Ozonation of o-phenylenediamine in the presence of hydrogen peroxide by high-gravity technology☆
2018-05-26MosesArowoZemengZhaoGuangjunLiGuangwenChuBaochangSunLeiShao
Moses Arowo ,Zemeng Zhao ,Guangjun Li,Guangwen Chu ,Baochang Sun ,*,Lei Shao ,*
1 State Key Laboratory of Organic–Inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
2 Department of Chemical&Process Engineering,Moi University,3900-3100 Eldoret,Kenya
3 Research Center of the Ministry of Education for High Gravity Engineering and Technology,Beijing University of Chemical Technology,Beijing 100029,China
4 Beijing Yanjing Beer Co.Ltd.,Beijing 101300,China
1.Introduction
High-gravity technology is an innovative process intensification technique that was originally suggested in 1979[1].The technology is achieved through multiphase reactors which can create a high-gravity condition of several orders of magnitude greater than the Earth's gravitational field.A classic example of such reactors is a rotating packed bed(RPB)which has hitherto been employed in many applications[2,3],and is widely acknowledged to exhibit good mass transfer characteristics[4,5].However,RPBs still suffer some intrinsic limitations which hinder their application in the industry[6,7].
Owing to the challenges associated with RPBs,another high-gravity multiphase device – a rotor–stator reactor(RSR)– which is also regarded as an efficient gas–liquid contactor was developed on the basis of an RPB[8].In an RSR,a high-gravity environment is created by large centrifugal force generated by rotation of a rotor.As a result,fluid flowing through the reactor is split into fine liquid elements and films.Also,there is strong turbulence of gas and liquid streams as well as fast renewal of gas–liquid interface.All of these can significantly contribute to intense mixing and increased mass transfer efficiency in the reactor[9,10].Consequently,RSRs have been successfully employed in several applications in recent years[11–13].
o-Phenylenediamine(o-PDA),also known as 1,2-benzenediamine,is an aromatic amine with wide spread applications in the chemical industry[14,15].However,it is also highly toxic and bio-recalcitrant compound that must be eliminated from industrial effluents[16].Some methods to remove o-PDA from waste waters have been reported in recentyears[17,18].However,these methods suffer the limitations of low removal efficiency,difficulty to operate,inability of continuous operation and low throughput capacity.Thus,there is a need to develop a simple and efficient alternative approach to remove o-PDA from effluents.
Ozonation is an advanced oxidation process that is widely recognized as an effective approach to degrade refractory organics in wastewater owing to the high oxidation potential of ozone(2.08 V)as well as its excellent disinfection ability[19,20].Ozonation can also increase the amount of dissolved oxygen necessary for subsequent bio-treatment[21].Nonetheless,the selectivity of ozone in reaction limits its application and ability to achieve complete mineralization of compounds[22].More efforts to enhance ozonation performance in order to improve removal efficiency of pollutants are therefore necessary.Although combined use of O3with H2O2has been applied to treat several bio-recalcitrant organics[23,24],there is hardly any report regarding itsapplication to treat o-PDA effluents.Moreover,the ozonation processes are limited by ozone-liquid mass transfer rate since ozone is rapidly consumed in aqueous solution[25].Consequently,a gas–liquid contacting unit with good mass transfer performance is desirable.
This study therefore supposes that simultaneous use of ozone and hydrogen peroxide(O3/H2O2process)can enhance ozonation performance and consequently improve degradation rate of ophenylenediamine(o-PDA).A high-gravity environment to intensify ozone-liquid mass transfer rate was achieved through large centrifugal force generated by rapid rotation of a rotor in an RSR.The degradation efficiency of o-PDA(η)as well as the overall gas-phase volumetric mass trans ferco efficient(KGa)were determined under different operating conditions including H2O2concentration(CH2O2),initial o-PDA concentration(CAO),initial pH,temperature of reaction(T)and rotation speed(N)of RSR,and the results were validated by comparison with those of sole use of ozone(O3process).Intermediate products of degradation of o-PDA under each of the ozonation processes were also identified using a gas chromatography–mass spectrometer(GC/MS)to further evaluate the extent of o-PDA degradation as well as establish its possible degradation pathway.
