Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
2018-05-26NorhaziyanaHamzahChoePengLeo
Norhaziyana Hamzah,Choe Peng Leo*
School of Chemical Engineering,Engineering Campus,Universiti Sains Malaysia,14300 Nibong Tebal,Pulau Pinang,Malaysia
1.Introduction
Propolis is a bee product which contains pollen,wax,water,essential oils,resins and balsams for sealing and protection purposes.Due to the high content of phenolic and flavonoid compounds,propolis exhibits a wide range of biological attributions including antioxidant[1],antibacterial[2],anticancer[3],antifungal[4],anti-in flammatory[5]and antiviral effects[6].However,the extraction of bioactive constituents from raw propolis is required before oral consumption or other applications.Propolis extraction can be easily achieved by maceration and Soxhlet extraction using ethanolic solution[7].The advanced extraction methods such as ultrasonic assisted extraction(UAE)[7,8]and microwave-assisted extraction(MAE)[9,10]can be adopted to reduce the time and solvent used in propolis extraction.
Most of these extraction methods require an excessive volume of organic solvent during extraction.The subsequent reduction of solvent in the propolis extract involves several types of energy intensive unit operations,including vacuum distillation,lyophilization and evaporation[11].Vacuum distillation not only requires a great energy amount,but also causes the loss of low molecular weight compounds.Lyophilization is also energy exhaustive since the system has to be maintained at−20 °C for more than 24 h.Although evaporation at 70 °C only needs a small capital cost,it usually causes the degradation of phenolic and flavonoid compounds.Thus,nano filtration has been proposed to concentrate propolis extract[12,13].The high pressure used in nano filtration,however,can be translated into high capital cost.In membrane distillation(MD),a hydrophobic and macroporous membrane works as the barrier for liquid phase,allowing only the solvent vapor to be transported and removed through the membrane pores.The partial pressure difference because of temperature difference serves as the driving force for the process.Comparatively to vacuum evaporation,MD only requires moderate temperature and energy consumption[14].
In the production of tomato paste,MD and osmotic distillation retained better color value and ascorbic acid compared to evaporation[15].MD had also been successfully applied in the concentration of aloe vera juice and black currant juice.The flux of 14–18 kg·m−2·h−1was achieved in the concentration of aloe vera,but the flux decline over time was observed at high concentration[16].The black currant juice with an initial content of 22°Brix could be concentrated up to 58.2 °Brix using MD[17].An integrated membrane system with MD was predicted to further reduce cost in the concentration process of black currant juice as much as 43%[18].MD is actually less popular in the concentration of food and beverages compared to osmotic distillation.Alves and Coelhoso[19]commented that membrane distillation produced a lower permeate flux and retained less aroma compounds in orange juice atelevated temperature compared to osmotic distillation.However,Khayet and co-workers[20–22]recovered the phenolic compounds successfully from olive mill wastewater using MD.The direct contact membrane distillation(DCMD)operated at temperature as high as 60°C showed the insignificant effects on the phenolic content and antioxidant activity of retentate[20].In the laterstudy,they observed more decline of permeate flux using PTFE membrane with bigger pore in MD.However,the membrane fouling had not been studied in detail.
In this work,the potential of MD in concentration of propolis extract was investigated.The hydrophobic membrane with water contactangle larger than 90°is widely used in MD.However,near hydrophobic or hydrophilic membranes in MD were also reported in the past[23,24].These near hydrophobic or hydrophilic membranes with small pore size were successfully applied in MD without wetting.This is because the small pores resulted in liquid entry pressure(LEP)similar to the hydrophobic membranes.It is important to learn on the organic fouling on hydrophobic and near hydrophobic membranes since membrane fouling had been extensively related to the surface hydrophobicity in the pastresearch works[25,26].Besides a commercial PVDF membrane,three types of PVDF membranes are synthesized via phase inversion with varied composition of ethanol in coagulation bath for this investigation.The performance of these membranes is evaluated in this work in terms of phenolic and flavonoid rejection,permeation and fouling.
