Effect of endogenous hydrogen utilization on improved methane production in an integrated microbial electrolysis cell and anaerobic digestion:Employing catalyzed stainless steel mesh cathode☆
2018-05-26KirosHagosChangLiuXiaohuaLu
Kiros Hagos ,Chang Liu *,Xiaohua Lu *
1 State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Mizan-Tepi University,Mizan-Aman 260,Ethiopia
1.Introduction
Methane(CH4)production using anaerobic digestion(AD)of organic byproducts plays an essential role in achieving the goal of renewable and sustainable energy and environmental protection.However,AD efficiency and CH4yield remain low and in most processes,only 50%–60%of the organic materials converted to biogas while the remaining part converts to CO2and other intermediate products during the acetogenesis and aceticlastic methanogenesis stages[1–3].Even though,many researchers have been using different methods to improve the CH4yield and production rate from different low-grade biomass[4–9],improving the production of methane,while maintaining a significant level of process stability,is the main challenge in the AD process of the research area.
In the AD process,the types of bacteria availability depend upon the degradability of organic materials.The four stages of biological processes(hydrolysis,acidogenesis,acetogenesis,and methanogenesis)involved in AD require various optimal operating conditions.The two main paths of AD process of methanogens phase,which were proposed by Pohland F.G,et al.[10],are acetoclastic(acetate degrading)and hydrogenotrophic(utilization H2)[11,12].

The Gibbs energy value of the hydrogenotrophic path is more negative(Eq.(2))which is exothermic,thermodynamically more favorable and stable process compared to the acetoclastic path(Eq.(1)).The hydrogenotrophic methanogenesis is the mostcommon route ofhydrogen(H2)in the anaerobic process which contains the high growth rate and less sensitive microorganisms[13–16].Some reports indicate that the acetate degradation is one of the rate-limiting phases in AD process because of its extremely slow growth and more sensitive to inhibitory factors compared to hydrogenotrophic methanogens[2,17].In principle,utilizing exogenous or endogenous hydrogen can facilitate the hydrogenotrophic methanogenesis path of the process and enhance the CH4yield by reducing the CO2[13,18,19].Luo etal.[20]proposed a novel process for biogas upgrading by adding exogenous H2since H2and CO2are biologically converted to CH4.This is an interesting insight since H2is an intermediate product generated in the acidogenic and acetogenic phases of the AD process.Even though the addition of exogenous H2increased the dissolved hydrogen concentrations,the degradation of propionate is still thermodynamically feasible at the reactor condition.As the utilization efficiency of H2increases,the accumulation of volatile fatty acids(VFAs)decreases,which can control the optimum pH value[21].This process can reduce the cost of biogas upgrading technology.However,the injection rate of exogenous H2is the limiting factor of utilizing external H2to enhance the biogas production and is not cost effective[16],and adding the exogenous H2to anaerobic reactors cause a problem due to the increase of the H2partial pressure(PH2)and depletion of CO2in the system,which can lead to the inhibition of VFAs(acetate,butyrate and propionate)degradation and causes a possible process disturbance or failure due to acidification caused by accumulation of VFA[16,21,23,24].The gas–liquid mass transfer is also another limiting factor of the process[4,25].Besides,H2is a gaseous substrate and it is difficult to capture by microorganisms in the liquids phase.In the previous study,the utilization of H2by the process to produce biogas was about 20%of the total injected exogenous H2[20].Therefore,investigating an alternative source of hydrogen and simultaneous process of hydrogen production could be an interesting area to mitigate these drawbacks.