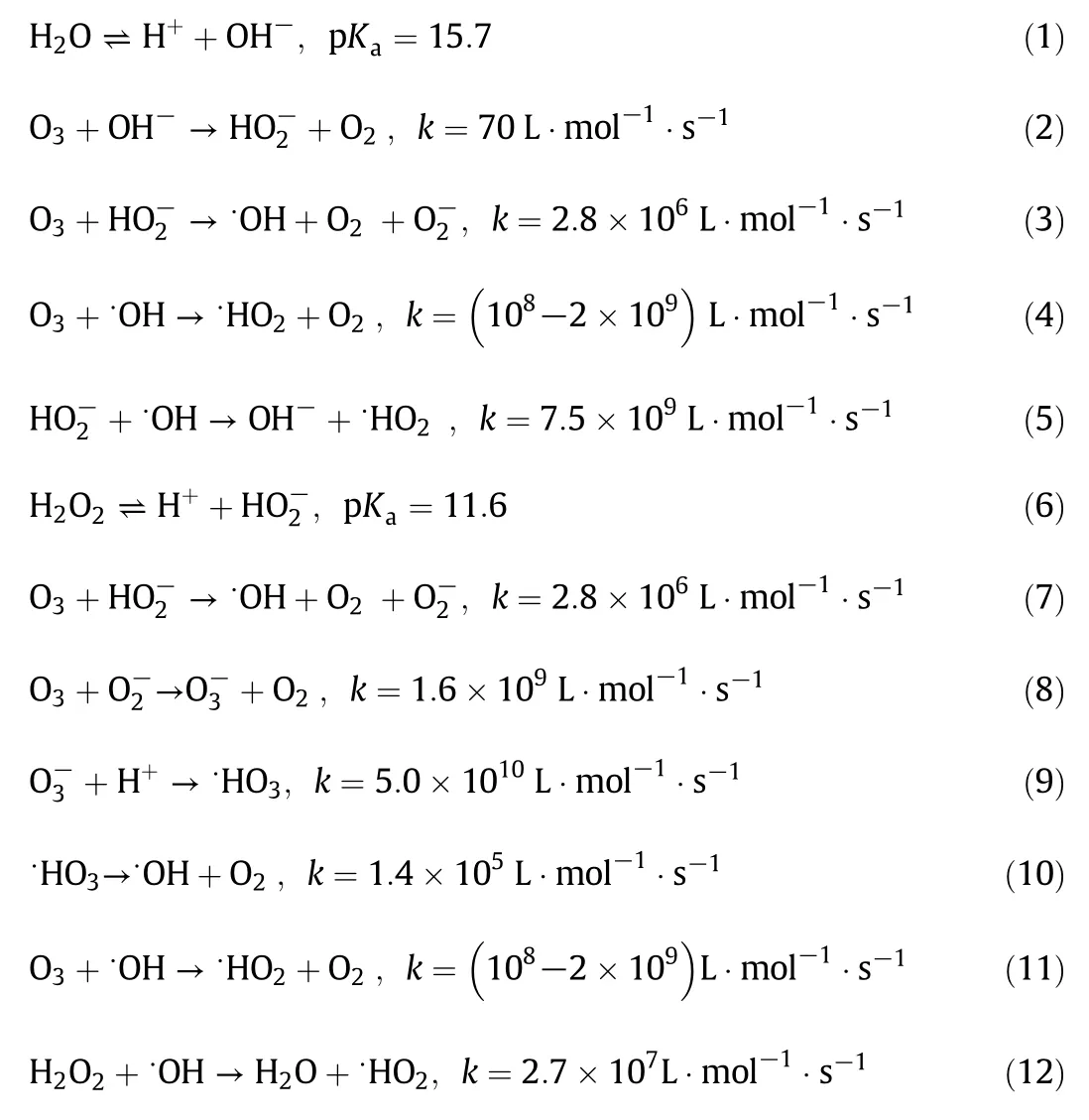
Ozonation of compounds in aqueous solution involves mass transfer with chemical reactions,i.e.,ozone mass transfer from gas phase into liquid phase,decomposition of ozone and finally mineralization of the contaminants[26].Ozone can react directly with the target compound via electrophilic attack or indirectly by first decomposing to yield hydroxyl radical(•OH),which then reacts with contaminants through abstraction of hydrogen,addition of OH or its substitution,or by means of electron transfer[27].The decomposition mechanism of O3in the presence of H2O2in water and its subsequent oxidation of o-PDA can be described by the following equations[28,29].

2.Materials and Methods
2.1.Structure of an RSR
Fig.1(a)and(b)shows a schematic structure of a rotor–stator reactor.It comprises a series of concentric rotor rings and stator rings arranged consecutively in a radial direction with a spacing of about 1 mm.The rotor rings are fixed on a motor-driven seat whereas the stator rings are fixed on a cover cap.As shown in Fig.1(b),the open slots on the stator rings and holes on the rotor rings are passages for fluid flow.Rotation of the rotor generates large centrifugal force which causes liquid to flow radially outwards through the open slots and holes.The employed RSR measures 120 mm both in radius and axial length.More details on its specifications are available in our previous work[30].
2.2.Experimental set-up and procedures
Fig.2 shows a diagram of the experimental setup.It mainly comprises an RSR,oxygen gas cylinder,ozone generator,three liquid tanks,two peristaltic pumps and flow meters.Ozone was produced from pure oxygen using an ozone generator(Tonglin Sci.and Tech.Ltd.,China).Concentrations of ozone at the inlet and outlet gas streams were monitored and measured using ozone analyzer(Double UV Light Ozone Meter,Limicen Ozone R&D Center,China).Simulated o-PDA wastewater was prepared by dissolving o-PDA(purity 99.8%)into de-ionized water and adjusting its pH to a desired value using either sodium hydroxide(0.1 mol·L−1,NaOH)or sulfuric acid(0.1 mol·L−1,H2SO4),both supplied by Beijing Chemical Works Co.Ltd.,China.Hydrogen peroxide solution was also obtained from Beijing Chemical Works Co.Ltd.,China.The solutions of o-PDA and H2O2were introduced into the RSR at volumetric flow rates of 18 and 2 L·h−1respectively(total liquid volumetric flow rate=20 L·h−1)through different liquid inlets.The two liquid streams sprayed uniformly via the respective nozzles of the liquid inlets to the innermost layer of the rotor where they merged into a single stream and then flowed radially outwards through the rotor and stator under the influence of centrifugal force generated by the rotation of the rotor.The concentrations of o-PDA and H2O2solution were adjusted accordingly to ensure that the mixed liquid stream achieved the desired concentrations of o-PDA and H2O2solution.At the same time,the ozone-containing gas stream was fed into the RSR at a volumetric flow rate of 240 L·h−1through a gas inlet and flowed inwardly through the rotor and stator.The gas and liquid streams contacted counter-currently and mixed rapidly inside the RSR,leading to absorption of ozone into the liquid stream and subsequent degradation of o-PDA.Finally,the gas and liquid streams left RSR via the gas outlet and liquid outlet respectively.When the concentration of ozone in the gas outlet stream reached a steady state,the outlet liquid samples were collected and immediately analyzed.The system was operated in a continuous mode without recycle.The gas stream was introduced into the RSR when the desired ozone concentration had been achieved.A comparison experiment(O3process)was performed in the same way though without introduction of hydrogen peroxide.