2.Materials and Methods
2.1.Materials
The commercial propolis extract(Eu Yan Sang Brazilian Green propolis)was used as the feed.PVDF powder(Solef®6010 PVDF)was supplied by Solvay Solexis(France).Commercial PVDF membrane pore size 0.1μm in diameter(VVHP 04700),N-methyl-2-pyrrolidone(NMP)(>99.5%),or tho-Phosphoric acid(H3PO4)(>85%),methanol(>99.9%),ethanol(>99.9%),acetone and sodium carbonate(NaCO3)were purchased from Merck(Darmstadt,Germany).Folin–Ciocalteu reagent,aluminum chloride(AlCl3),2,2-diphenyl-1-picrylhydrazyl(DPPH),gallic acid and quercetin were acquired from Sigma-Aldrich(St.Louis.MO,USA).
2.2.Membrane preparation
The preparation of membrane was done following literature[23]with some modifications.PVDF powder was dried at 100°C for 24 h to remove its moisture content.The dried PVDF powder was weighed at desired amount and poured into a mixture of NMP solvent and nonsolvent additives(acetone and H3PO4).The mixture was stirred continuously for 24 h at 50°C.Afterwards,the stirring was stopped and degassed for 24 h.The degassed mixture was cast on a glass plate at room temperature(Elcometer 4340 automatic).The cast film was immersed immediately into a coagulation bath containing distilled water for 24 h.The ethanol content in the coagulation bath was varied.The membranes were designated according to the ethanol content in the coagulation bath,namely P-0 membrane immersed in the pure water bath,P-25 membrane immersed in 25%ethanolic solution,and P-50 immersed in 50% ethanolic solution.The precipitated membranes were removed from the coagulation bath and rinsed with distilled water to remove the residual solvent and non-solvent additives.The wet membranes were further dried in the dry cabinet for 24 h.
2.3.Membrane characterization
The membrane hydrophobicity was compared in terms of water contact angle measured using a goniometer(Ramé-Hart Instruments Co.,USA).Deionized(DI)water with a volume of 0.1 μl was carefully dropped onto the membrane surface under ambient temperature.The water droplets were placed at five different positions of each membrane sample to obtain an average value of water contact angle.Porosity was measured by percentage volume of oil entrapped per volume of membrane.Membrane samples with an area of 1 cm2were immersed in the palm oil for 3 h.The mass difference between dry and wet membranes was measured in order to determine the volume of oil entrapped in the membrane and the membrane porosity.The surface morphology before and after use was observed using scanning electron microscopy(SEM)(HITACHI S-3000N,Hitachi Ltd.,Japan).The topography images on the membrane surfaces were observed using atomic force measurements(AFMs)(XE-100 PARK SYSTEM,Korea).Further analysis on pore size,pore size distribution and liquid entry pressure(LEP)of the membranes was conducted using a porometer(Porolux 1000,IB-FT GmbH,Germany)with an accuracy of±0.001 μm within the measurable pore size range of 15 nm–300 μm.The LEP measurement was conducted with liquid 70%ethanol–water mixture until the pressure reached 1.0 MPa.
2.4.Direct contact membrane distillation(DCMD)
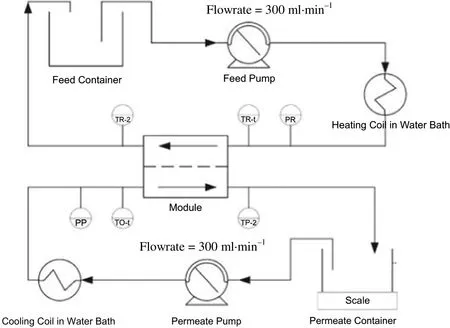
Fig.1.Schematic of MD set-up.