The AD process is the microbially catalyzed conversion of organic substrates into a gas mixture primarily consisting of methane and carbon dioxide.The occurrences of methane(CH4)generation from H2and CO2on the surface of bio-cathode have been confirmed and nearly all of them are associated to hydrogenotrophic methanogens[4,19].Bioelectrochemical systems(BES)such as microbial electrolysis cells(MECs)have emerged as another highly versatile technology,which supplies a new pathway for H2evolution from organic materials[26–28].In the MEC,the voltage generated by bacteria degrading organic matter on the anode can resultin the electrical currentgeneration and hydrogen production at the cathode when additional power is applied to the system since the process is a nonspontaneous process[29,30].Hydrogenotrophic methanogens would be most effective when using the H2during the growth on the cathode,and electrotrophic methanogens must necessarily attach to the cathode.The single chamber MEC without a membrane is preferable since it can potentially reduce the cost of the systems[31,32].Therefore,the biocathodes in the MEC can potentially be employed to simultaneously catalyze the CH4production and stimulate the growth of hydrogenotrophic methanogens[33,34].Recently,determining the potential advantages of hydrogenotrophic methanogens pathway to improve the CH4production rate and yield in a hybrid reactor have been attracting the attention of researchers.The introduction of MEC system into the AD process has great potential to regulate the microbial metabolism to toward a higher methane production rate with a fast growth rate of hydrogenotrophic.Cai et al.[33]conducted the hybrid reactor that coupled the MEC and AD,employing carbon cloth coated with Pt/C as a cathode,in terms of CH4production and organic degradation using waste-activated sludge(WAS)as the substrate and showed the methane production rate improved significantly.There have been reported different commercially available and environmentally friendly biocatalyst materials for MECs[32,35–41].Nevertheless,due to the lack of knowledge in this field,the cost-effective and optimal electrode potential for MEC-AD reactors are still under investigation.Generally,the integration of MEC with AD reactor is a promising technology regarding the energy recovering efficiency,self-buffering,optimizing the AD performance,and enhancing the methane production[42,43].The design parameters of electrode sizes,arrangement,and operational parameters affect the methane production of the system[44,45].The cobalt phosphate(CoP)catalysts,which,normally,they have been using for the purpose of water splitting-biosynthetic systems,are promising environmentally-friendly and cost-effective catalyst[46,47].Integrating the MEC and AD system using the biocompatible electrodeposited CoP as a catalyst of the electrodes could be less energy-intensive and efficient method to produce endogenous H2which could facilitate the hydrogenotrophic methanogenesis phase and improve the CH4yield.
Modeling is an essential method for evaluating aspects of bioprocesses and optimizing biologicalsystems.With the aimofproviding a common modeling platform,ADM1[48]model and its modified version have a greatpotentialfor simulating,optimizing,and predicting the ADprocess.Among the modified versions ofADM1,ADM1xp,which was proposed by Wett et al.[49],is more applicable for large-scale biogas plants.Accordingly,the update of the ADM1 proposed by Wett et al.was also further modified to ADM1da which assumes a low disintegration constant rate and high hydrolysis constant rates for carbohydrates,lipids,and proteins[50].One of the most important processes in the ADM1da is the decay of biomass,which is responsible for the production of the particulate organic material.Typically ammonia and hydrogen inhibitions for acetoclastic methanogenesis and acetogenesis are calibrated in ADM1da.Modeling the effect of endogenous H2utilization using MEC-AD coupled reactorcould help to predict and understand the process and its industrial feasibility in the future.
The objective of this study was to improve the methane production rate by utilizing endogenous H2using MEC-AD coupled reactor employing catalyzed stainless steel mesh cathodes and the synthesized medium as substrate.The cathode and anode electrodes were prepared and then improved their catalytic properties using the Co-P catalyst based on the previous report[46].In addition,the effect of the endogenous hydrogen was simulated using ADM1da in SIMBA#biogas 2.0.0 software.The ADM1da model was applied to determine the influences of the endogenous hydrogen in the process.The electrodeposited on the electrode was characterized using Scanning electron microscopy(SEM).This study may provide a good conformation to the discovered process of methane production and upgrade from the bioelectrochemical CO2reduction process.
2.Materials and Methods
2.1.Preparations catalyzed electrodes
All the chemicals were used as received without any modification.Cobalt nitrate hexahydrate(Co(NO3)2·6H2O),cobalt chloride hexachloride(CoCl2·6H2O)(Sinopharm Chemical Reagent Co.,Ltd.),Na2HPO4·12H2O,NaCl,and NaH2PO2·H2O(Xilong Chemical Reagent Co.,Ltd.);K2HPO4and H3BO3(Shanghai Lingfeng Chemical Reagent Co.Ltd.).The 316 L stainless steel(SS)meshes(Tianhong Mesh Company,China),carbon clothes were also purchased from(Toray Industries,Inc.,Japan).The 1000 Ω resistors,crocodile Clippers,and the stainless steel wires were used for circuit connection.The dimensions 3 cm×4 cm of both SS mesh and carbon cloth were prepared and cleaned using distilled water and dried in an open air before use.The catalysts for hydrogen evolution reaction(HER)were created by electrochemical deposition methods with help of potentiostat(PGSTAT302N,CH Instruments,Inc.,Switzerland).The conventional three-electrode cell was used,including the SS mesh and carbon cloth(CC)(geometric area=12 cm2)as working electrode,platinum plate as a counter electrode and an Ag/AgCl(saturated KCl)electrode as the reference electrode.All the experiments were carried out at ambienttemperature.The cathodes and anodes connected with the silver wire(0.4 mm diameter)using conductor silver adhesive sealant dried in a vacuum oven.Then the electrodes covered with silicon adhesive sealant to avoid shortcircuit and toxicity of the adhesive material and dried using vacuum oven.