Fig.1.Schematic structure of an RSR.(a)Structure of an RSR(b)An illustration of rotor rings and stator rings:(1)Gas inlet;(2)Cover cap;(3)Stator;(4,7,11)Bolts;(5)Nozzle;(6)Gas outlet;(8)Liquid outlet;(9)Seal;(10)Shaft;(12)rotor;(13)Rotor seat;(14)Casing.
2.3.Analytical methods
Ozone concentration at the inlet(Ci)and outlet streams(Co)was monitored and measured by ozone analyzer(Double UV Light Ozone Meter,Limicen Ozone R&D Center,China).The degradation efficiency of o-PDA was calculated based on the concentrations of o-PDA before and after treatment as measured by a UV–Vis spectrophotometer(UV2600,Shimadzu,Japan)at 416 nm.The COD of the wastewater was determined using a COD analyzer(5B-3A,Lian-hua Tech.Co.,Ltd.,China)according to the Chinese standard(HJ/T 399-2007)while the intermediate products of degradation were identified by a gas chromatography-mass spectrometer(GC/MS,5977A,Agilent-USA).The degradation efficiency(η),which indicates the extent of removal of o-PDA,was calculated by Eq.(17)while rCOD,which signifies the degree of removal of organic contaminants from the effluent,was obtained by Eq.(18).The overall gas-phase volumetric mass transfer coefficient(KGa),which indicates the degree of ozone absorption into the solution,was calculated by Eq.(19)which is a modification of the formula proposed by Li et al.[31].where CAoand CAfare the concentrations of o-PDA(mg·L−1)before and after treatment respectively while CODoand CODfrepresent initial and final COD(mg·L−1)of the wastewater respectively.G is the gas volumetric flow rate(L·h−1),H is the axial length of RSR,R is the inner radius of the RSR casing while Ciand Corepresent the concentrations of ozone(mg·L−1)at the inlet and outlet gas streams respectively.


Fig.2.Experimental set-up for degradation of o-PDA by O3/H2O2 process in an RSR.
3.Results and Discussion
3.1.Effect of H2O2 concentration
Fig.3 illustrates the effect of H2O2concentration(CH2O2)on KGa andη respectively under a constant supply of ozone.It was noted that η increased with increase in H2O2concentration,reaching a peak value at CH2O2=30 mg·L−1,and thereafter slightly declined with further rise in H2O2concentration.On the other hand,KGa increased throughout with increase in H2O2concentration.Higher H2O2concentration led to more HO2–in the solution[Eq.(6)],which then reacted rapidly with the absorbed ozone in the solution to generate more•OH radicals(Eq.(7))besides those generated as a result of ozone decomposition alone[Eq.(3)].Consequently,there was reduction in the concentration of ozone in the solution,leading to increased mass trans ferdriving force and therefore larger KGa.The availability of extra•OH radicals in the solution resulted in increased chance of reaction with o-PDA leading to higher η.Nonetheless,H2O2can also acts as a scavenger for •OHradicals according to Eqs.(12)and(13),leading to reduction in the amount of•OH radicals in the solution and hence degradation rate.This phenomenon seemingly predominated at CH2O2beyond 30 mg·L−1,and hence the slight reduction in η observed.