Table 1 The characteristics of PVDF membranes prepared using varied ethanol content in coagulation bath
DCMD experiments were carried out to evaluate the separation performance of the prepared membranes.The schematic of the DCMD set-up is shown in Fig.1.The membrane module consisted of two chambers,one for the feed and the other for distillate.The selected porous membrane was tightly clamped between the two chambers and had an effective area of 1.26×10−3m2.The hot feed at 35°C with an initial vapor pressure of 127.1 mbar and cold distillate at 20°C with an initial vapor pressure of 23.4 mbar were circulated in the membrane module with the help of two peristaltic pumps(Master-Flex,Cole Parmer,USA).The distillate consisted of water initially,but the vapor mixture of water and ethanol(84%)later was transferred into distillate.The initial feed composition was 1 wt%propolis in the mixture of ethanol of water,containing 70%of ethanol.The vapor pressure and the vapor composition were determined based on UNIFAC(UNIQUAC Functional-group Activity Coefficients)method using Aspen Plus V8.2.The circulation feed rate and permeate rate were adjusted by two rota meters(Dwyer)and kept constant at 300 ml·min−1.The feed and cold permeate flowed co-currently through the module and their temperatures were controlled using two water baths.The hot water bath was heated using a stirring hot plate(Wise Stir,witeg,Germany)while the temperature of cold bath was maintained using a chiller(Minichiller,Huber,Germany).The temperature of both fluids was monitored at the inlet and outlet of the membrane module using four Pt-100 thermoresistances connected to a digital meter(Digi-Sense,model 20250-03).The permeate flux of the prepared membrane J was calculated by the following equation:
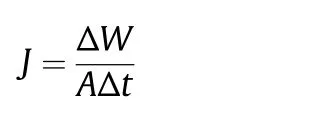
where J is the permeate flux(kg·m−2·h−1),ΔW is the quantity of distillate(kg),A is the effective area of flat-sheet membrane(m2)and Δt is the sampling time(h).The rejection coefficient R was calculated according to the following equation:

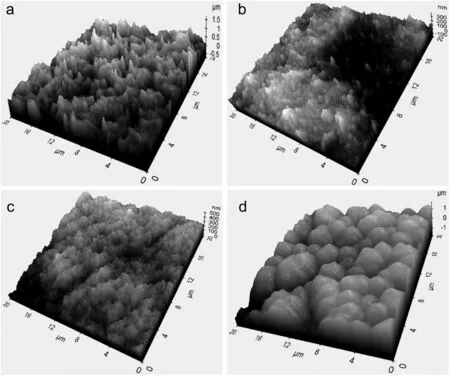
Fig.2.AFM images of(a)P-C,(b)P-0,(c)P-25 and(d)P-50 membranes.
where Cfis the concentration of the feed and Cpis the concentration of the permeation.The concentrations were calculated by total phenolic and total flavonoid contents on the feed and permeate sides which were determined following the method in[10].The feed and the permeate samples(0.25 ml)were mixed with 0.25 ml of Folin–Ciocalteu(diluted in water,1:1),0.5 ml of 20%Na2CO3and 4 ml of distilled water.Then,the mixture was incubated for 20 min and centrifuged for another 10 min at 3000 r·min−1.The absorbance was measured at 760 nm and compared to the calibration curve to determine the phenolic content.For the determination of total flavonoid content,0.5 ml of feed and permeate samples were separately mixed with 0.25 ml of 5%aluminum chloride solution and 4.25 ml of methanol.The mixture was incubated for 30 min and absorbance was measured at wavelength 425 nm.
3.Results and Discussion
3.1.Membrane characterization
As summarized in Table 1,the water contact angles on P-0 and P-25 membranes were comparable due to their similar roughness and pore size.The water contact angle on PVDF membranes fabricated in this work increased to near hydrophobic after increasing the ethanol content to 50 wt%in the coagulation bath.The improvement was due to the growth of the surface roughness for P-50 membrane as shown in Fig.2.At the same time,the membrane pores enlarged slightly with the increasing ethanol content in the coagulation bath.The slow demixing was promoted by the addition of ethanol,resulting pore enlargement and surface roughness.The ethanol coagulation bath promoted crystallization time and nucleation rate by delaying membrane precipitation[27].