The synthesized cobaltphosphorous(CoPi)catalysts were deposited using electro-deposition method followed the procedures of Liu et al.[46]and Esswein et al.[47].The solution contains 10 mmol·L−1Co(NO3)2and 0.1 mol·L–1MePi(contains 0.1 mol·L−1Na2HPO4.12H2O,0.1 mol·L−1K2HPO4)buffer(pH=8.0).Two different conducting materials:316 L SS meshes(with sizes#80 and#200)and CC as working electrodes,and reference electrode Ag/AgCl,as well the platinum plate for counter/auxiliary electrode were used to prepare the catalyzed anode electrodes.The deposition was performed at 0.85 V vs.reference until the desired amount of charge was passed through.For carbon cloth,500 mC·cm−2charges were passed,and on to stainless steel 50 mC·cm−2was passed before the decomposition process stopped.
The cobalt phosphorous(Co-P)alloy catalysts for cathode were deposited using a cathodic electrodeposition method adopted from the previous report[46,51].The deposition solution(PBS)(pH=7.15)contains 0.15 mol·L−1H3BO3,0.1 mol·L−1NaCl,0.33 mol·L−1and NaH2PO2·H2O,and the 0.2 mol·L−1CoCl2·6H2O.The clean mesh SS of size 80 and 200 were biased at−1.2 V vs.reference for 900 s.
2.2.Electrode characterization
Scanning electron microscopy(SEM)measurements(FEI QUANTA 200)at an accelerating voltage of 20 kV was used to characterize the catalyzed electrodes.For characterization by SEM,the SS mesh,and CC(0.5 cm×0.5 cm)were placed on the sample holder directly from the electrochemical deposition process.The morphologies of the synthesized catalysts were imaged using scanning electron microscope(SEM)equipped with the standard SE and BSE detectors.
2.3.Medium preparation
The synthetic medium was used as input substrate,and prepared by weighing the specified amounts of ammonium chloride(Xilong Chemical Reagent Co.Ltd.),glucose,starch granule,beef extract,xylose,and cellulose(Sinopharm Chemical Reagent Co.Ltd.)with the desired volume of the reactor.The concentrations of the constituents of the C/N=20 feed were as follows(g·L−1)[52]:Glucose(1.875),Ammonium chloride(1.3),Starch soluble(19.25),Beef Extract(7.875),Xylose(7.175)and Cellulose(68.75).The inoculum for this study was obtained from biogas plant(Nanjing Tech University,Nanjing,China)operating at a mesophilic temperature range[(38 ± 1)°C].The inoculum was prepared for the experiment by reserving in 4°C for two days.
2.4.Experimental reactor construction and operation
The single-chamber membrane free MECs were prepared in the laboratory using bottle glass with total volume of 600 ml(as shown in Fig.1).The cathodes(4.0×3.0 cm2)and the anodes(4.0×3.0 cm2)were made of SS mesh and CC.The reactors were equipped with the gas and sampling port.The bottle without electrode and the stainless steel(of 0.8 mm diameter)wire were used as a control and to connect the cathodes and anodes with rubber stoppers[53],respectively.Crocodile clippers were used to fix.The space between the anode and cathode was 2.0 cm.The crocodile electronic wires(of 55 cm length)were used to connect the power supply(HSPY-120-01).The DC power source is ready with the range from 0 to 120 V,the external resistor is R=1000 Ω.To operate the reactors,the positive terminals were connected to the anodes and the negative terminals were connected to the cathodes.We used a single power source for all reactors in parallel circuit connection system[53].The experimental setup was as shown in Fig.1.The experimental procedure was performed using the biochemical methane potential(BMP)experiments(AMPTS II,Bioprocess control,Sweden)of the total volume of 600 ml and 400 ml working volume at a temperature of 38.0°C.The over headspace was 200 ml which was flashed using the 99.9%N2to prepare the condition for anaerobic digestion environment[54].The methane accumulation was obtained using BMP experiments.The bottles were filled with 376.7 ml inoculums and 23.3 g substrate(synthesized medium)and each in duplicated bottles.A digitalmultimeter(modelDT-9205A)was used to record the voltage drop in the anode within two days interval.