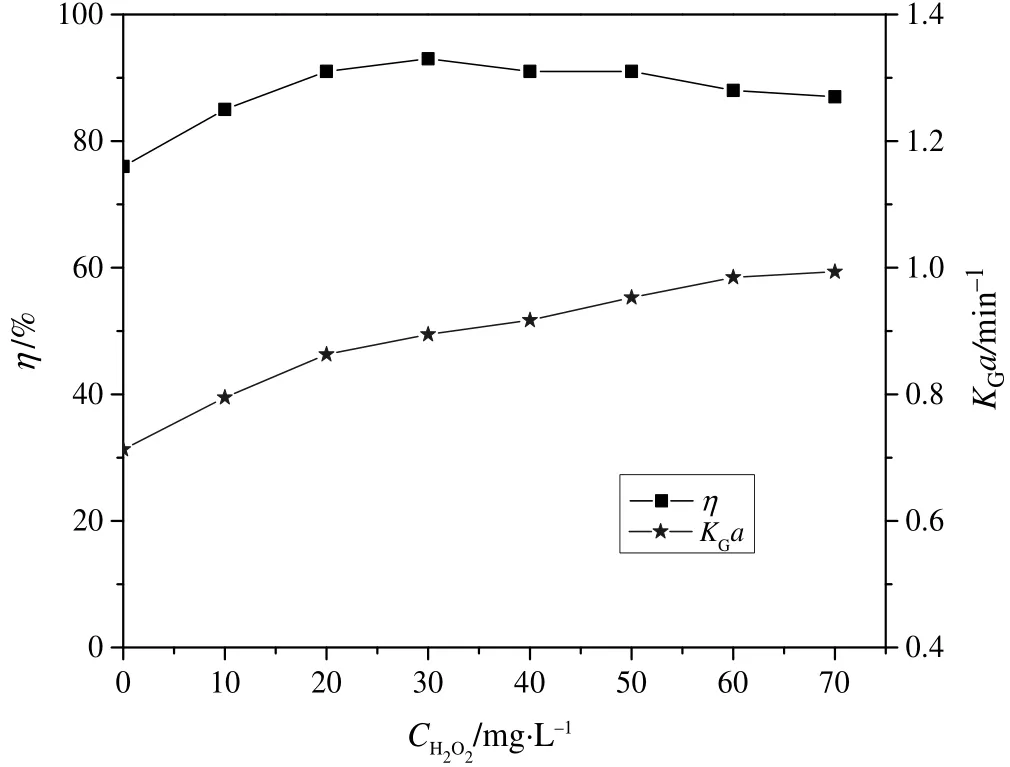
Fig.3.EffectofH2O2 concentration on η and K G a:(T=25°C;pH=6.5;C i=51.0 mg·L−1;C Ao=60 mg·L−1;N=1000 r·min−1).
3.2.Effect of initial o-PDA concentration
Fig.4 shows the variation of η and KGa with initial o-PDA concentration(CAo).It is evident that higher initial o-PDA concentration boosted KGa while it led to a reduction in η.The results can be attributed to the following reasons.Firstly,since H2O2concentration and ozone supply was fixed during the experiment,the amount of generated hydroxyl radicals in the solution remained almost constant with a varying initial o-PDA concentration.Thus,a lower initial o-PDA concentration favored η.Also,the degradation by-products of o-PDA in the solution increased accordingly with increase in initial o-PDA concentration,leading to increased competition for ozone and the generated hydroxyl radicals between the by-products and o-PDA,and hence the decline in η.However,the increase in the concentration of o-PDA and its degradation byproducts also resulted in increased consumption rate of absorbed ozone in the solution.Consequently,there was reduction in the concentration of ozone in the solution,leading to increased mass transfer driving force and hence larger KGa.
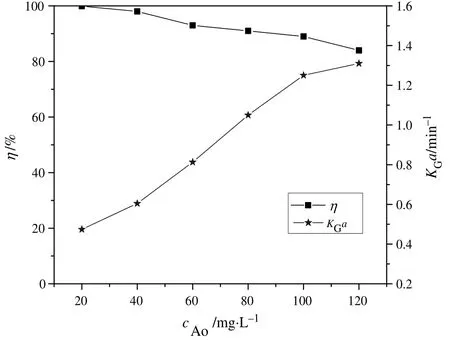
Fig.4.Effect of initial o-PDA concentration on η and K G a:(T=25 °C;pH=6.5;C i=51.0 mg·L−1;C H2O2=30 mg·L−1;N=1000 r·min−1).
3.3.Effect of initial pH
Fig.5 illustrates the effect of initial pH of the solution on ηand KGa.It was noted that higher initial pH favored both η and KGa.Higher pH means more hydroxyl ions in the solution and thus favored ozone decomposition in the solution to generate more•OH radicals.Higher pH also favors decomposition rate of H2O2to yield hydroxyl radicals[32].Consequently,there was increased rate of reaction between o-PDA and •OH radicals,leading to higher η.The enhanced decomposition rates of both ozone and H2O2also resulted in a reduction in the concentration of absorbed ozone in the solution,leading to increased mass transfer driving force and consequently increased absorption of ozone.The optimal initial pH was determined as 6.5 in view of extra costs associated with pH adjustments,since it is also the natural pH of o-PDA effluent.

Fig.5.Effect of initial pH on η and K G a:(T=25 °C;C Ao=60 mg·L−1;C i=51.0 mg·L−1;C H2O2=30 mg·L−1;N=1000 r·min−1).
3.4.Effect of rotation speed
Fig.6 shows the effectof rotation speed(N)of RSR onη and KGa.The rotation speed was varied from 400 to 14000 r·min−1.It is evident from the figure that both η and KGa increased throughout with increase in rotation speed.This phenomenon can be attributed to the fact that higher rotation speed enhanced the relative movement of rotors and stators of the RSR and as a result created a high-gravity environment through generation of larger shear force.Consequently,liquid flowing through the reactor was splitin to finerelements and thinner films,leading to increased turbulence,larger effective gas–liquid contact area as well as faster gas–liquid surface renewal rate.All of these led to significant intensification of mass transfer rate between the gas and liquid phase,which is the limiting step in the ozonation process of o-PDA,leading to increased ozone absorption and subsequently higher degradation rate of o-PDA.