The changes mentioned earlier also resulted in a variation of LEP for the fabricated membranes(P-0,P-25 and P-50).LEP is an important indicator as the membrane applicability in MD.When the operating pressure difference is lower than the LEP of a membrane,the membrane can prevent the transfer of liquid feed but permit the transportation of vapor.As stated in the Laplace–Young equation,LEP is correlated to the liquid surface tension,the contact angle of the liquid on membrane,the pore size and the pore shape.The LEPs of the fabricated membranes(P-0,P-25 and P-50)are generally higher than the LEP of the commercial membrane(P-C).The observation could be explained by the great difference in pore size.Even though the commercial membrane possesses bigger pore size and smoother surface than P-50 membrane,the commercial membrane was found to be more hydrophobic.This difference may be due to the variation of membrane material,especially different types of PVDF polymers with variation in trade and characteristics were used by the membrane fabricators.P-0,P-25 and P-C membranes exhibited the comparable porosity beyond 50%.However,the porosity of P-50 membrane(36.8%)was greatly reduced and it could affect the separation performance in DCMD.
3.2.Membrane performance in MD
All the membranes tested in this work achieved the satisfactory rejection of phenolic and flavonoid compounds.The membranes rejected more than 95%of phenolic and flavonoid compounds in MD process despite the difference in pore size and surface hydrophobicity(Fig.3).Hence,the osmotic and hydrolytic pressure in these MD tests was expected to grow less than LEPs during the concentration process.The rejection of both compounds using MD is higher than the rejection of polyphenols using nano filtration to concentrate ethanolic extracted propolis.In nano filtration,10%loss of the total flavonoids and nearly 47%loss of the total phenolic content into the permeate side were reported[13].MD is proven to be more practical to concentrate the polyphenol rich solution compared to nano filtration.
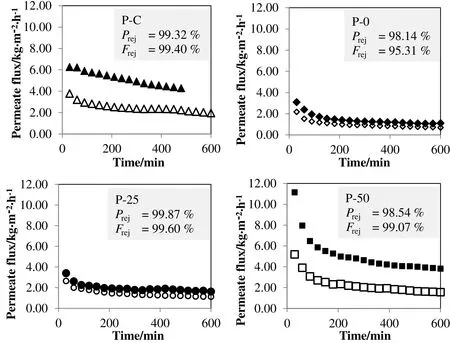
Fig.3.Membrane performance in MDusing blank solventas feed( filled marker)or propolis extractas feed(blank marker)where P rej indicates the phenolic rejection and F rej indicates the flavonoid rejection.
As mentioned earlier,MD is still less popular to be used in the concentration food and beverages due to its low permeate flux compared to osmotic distillation.The permeate flux pattern of PVDF membranes in MD can be observed from Fig.3.Both P-0 and P-25 membranes achieved a steady flux around 1 kg·m−2·h−1when propolis extract was fed into the separation system.The low permeate flux could be related to the small pore size and membrane wetting which always cause a poor mass transfer rate in MD.Meanwhile,P-50 membrane showed the permeate flux of 2 kg·m−2·h−1at steady state,which is quite similar with the commercial membrane(P-C)in the concentration of propolis extract.The observation is reasonable only if the fouling on P-C membrane is more severe than P-50 membrane.This is because P-C membrane with great hydrophobicity,big pore size and thin structure was expected to produce the higher permeate flux than P-50 membrane.Moreover,the permeate flux obtained in this work is much lower than the permeate flux reported in another study which involved the concentration of phenolic compound from table olive wastewater at a feed temperature of 50°C using PTFE membrane[21].Kai et al.[21]reported a very high permeate flux,about 15 L·m−2·h−1.Besides the difference in temperature profiles,the low permeate flux of P-0,P-25,P-50 and P-C membranes could be related to the presence of ethanol in the feed.The ethanolic aqueous solution causes the wetting on membrane to be more severe than the aqueous solution since the mixture of ethanol and water solution has a much lower surface tension of24.21 mN·m−1at35°C[28].Also,the ethanolic aqueous solution could result in the reduced pore size in P-0,P-25,P-50 and P-C membranes due to membrane swelling or dilation[13].The flux decline patterns of these membranes,especially P-50 and P-C membranes could be observed from Fig.3 as well.The flux decline could be associated with the gradual fouling on P-C and P-50 membranes over the test duration.
