJ,Liebermann J, editors. Vitrification in assisted reproduction: A user’s manual and trouble-shooting guide. London, UK: Taylor & Francis;2007, p. 107-117.
[24] Men H, Monson RL, Rutledge JJ. Effect of meiotic stages and maturation protocols on bovine oocyte’s resistance to cryopreservation.Theriogenology 2002; 57: 1095-1103.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Missed estradiol determination resulting in oocyte retrieval and embryo development following controlled ovarian hyperstimulation at early pregnancy: Case report
- Role of preputial washing in reducing microbial load and improving bovine semen quality
- Effect of different concentration of fish oil in skim milk-egg yolk extenders on postthawed semen qualities of Kalang swamp buffalo bull
- Prediction of protein structure of novel protein (116 kDa) from human sperm membrane
- Antiandrogenic activity of Calotropis procera latex in rats
- Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats