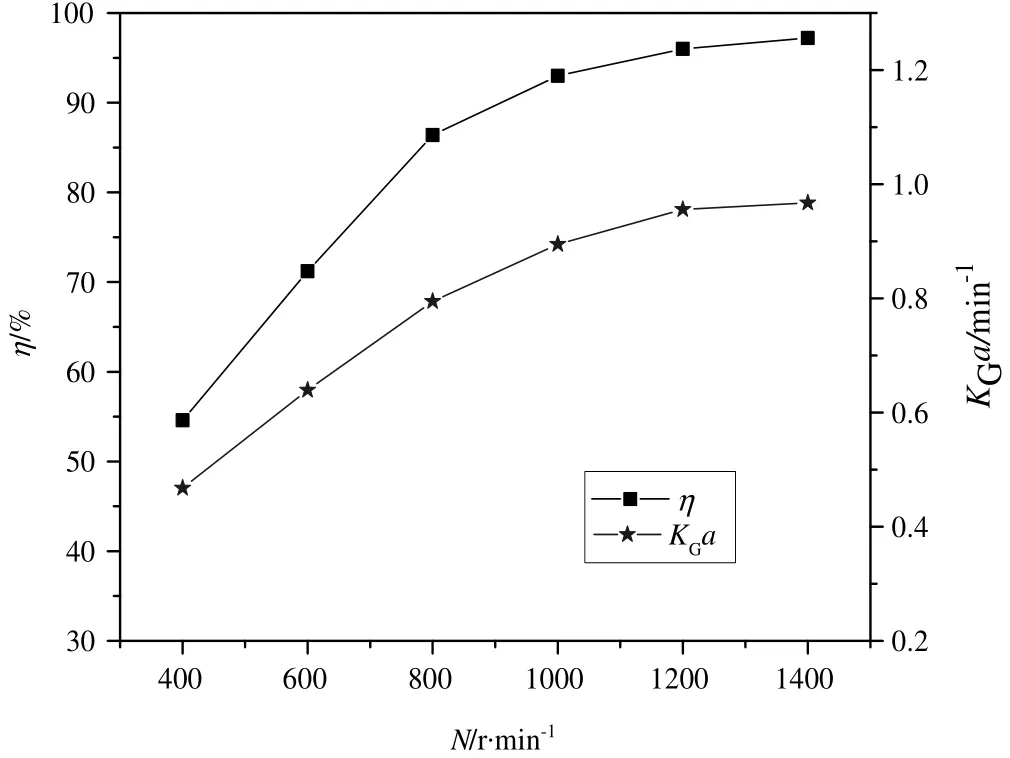
Fig.6.Effect of rotation speed on η and K G a:(T=25 °C;C Ao=60 mg·L−1;C i=51.0 mg·L−1;C H2O2=30 mg·L−1;pH=6.5).
3.5.Effect of reaction temperature

Fig.7.Effect of reaction temperature on η and K G a:(C Ao=60 mg·L−1;C i=51.0 mg·L−1;C H2O2=30 mg·L−1;pH=6.5;N=1000 r·min−1).
The variation of η and KGa with reaction temperature(T)is presented in Fig.7.The temperature was varied from 15 to 65°C by circulating water at a particular temperature around the reactor casing as well as adjusting the temperature of the solution accordingly prior to pumping into the reactor.It is evident that both η and KGa increased with increase in temperature,reaching peak values separately at 35°C and afterwards gradually declined with further rise in temperature.Higher temperature increased the rate of ozone decomposition to generate more•OH radicals.As a result,the concentration of the absorbed ozone in the solution decreased,leading to greater ozone-liquid mass transfer driving force and hence increased absorption of ozone.Besides,increasing the amount of•OH radicals through ozone decomposition,higher temperature increased the reaction rate between o-PDA and•OH radicals,resulting in higher degradation efficiency.Nonetheless,higher temperature can also lead to a reduction in solubility of ozone in the solution,and thereby limit ozone-liquid mass transfer rate.The effects of the latter factor superseded the aforementioned benefits of higher temperature at temperatures beyond 35°C,which probably explains the drop in the overall gas-phase volumetric mass transfer coefficient as well as degradation efficiency of o-PDA.
3.6.Comparison experiment
Based on the above results,another experiment to determine the achievable η,KGa and rCODat a particular set of operating conditions of CAo=60 mg·L−1;(at CAo=60 mg·L−1,CODo=116.3 mg·L−1),Ci=51.0 mg·L−1,pH=6.5,T=25 °C,N=1000 r·min−1and CH2O2=30 mg·L−1was performed,and results compared with those of a different experiment using ozone alone(O3process)carried out under the same set of conditions but without introduction ofH2O2in the solution.The comparison results,as illustrated in Fig.8,show that η,KGa and rCODachieved by O3/H2O2process were 24.4%,31.6%and 28.9%respectively higher than those of O3process,indicating the synergistic role played by O3and H2O2in ozonation.H2O2dissociates to yield HO2−ions which then react with ozone to generate more•OH radicals.Consequently,there was increased rate of reaction between o-PDA and•OH radicals,leading to higher degradation efficiency.The increased rate of reaction between HO2−and ozone also resulted in reduced concentration of absorbed ozone in the solution,leading to increased ozone-liquid mass transfer driving force and therefore increased ozone absorption.