3.3.Membrane fouling and autopsy
Theoretically,100%rejection of nonvolatile compounds and a steady permeate flux can be achieved using MD because only the solventvapor is allowed to be transferred through the membrane pores.This perfect separation is actually hard to be achieved in the long run because of few reasons,such as the degradation of membrane material,the instable hydrophobicity on membrane surface and the membrane fouling[29].In this work,the flux decline is more severe than the loss of phenolic.Hence,the flux decline was further studied using the relative flux(J/J0)as shown in Fig.4.The relative flux patterns could be approximately divided into two categories,the mild decline of flux with a relative flux of 0.7 and the severe decline of flux with a relative flux as low as 0.45.The hydrophobic P-C membrane and the near hydrophobic P-50 membranes exhibited a very low relative flux.The fouling was more noticeable in comparison to hydrophilic membranes,P-0 and P-25.The similar decline in permeate flux over time was also noted in other studies on the concentration of food or beverages using hydrophobic membranes in MD[16,19,21].

Fig.4.The relative flux of P-0,P-25,P-50 and P-C membranes.
The water contact angle on P-0,P-25,P-50 and P-C membranes after MD was measured to determine the changes of surface hydrophobicity due to fouling(Fig.5).The P-C membrane experienced a huge deterioration of surface hydrophobicity,almost 35%after being tested in MD for 10 h.The reduction in membrane hydrophobicity indicates the membrane wetting due to fouling[30].The penetration of small compounds into the membrane pores would enhance the wetting,thus increasing the membrane resistance towards mass and heat transfer[21].The other membranes(P-0,P-25 and P-50)experienced insignificant changes in water contact angle after they were used in MD.Hence,the membrane fouling or wetting could not be confirmed for P-0,P-25 and P-50 membrane using the goniometer.The membranes with smaller pore size were reported to have a better resistance to irreversible fouling compared to the membranes with large pore size[21].The FT-IR patterns of the used membranes(Fig.6)also confirmed on the foulant accumulation on P-C membrane.Phenolic compounds consist of aliphatic and aromatic fraction showed a significant absorption peak around 3400 cm−1.However,changes on the FT-IR patterns of other membranes after MD could not be detected.