2.5.Analytical methods
The total solids(TS)and volatile solids(VS)were measured according to the standard methods[55]and the result is shown in Table 1.The inoculum and the substrate ratio(I/S ofwt%/wt%)was 0.6.The chemical oxygen demand(COD)(mg·L−1)was determined using fast COD analyzer 5B-3C(Lian-Hua Tech Co.,Ltd.,China).The concentration of methane was determined using Gas chromatography(GC)(SP-6890 gas chromatograph),and also,the concentration of volatile fatty acid(VFA)was determined using a GC(Agilent Technologies,120 in finity series).The pH value of the process was measured using pH meter(Xinksite INC,China).The statistical difference of the methane production was determined using Origin-Lab(version 8.0773)with tolerance 95%.The removal(%)of VS and COD removal was calculated:
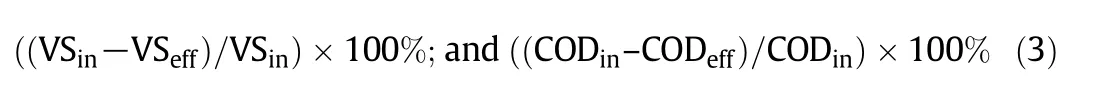
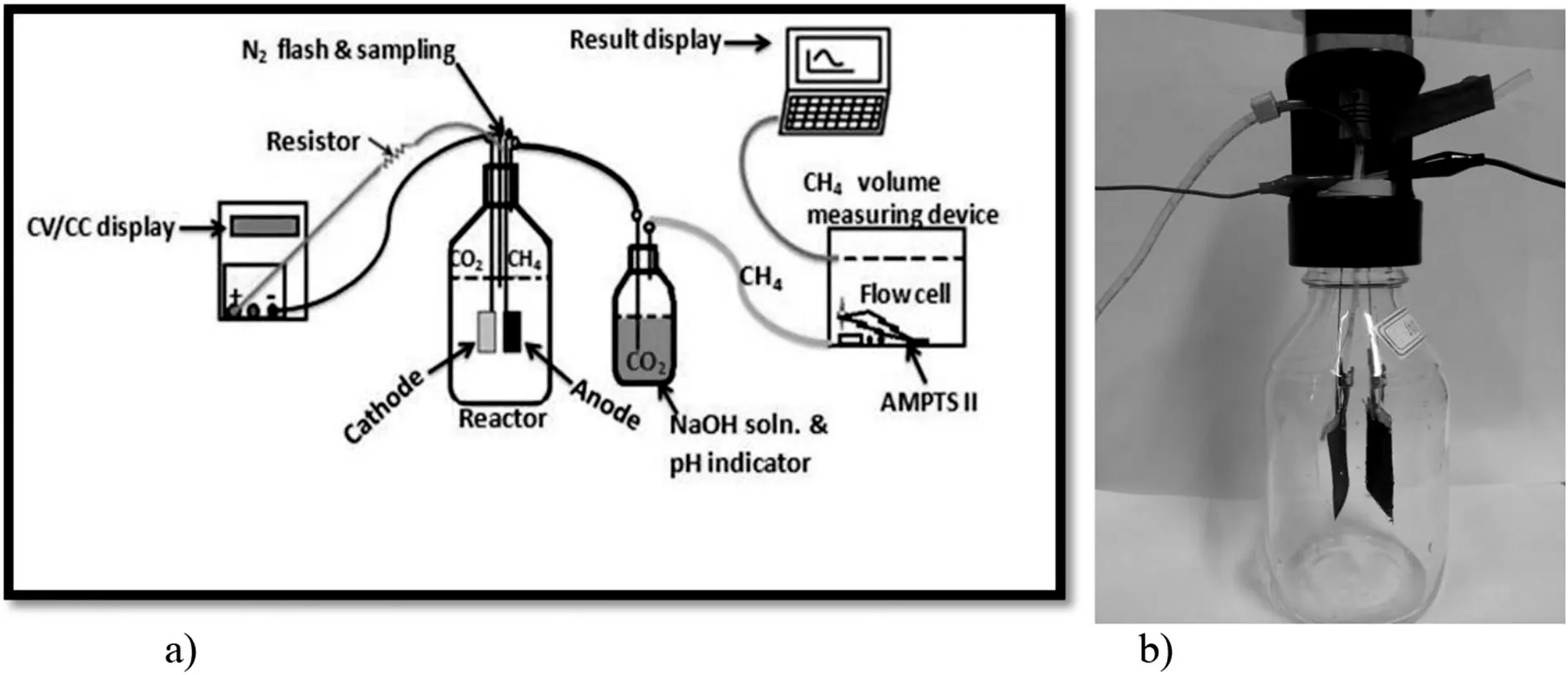
Fig.1.The schematic diagram a)and photo of the construction b)of the experimental setup using single power supply.