Fig.8.Comparison of η,r COD and K G a achieved by O3/H2O2 and O3 processes in RSR.
3.7.Intermediate products
Table 1 shows the degradation intermediate products of o-PDAunder each of the oxidation approaches as identified by GC/MS analyses.It is evident that both processes can degrade o-PDA(1,2-benzenediamine)owing to the absence of its peak in both total ion chromatograms as shown in Fig.9(a)and(b).
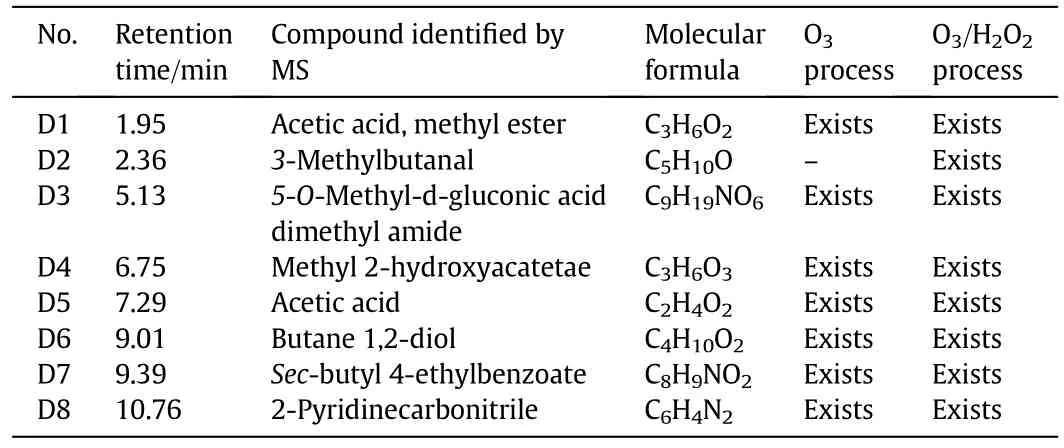
Table 1 Identified intermediate products in O3 and O3/H2O2 processes
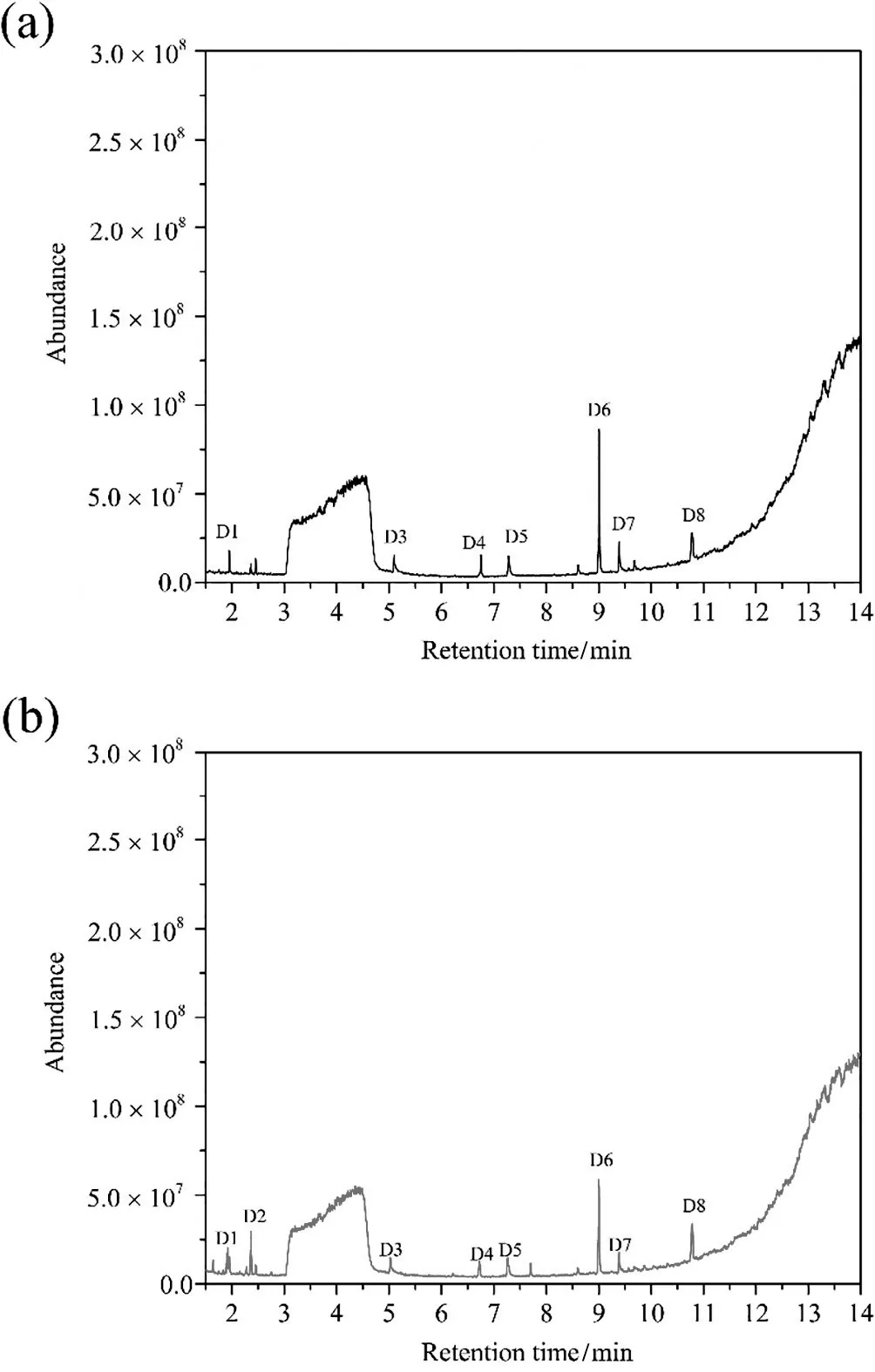
Fig.9.GC/MS total ion chromatograms for o-PDA effluent:(a)treated by O3 process and(b)treated by O3/H2O2 process.