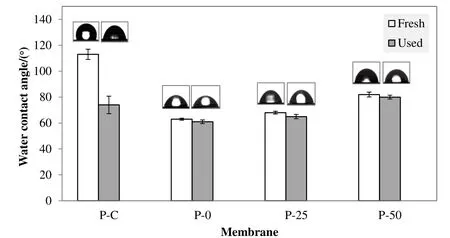
Fig.5.Changes on membrane hydrophobicity after MD.

Fig.6.FTIR pattern changes of membrane after MD.
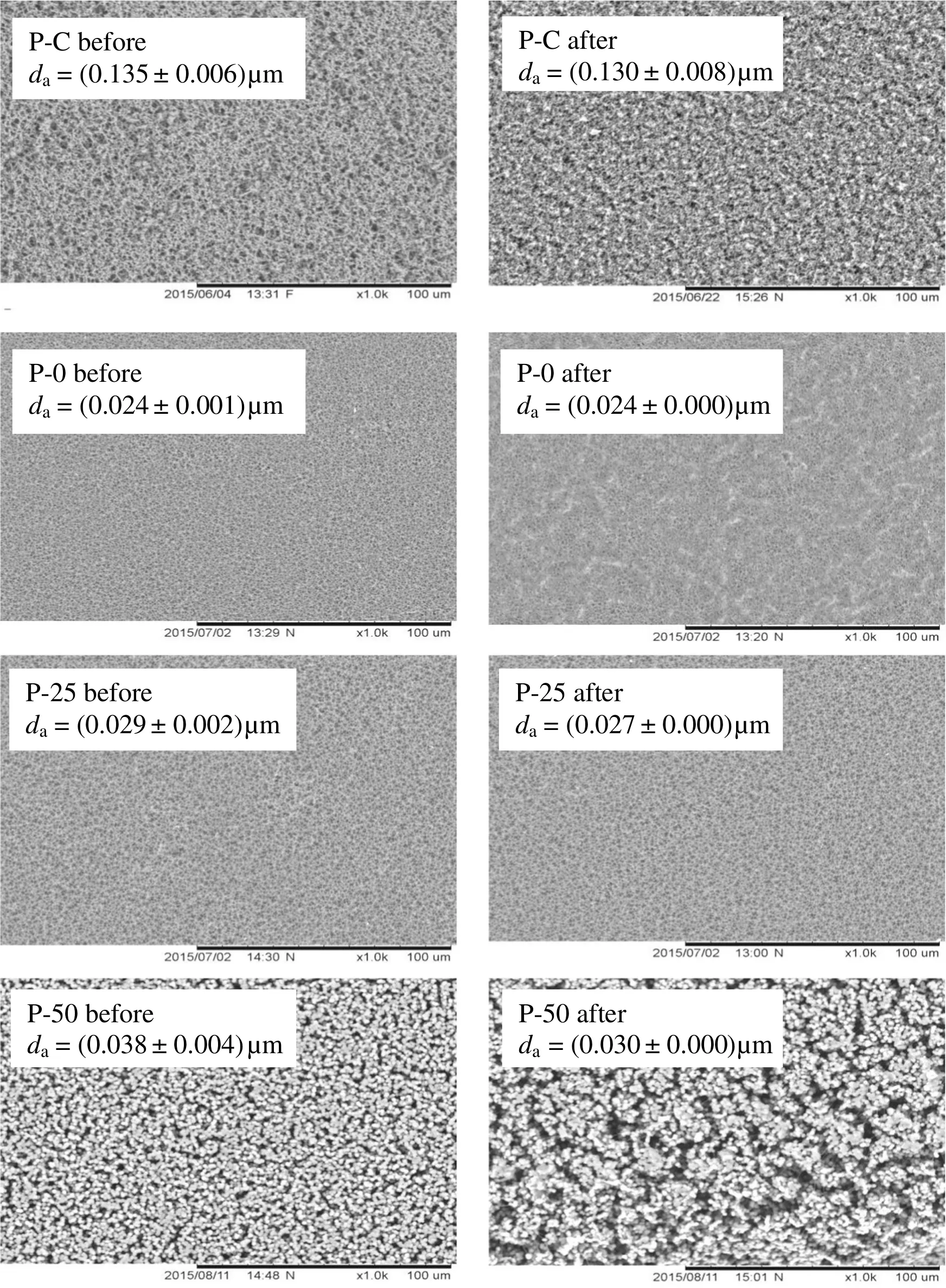
Fig.7.SEM images of membrane morphology before(left)and after(right)MD.
The changes on membrane pore size and surface morphology before and after MD operation are shown in Fig.7.The average pore size of P-C and P-50 membranes reduced significantly after MD.Pore plugging occurred on the surface of P-C membrane while the swelling of P-50 membrane was noticeable in the SEM images.The observation explains the severe declining pattern of permeate flux for both membranes.The pore plugging on P-C membrane could be related to its large pores with poor resistance of fouling as discussed earlier.Besides reducing the vapor pressure,fouling also caused increment of resistance for vapor transfer[25].P-50 membrane was the only sample which exhibited serious swelling by the ethanolic aqueous solution.The membrane wetting was expected as a high degree of swelling was caused by the absorption of solvent[31].Membrane swelling could induce the shrinkage or growth of pore size and affect the separation performance[32].The SEM image of P-50 membrane in Fig.5 showed a growth of pore size but the pores were fouled after MD.Hence,P-50 membranes experienced a great reduction of permeate flux over time.Although the pore size of P-0 membrane was well-maintained after MD,membrane fouling was observed in the SEM images.The foulants on P-0 membrane should be hydrophilic compounds because the surface hydrophilicity remained as discussed in the previous paragraph.With a very small pore size,P-0 membrane allows the foulant to accumulate on the surface instead of penetrating into its pores.Among the membranes,only P-25 membrane maintained its morphology and pore size after being tested for 10 h.The observation could be related to the minimum fouling on P-25 membrane as previously discussed.