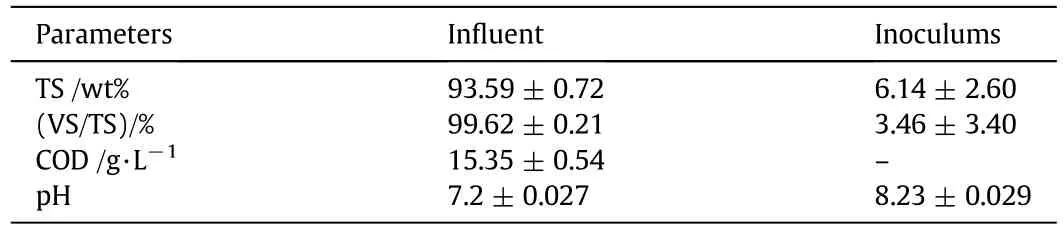
Table 1 The substrate compositions characterization
where VSinand CODinare initial values of in fluent,and VSeffand CODeffare the final values of the effluent after the completion of the experiment.
2.6.Process modeling(description,sensitive analysis,and calibration)
All simulations were performed in SIMBA#biogas 2.0.0 that is based on Matlab®/Simulink™ (the SIMBA 2.0.0 software package(IFAK system GmbH,Germany)),and ADM1 including a separate fraction for inertparticulate decay products(Xp)was used for modeling the reactor processes(ADM1da)[50].The parameter value set recommended in ADM1da is used as the default value for the model calibration and parameter estimation.At the beginning,the parameters having the greatest impact on the simulation results were estimated by means of a sensitivity analysis.The concentration value and flow rate of the H2,which were used in the simulation process,are 200 kg COD·m−3and 0.6 m3·d−1,respectively.
The standard values in ADM1da for disintegration rate,hydrolysis rate coefficients(ksdis=0.04 d−1,kfdis=0.4 d−1,khyd,ch,khyd,pro,khyd,li=4 d−1)are given as average optimum values.To determine which kinetic parameters of the model needed to be calibrated,in addition to the hydrolysis rate coefficients,a sensitivity analysis was performed.The considered parameters were the first-orderparameters,the monod maximum specific uptake rates,and the half-saturation values.After the sensitivity analysis,the five parameters that had the largest impact on the studied simulation output variables(methane yield,methane content,pH,VFA)were selected for calibration.The calibrated parameters were the hydrolysis rate coefficients for the substrates and the half-saturation values for hydrogen.These parameters were standardized manually by testing different values for each parameter(−50%to+50%)and for all combinations of the parameters.The fit between experimental and simulated values was calculated using the normalized root-mean-square deviation(NRMSD)[56,57]
The Modeling efficiency(ME)

To evaluate the model result,the index of agreement(IoA)between simulated and measured values was calculated using[56,57]:
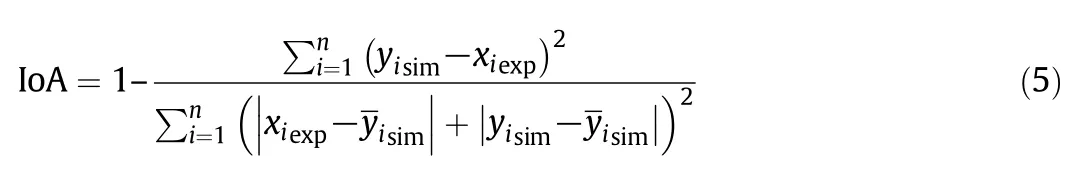
where xiexpis experimental value,yisimis simulated value andis mean simulated value;n is the total number of measurements.
2.6.1.Sensitivity analysis
The sensitivity analysis is an essential step aimed at defining the most sensitive kinetic parameters for the ADM1 calibration.The evaluated kinetic parameters were the composites particulate disintegrations(kdis),carbohydrates,proteins and lipids first hydrolysis rate(khyd,ch,khyd,prand khyd,li),sugars,amino acids,LCFAs,acetate,volatile fatty acids and hydrogen maximum uptake rate(km,su,km,aa,km,fa,km_c4,and biomass decaykdec,c4,kdec,xpro,kdec,xac,kdec,xh2).Therefore,all the biochemical processes included in the ADM1da have been assessed.Sensitivity analysis was carried out with cumulative CH4production as focused variable;the simulations were performed varying the kinetic parameters within−50%and+50%of the default value,similarly to the ADM1da and ADM1 model[48,50].The sensitivity index(SI)for N number of data was calculated as[58,59]:

whereis the cumulative methane production at time t calculated with the parameter ‘default value’.is the cumulative methane production at time t calculated with the parameter ‘calibrated value’.