The relative abundance of compounds D1,D3,D4,D5 and D7 in each of the oxidation processes is shown in Table 2.Although the products D1,D3,D4,D5 and D7 are presentin both oxidation processes,their relative abundance in the O3/H2O2process is much less than that in the O3process,suggesting that the degradation of o-PDA was more enhanced in the O3/H2O2process than in the O3process.This phenomenon further,perhaps explains the difference in CODreduction rates achieved by both processes.

Table 2 Relative abundance of compounds D1,D3,D4,D5 and D7 in the O3 and O3/H2O2 processes
Proposed degradation pathways for o-PDA by O3/H2O2and O3processes,based on the identified intermediates,are respectively shown in Fig.10.

Fig.10.Proposed degradation pathways of o-PDA by O3/H2O2 and O3 processes.
4.Conclusions
This study investigated the effectiveness of O3/H2O2process to degrade o-PDA in a rotor–stator reactor(RSR).The degradation efficiency of o-PDA(η)and the overall gas-phase volumetric mass transfer coefficient(KGa)were separately determined as a function of various operating parameters.Results reveal that higher initial o-PDA concentration favored the overall gas-phase volumetric mass transfer coefficient while it resulted in reduced degradation efficiency.Higher rotation speed of RSR as well as higher initial pH of o-PDA effluent favored both η and KGa.On the other hand,both η and KGa only increased up to peak values with increase in reaction temperature and H2O2concentration.Comparison experiment results show that η,KGa and rCODachieved by O3/H2O2process were 31.6,24.4 and 28.9%respectively higher than those of O3process.Analyses of the intermediate products of degradation further reveal that O3/H2O2process achieved faster and more complete degradation of o-PDA as compared to O3process,a phenomenon which can be attributed to the generation of more hydroxyl radicals in O3/H2O2process than in O3process.These results indicate that H2O2can greatly enhance ozone oxidation process,and confirm that simultaneous use ofozone with H2O2is a promising alternative approach to pre-treat o-PDA effluents.This work further demonstrates that RSR can significantly boost ozone absorption and thus provides a feasible intensification means for the ozonation of o-PDA as well as other recalcitrant organic effluents.
[1]C.Ramshaw,R.H.Mallison,Imperial chemical industries limited,Euro.Pat.,0002568(1979).
[2]Y.Luo,G.W.Chu,H.K.Zou,Y.Xiang,L.Shao,J.Chen,Characteristics of a two stage counter-current rotating packed bed for continuous distillation,Chem.Eng.Process.52(2012)55–62.
[3]W.H.Chen,Y.Syu,Hydrogen production from water gas shift reaction in a high gravity(Higee)environment using a rotating packed bed,Int.J.Hydrog.Energy 35(2010)10179–10189.
[4]A.Chandra,P.Goswami,D.P.Rao,Characteristics of flow in a rotating packed bed(Higee)with split packing,Ind.Eng.Chem.Res.44(2005)4051–4060.
[5]H.Zhao,L.Shao,J.F.Chen,High-gravity process intensification technology and application,Chem.Eng.J.156(2010)588–593.
[6]G.Q.Wang,Z.C.Xu,Y.L.Yu,J.B.Ji,Performance of a rotating zigzag bed—A new HIGEE,Chem.Eng.Process.47(2008)2131–2139.
[7]J.Burns,C.Ramshaw,Process intensification:Visual study of liquid maldistribution in rotating packed beds,Chem.Eng.Sci.51(1996)1347–1352.
[8]Y.H.Song,G.W.Chu,J.M.Chen,J.F.Chen.,A rotor-stator reactor and its application,China Patent,200410042631.6(2006).
[9]G.W.Chu,Y.Song,H.Yang,J.M.Chen,H.Chen,J.F.Chen,Micromixing efficiency of a novel rotor-stator reactor,Chem.Eng.J.128(2007)191–196.
[10]Z.Zhao,B.Sun,M.Arowo,G.W.Chu,J.F.Chen,L.Shao,Study on the hydrodynamic characteristics of a rotor-stator reactor by electrical conductance and response time technique,Chem.Eng.Process.109(2016)158–163.