4.Conclusions
The commercial and synthesized PVDF membranes could be used to reduce solvent in propolis extract.This is because good rejection and minimum loss of bioactive compounds were recorded.The hydrophobic membranes with big pore size offered high permeate flux due to lower mass transfer resistance.However,the bigger pore size could induce more severe fouling due to pore plugging.The hydrophilic membranes showed the lower permeate flux than the hydrophobic membrane even though the serious fouling was not detectable.The poor performance could be related to the low mass transfer rate through the wet membrane.Thus,the superhydrophobic membrane with an adequate pore size should be used to further reduce membrane fouling and improve the mass transfer.The minimum contact between the superhydrophobic surface with the polyphenol rich feed is required for such improvement.The superhydrophobic surface is proposed in the future works as the air gap appeared at the Cassie–Baxter state to avoid wetting by feed and further interaction with fouling agents.
Acknowledgments
The author would like to acknowledge the Ministry of Higher Education,Malaysia for providing MyPhD.The author would also like to thank the Universiti Sains Malaysia for funding the research through Membrane Science and Technology Cluster.
[1]J.Bonvehí,A.Gutiérrez,Antioxidant activity and total phenolics of propolis from the Basque Country(Northeastern Spain),J.Am.Oil Chem.Soc.88(2011)1387–1395.
[2]L.G.Dias,A.P.Pereira,L.M.Estevinho,Comparative study of different Portuguese samples of propolis:Pollinic,sensorial,physicochemical,microbiological characterization and antibacterial activity,Food Chem.Toxicol.50(12)(2012)4246–4253.
[3]T.Kimoto,M.Aga,K.Hino,S.Koya-Miyata,Y.Yamamoto,M.J.Micallef,et al.,Apoptosis of human leukemia cells induced by Artepillin C,an active ingredient of Brazilian propolis,Anticancer Res.21(1A)(2001)221–228.
[4]A.N.Koc,S.Silici,D.Ayangil,A.Ferahbaş,S.Cankaya,Comparison of in vitro activities ofantifungaldrugs and ethanolic extractofpropolis against Trichophyton rubrum and T.mentagrophytes by using a microdilution assay,Mycoses 48(3)(2005)205–210.
[5]B.Bueno-Silva,S.M.Alencar,H.Koo,M.Ikegaki,G.V.Silva,M.H.Napimoga,et al.,Anti-in flammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis,J.Agric.Food Chem.61(19)(2013)4546–4550.
[6]N.El-Deen,M.AI,S.Zaki,S.I.Shalaby,S.Nasr,Propolis,with reference of Chemical composition,antiparasitic,antimycotic,antibacterial and antiviral activities:A review,Life Sci.J.10(2)(2013)1778–1782.
[7]B.Trusheva,D.Trunkova,V.Bankova,Different extraction methods of biologically active components from propolis:Apreliminary study,Chem.Cent.J.1(1)(2007)13.
[8]K.L.Yeo,C.P.Leo,D.J.C.Chan,Ultrasonic enhancement on propolis extraction at varied pH and alcohol content,J.Food Process Eng.38(2015)562–570.
[9]F.Pellati,F.Prencipe,D.Bertelli,S.Benvenuti,An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology,J.Pharm.Biomed.Anal.81-82(2013)126–132.
[10]N.Hamzah,C.P.Leo,Microwave assisted extraction of Trigona propolis:The effect of processing parameters,Int.J.Food Eng.11(2015)861–870.
[11]R.Krell,Value-added products from beekeeping,FAO Agric.Serv.Bull.(124)(1996).
[12]B.C.B.S.Mello,J.C.C.Petrus,M.D.Hubinger,Nano filtration of aqueous propolis extracts and the effects of temperature,pressure and pH in the concentrated product,Stud.Chem.Process Technol.1(4)(2013)55–65.
[13]B.C.B.S.Mello,J.C.C.Petrus,M.D.Hubinger,Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nano filtration,J.Food Eng.96(4)(2010)533–539.