2.6.2.Calibration
Total methane yield was selected as focused variable,in analogy with sensitivity analysis.As a costfunction,the Nash–Sutcliffe efficiency coefficient(NE)was selected,expressed by the following equation[58,60]:

where xexpis cumulative CH4yield observed;xsimis the cumulative CH4yield calculated by the model;is the cumulative CH4yield average and n is the number of data.The NE can be calculated for different kinetic parameters combination.For this calibration,the j exponent can be set equal to 1.The coefficient can range from−∞to 1;an efficiency of 1(NE=1)shows an exact match of measured and simulated data,but when NE=0 means that the simulation results are as accurate as the average of the measured data.So,the closer to 1 shows the efficiency coefficient of the model is more accurate[58].
万千年来,人类社会总是沿着文明、富足的轨迹在前进。此外,人与人之间的交往日益密切。当然,我们的内心也因此变得更加孤独。
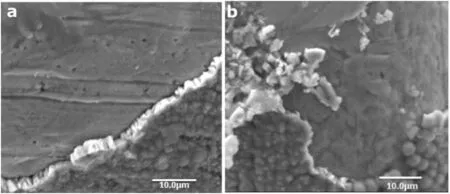
Fig.2.The electrodeposition on the SS meshes of size a)#80,b)#200;with scale bar of 10.0 μm(a),and 10.0 μm(b)at−1.2 V vs.Ag/AgCl reference electrode.

Fig.3.The electrodeposition on the a)CC,b)and c)SS mesh anodes,(SEM images of CoPi OER catalyst,deposited on carbon cloth as a high surface area conductive support,Scale bar:5.0 μm(a),10 μm(b)and 10 μm(c)at 0.85 V vs.Ag/AgCl reference electrode.
3.Results and Discussion
3.1.Preparation and characterization ofthe Co-P catalystson the electrodes
The performance ofthe electrodes was improved using the catalysts.The electrode for cathode and anode from 316 L SS meshes with sizes of#80 and#200,and the untreated CC,with dimensions of 3 cm×4 cm for both electrodes,were prepared.The biocompatible catalyst materials which are not toxic to the microbial community and inexpensive catalysts compared to platinum(Pt)were prepared[61].The biocompatible 10 mmol·L−1of cobalt nitrate hexahydrate,0.2 mol·L−1cobalt chloride hexachloride,and 0.1 mol·L−1methyl phosphate(MePi)electrolytes were used as catalysts to improve the performance of the electrodes activities.We used the electrodeposits method to enhance the performance of the electrodes using potentiostat.Figs.2 and 3 show that the SEM image of the electron-deposit on the electrodes(cathodes and anodes).The phosphate buffer(PBS)can lead to increase the performance of the cathodes and anodes which can affect the HER on the cathode.The deposited of cost effective cobalt phosphorous(CoP)alloy cathode and cobalt phosphate(CoPi)alloy anode on SS mesh and CC as a high surface area conductive support was observed.
3.2.The performance of the BMP experiment tests
The Cop/CoPi catalyst was improved the methane production significantly,which is a new method of electrode catalyzing in MEC for the enhancement of methane production.The enrichment of hydrogenotrophic methanogens on the cathode resulted in a rapid conversion rate from hydrogen into CH4indicating a potential strategy for improving the methane production rate in the AD process with endogenous hydrogen.The coupled MEC-AD reactor was operated in the AMPTS II with improved electrodes and showed the significant improvement of performance of the methane yield and production rate compared to the control reactor without electrodes.The gas production was controlled and analyzed using BMP tests for 45 days.The initial pH value was adjusted to 8.5 using the 5.0 mol·L−1NaOHsolution to maintain the optimum condition of the hydrolysis and acidification stages[44].The result of CH4accumulated amount(NmL)and the flow rate of the CH4(ml·d−1)are shown in Fig.4a and b.The CH4production rate and methane yield increased significantly.The volume ofthe methane production has increased on average up to 48%ofthe control.This is mainly due to the utilization of the endogenous hydrogen and conversion of CO2to methane.The methane production rate is averagely 1.65 fold on average compared to the control.Likewise,Cai et al.[33]reported the similar result.
The enrichment of hydrogenotrophic methanogens on the cathode resulted in a rapid conversion rate from hydrogen/electron into methane,indicating a potential strategy for improving the methane production rate in the AD process with endogenous hydrogen.Compared with exogenous hydrogen injection,it is advantageous in gas–liquid mass transfer because atomic or molecular hydrogen are favorable for hydrogenotrophic methanogens[4].Generally,the introduction ofa microbial electrolysis system in the AD process showed tremendous potential to regulate the microbial metabolism toward a higher methane production rate of hydrogenotrophic methanogens[33].Cai et al.suggested that using MEC regulation can reduce the digestion time of AD and increase the methane production.The fraction of methane released daily as a ratio from the total accumulated CH4production over 46 days is presented in Fig.4b,and more than 70%of the CH4was produced within the first 15 days and 20 days in the MEC-AD reactor and AD reactor(withoutelectrode)respectively.This suggested that the shorthydraulic retention times(HRTs)may be profitable for the MEC-AD reactor[44].The hydrogenotrophic methanogens facilitate the biodegradability of the process due to the electron transfer and utilization of endogenous hydrogen.