[11]Y.Li,H.Wang,M.Arowo,B.Sun,J.F.Chen,L.Shao,Synthesis of nano-Ce0.5Zr0.5O2by absorption of ammonia into water-in-oil microemulsion in a rotor-stator reactor,J.Nanopart.Res.17(2015)51–61.
[12]G.W.Chu,Y.H.Song,J.M.Chen,H.Chen,Preparation of nano-CaCO3by rotor stator reactor,Chem.Ind.Eng.Prog.24(2005)545–548(in Chinese).
[13]H.Wang,Y.Li,Y.Zhang,J.F.Chen,G.W.Chu,L.Shao,Preparation of CeO2nanosupport in a novel rotor–stator reactor and its use in Au-based catalyst for CO oxidation,Powder Technol.273(2015)191–196.
[14]K.Ghasemi,A.Rezvani,A.Shokrollahi,I.Razak,M.Refahi,A.Moghimi,M.Rosli,Copper(II)ion catalytic oxidation of o-phenylenediamine and characterization,X-ray crystal structure and solution studies of the final product[DAPH][H3O][Cu(dipic)2].3H2O,J.Mol.Struct.1096(2015)102–109.
[15]L.Mei,L.Tai,F.Tao,S.Jie,L.Rong,A novel synthesis of 2,3-diaminophenazine,Res.Chem.Intermed.38(2012)499–505.
[16]A.N.Ejhieh,Z.Salimi,Heterogeneous photodegradation catalysis of ophenylenediamine using CuO/X zeolite,Appl.Catal.A Gen.390(2010)110–118.
[17]H.Liu,Z.Wang,Y.Liu,J.Xiao,C.Wang,Enthalpy change and mechanism of oxidation of o-phenylenediamine by hydrogen peroxide catalyzed by horseradish peroxidase,Thermochim.Acta 443(2006)173–178.
[18]T.Ono,K.Kawakami,M.Goto,S.Furusaki,Catalytic oxidation of ophenylenediamine by cytochrome c encapsulated in reversed micelles,J.Mol.Catal.B Enzym.11(2001)955–959.
[19]Z.Zeng,H.K.Zou,X.Li,B.Sun,J.F.Chen,L.Shao,Ozonation of phenol with O3/Fe(II)in acidic environment in a rotating packed bed,Ind.Eng.Chem.Res.51(2012)10509–10516.
[20]C.A.Quiroz,D.C.Barrera,M.G.Roa,B.P.Hernández,R.Romero,R.Natividad,Wastewater ozonation catalyzed by iron,Ind.Eng.Chem.Res.50(2011)2488–2494.
[21]S.C.Kwon,J.Y.Kim,S.M.Yoon,W.Bae,K.S.Kang,Y.W.Rhee,Treatment characteristic of 1,4-dioxane by ozone-based advanced oxidation processes,Ind.Eng.Chem.18(2012)1951–1955.
[22]P.Faria,J.O.O'rfa,M.Pereira,Ozonation of aniline promoted by activated carbon,Chemosphere 67(2007)809–815.
[23]S.Nelieu,L.Kerhoas,J.Einhorn,Degradation of atrazine into ammeline by combined ozone/hydrogen peroxide treatment in water,Environ.Sci.Technol.34(2000)430–437.
[24]J.L.Acero,S.B.Haderlein,T.C.Schmidt,M.F.S.Jr,U.V.Gunten,MTBE oxidation by conventional ozonation and the combination ozone/hydrogen peroxide:efficiency of the processes and bromate formation,Environ.Sci.Technol.35(2001)4252-4251.
[25]O.Chedeville,M.Debacq,C.Porte,Removal of phenolic compounds present in olive mill wastewaters by ozonation,Desalination 249(2009)865–875.
[26]W.Zhao,F.Liu,Y.Yang,M.Tan,D.Zhao,Ozonation of cationic red X-GRL in aqueous solution:degradation,kinetics and modeling,Chem.Eng.J.171(2011)528–539.
[27]Q.Dai,L.Chen,W.Chen,J.Chen,Degradation and kinetics of phenoxyacetic acid in aqueous solution by ozonation,Sep.Purif.Technol.142(2015)287–297.
[28]J.Staehelin,J.Hoigne,Decomposition of ozone in water:rate of initiation by hydroxide ion and hydrogen peroxide,Environ.Sci.Technol.16(1982)676–681.
[29]J.Hoigné,H.Bader,Rate constants of reactions of ozone with organic and inorganic compounds in water.I.Non-dissociating organic compounds,Water Res.17(1983)173–183.
[30]Y.Li,B.Sun,Z.Zeng,Y.Song,J.M.Chen,G.W.Chu,J.F.Chen,L.Shao,A study on the absorption of ammonia into water in a rotor-stator reactor,Can.J.Chem.Eng.93(2015)116–120.
[31]C.C.Lin,C.Y.Chao,M.Y.Liu,Removal of ozone from air by absorption in a rotating packed bed,Ind.Eng.Chem.16(2010)140–146.
[32]B.G.Kwon,D.S.Lee,N.Kang,J.Yoon,Characteristics of p-chlorophenol oxidation by Fenton's reagent,Water Res.33(1999)2110–2118.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