[14]A.Alkhudhiri,N.Darwish,N.Hilal,Membrane distillation:A comprehensive review,Desalination 287(2012)2–18.
[15]K.S.Bahçeci,H.G.Akıllıoğlu,V.Gökmen,Osmotic and membrane distillation for the concentration of tomato juice:Effects on quality and safety characteristics,Innovative Food Sci.Emerg.Technol.31(2015)131–138.
[16]S.K.Dershmukh,V.S.Sapkal,R.S.Sapkal,Dehydration of aloe vera juice by membrane distillation,J.Chem.Pharm.Sci.6(2013)66–71.
[17] Á.Kozák,E.Békássy-Molnár,G.Vatai,Production of black-currant juice concentrate by using membrane distillation,Desalination 241(2009)309–314.
[18]L.F.Sotoft,K.V.Christensen,R.Andrésen,B.Norddahl,Full scale plant with membrane based concentration of blackcurrant juice on the basis of laboratory and pilot scale tests,Chem.Eng.Process.54(2012)12–21.
[19]V.D.Alves,I.M.Coelhoso,Orange juice concentration by osmotic evaporation and membrane distillation:A comparative study,J.Food Eng.74(2006)123–133.
[20]A.El-Abbassi,H.Kiai,A.Ha fidi,M.C.García-Payo,M.Khayet,Treatment of olive mill wastewater by membrane distillation using polytetra fluoroethylene membranes,Sep.Purif.Technol.98(2012)55–61.
[21]H.Kiai,M.C.García-Payo,A.Ha fidi,M.Khayet,Application of membrane distillation technology in the treatment of table olive wastewaters for phenolic compounds concentration and high quality water production,Chem.Eng.Process.86(2014)153–161.
[22]A.El-Abbassi,A.Ha fidi,M.Khayet,M.C.García-Payo,Integrated direct contact membrane distillation for olive mill wastewater treatment,Desalination 323(2013)31–38.
[23]D.Hou,H.Fan,Q.Jiang,J.Wang,X.Zhang,Preparation and characterization of PVDF flat-sheet membranes for direct contact membrane distillation,Sep.Purif.Technol.135(2014)211–222.
[24]K.C.Chong,S.O.Lai,K.M.Lee,W.J.Lau,A.F.Ismail,B.S.Ooi,Characteristic and performance of polyvinylidene fluoride membranes blended with different additives in direct contact membrane distillation,Desalin.Water Treat.54(12)(2015)3218–3226.
[25]A.Hausmann,P.Sanciolo,T.Vasiljevic,M.Weeks,K.Schroën,S.Gray,M.Duke,Fouling of dairy components on hydrophobic polytetra fluoroethylene(PTFE)membranes for membrane distillation,J.Membr.Sci.442(2013)149–159.
[26]Y.H.Choi,H.S.Kim,J.H.Kweon,Role of hydrophobic natural organic matter flocs on the fouling in coagulation-membrane processes,Sep.Purif.Technol.62(2008)529–534.
[27]J.Cao,H.Zhang,W.Xu,X.Li,Poly(vinylidene fluoride)porous membranes precipitated in water/ethanol dual-coagulation bath:The relationship between morphology and performance in vanadium flow battery,J.Power Sources 249(2014)84–91.
[28]G.Vhquez,E.Alvarez,J.M.Navaza,Surface tension of alcohol+water from 20 to 50°C,J.Chem.Eng.Data 40(1995)611–614.
[29]M.Gryta,Fouling in direct contact membrane distillation process,J.Membr.Sci.325(2008)383–394.
[30]M.Gryta,Concentration of NaCl solution by membrane distillation integrated with crystallization,Sep.Sci.Technol.37(2002)3535–3558.
[31]M.Mulder,Membrane processes,Basic Principles of Membrane Technology,Kluwer Academic Publishers,Dordrecht 2000,p.308.
[32]O.M.Farid,Investigating Membrane Selectivity Based on Polymer Swelling,The University of Nottingham,Nottingham,2010.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
- Synthesis,adsorption and selectivity of inverse emulsion Cd(II)imprinted polymers☆