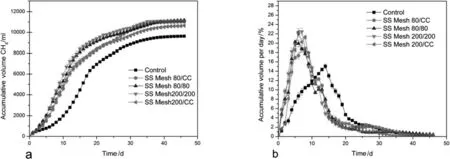
Fig.4.a)The CH4 accumulated volume,b)daily CH4 production as percentage of the total accumulated volume.

Table 2 The experimental and simulated AD performance(Average in fluent characteristics of the synthesized medium)
In this study Table 2,the removal efficiency of VS and COD of the experimental reactor are slightly greater than the control.This indicated that the utilization of endogenous hydrogen to convert CO2into methane was effective.One of the problems during external H2injection into the AD reactor is that the accumulation of VFAs leads to acidification of the process[20].In our system,there was a tolerable accumulation of VFAs.The VFA accumulation[(84.21 ± 1.40)mg·L−1]in the AD was slightly higher than the value in the control[(75.73±1.62)mg·L−1],and it might not have a negative effect on the methane production.This may be another advantage of endogenous hydrogen and MEC-AD coupled reactor of AD process and confirms the hydrogenotrophic methanogens less sensitive to the inhibitory factors[33].As shown in Fig.5b,there was a correspondence between the methane production and the flow of current which showed that the occurrence of the gas production rate.
The applied voltage used was 0.8 V which is lower than the voltage needed for water electrolysis(Eapp≥1.23 V).The applied external potential to the system is thermodynamically favorable for hydrogen evolution and methanogen growth in the presence of Co-P catalyst on SS mesh and carbon cloth.The H2production was intermediate which is convenient for methane production.When the electrolysis process started(Eapp=0.8 V),the process became stable,and the production of the methane increased rapidly.It is well known that the biogas production in the AD process normally contains 50%to 60%of CH4content[3].However,in this study,90.5%of CH4concentration was achieved,which indicated that the MEC-AD coupled reactor can be used as a promising method of biogas upgrading process[21,62].We noticed that the production of hydrogen within the AD reactor improved the methane production,and the pH of the system was also stable,this might be due to the consumption of bicarbonate by hydrogenotrophic methanogens,and the increase of ammonia concentration in the system.Operating the reactors in a single power supply can reduce the operating costs and allows for easy inclusion of replicating reactors[53].This could help to scale up the process into large-scale plants with more efficient and stable high methane yield.In the process,the pH value was maintained at the optimum value of AD process.The cumulated of CH4production and the pH value of whole the process is given in Fig.5a.
Finally,the CoP/CoPi catalyzed electrodes used in MEC-AD coupled reactor of the AD process showed remarkable potential to regulate the process and improve the methane production rate and methane yield.This could be confirmed that methane production is sustained and increased by the interspecies hydrogen transfer between electro-active hydrogen generating and utilizing microorganisms.This shows that the feasibility of endogenous hydrogen utilization in AD of biogas production and to scale up to the industrial level.However,the optimization of the coupled MEC-AD reactor for the improvement of methane production and the alternative source of external power supply need further investigation.
3.3.Modeling and parameter estimation
Mathematical models can be used for process prediction,optimization,in order to improve the understanding of the AcoD process,and to evaluate different control strategies.In this study,we used the modified ADM1 model,ADM1da using SIMBA#biogas 2.0.0 software,and the simulations were performed for all combinations of the eight parameters and four different values.The default values of the ADM1da model were used as the initialvariables(parameters)forthe simulation process.The ksdis,km,ac,and km,h2were found to be the most sensitive and influential parameters.

Fig.5.a)The average cumulative CH4 production and the pH value b)Current per area of the anodes(E app=0.8 V)of the MEC-AD coupled reactors.
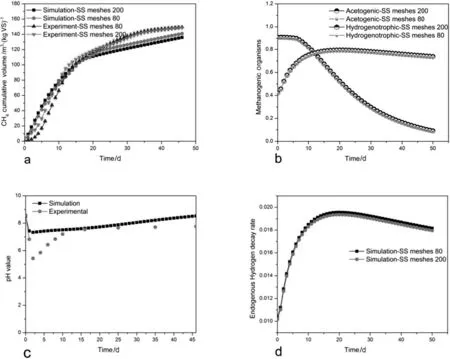
Fig.6.a)The comparison of simulation and experimental result of average specific methane yields b)the shifting of microorganisms c)average pHvalue and e)the hydrogen decay rate of the process of the MEC-AD coupled reactors.
As described in Table 4,the simulation was made for combinations of the eight parameters.Based on the result of sensitivity analysis,the calibration was focused on the first-order disintegration rate constant“slowly and rapidly disintegrable portions”(ksdis,kfdis),hydrolysis rate constant of carbohydrate(khyd,ch),hydrolysis rate constant of propionate(khyd,pro),hydrolysis rate constant of lipids(khyd,li),the maximum hydrogen uptake rate(km,h2),yield uptake hydrogen(Yh2),and half saturation coefficient of hydrogen(ks,h2).The rest ADM1da kinetic parameters corresponded the default values proposed in ADM1da(ADM1xp)(SIMBA#biogas 2.0.0)and the original ADM1 model[48–50].In the mathematical calibration,based on ADM1da,the disintegration rate constant(ksdis)is the most sensitive parameter.Including the disintegration rate in ADM1 modelcan improve the hydrolysis rate of the compositions which leads to the enhancement of the biodegradability of the organic materials and confirms that disintegration is the limiting stage of the AD system[63].Both km,h2and ks,h2turning up as influential at the same time are notsurprising since they are both part of the equation for the hydrogen uptake rate.For this fact,the uptake rate of hydrogen looks like to be of great importance for the fit model;khyd,pro,khyd,ch,and khyd,liparameters have less sensitivity,and the default values are at their optimum.In this paper,we observed that the disintegration rate constant(ksdis)is more sensitive compared to the hydrolysis rate constant in the modified ADM1 model(ADM1da).The hydrolysis rate constants can vary from 2 to 10 values which could not affect the process significantly.However,the slow disintegration rate constant(ksdis)showed a large difference with a small change of the value.
Methanogenesis and acetogenesis are two main paths engaged in AD processes which are very important for the final step of the bio-methanation.The Fig.6b,indicated that the shifting of the process from acetogenic methanogens to hydrogenotrophic methanogens.The methane was primarily produced from the hydrogen route,in this case,the endogenous hydrogen was used to reduce CO2and enhance the CH4production.The hydrogenotrophic methanogens,most probably,depend only on the hydrogen concentrations.The consumption of endogenous hydrogen in the anaerobic reactor promoted the population of the hydrogenotrophic methanogenic microorganisms.The hydrogenotrophic methanogens use hydrogen as an electron acceptor to produce CH4,whereas many hydrogen-using methanogens can also use formate,with low concentrations,at the same time as an electron donor to convert CO2to methane.The interspecies electron transfer by hydrogen in the AD process is a well-studied phenomenon[19,64].The activity of the hydrogenotrophic methanogens is essential for the efficient and stable performance of AD process.

Table 3 The index of agreement between the experimental and simulated values
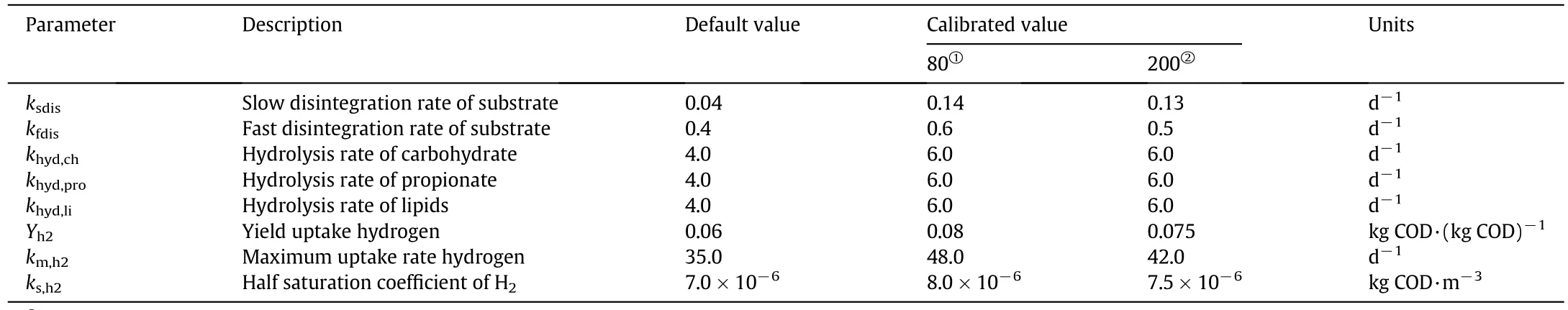
Table 4 The estimated result of the model parameters
From Fig.6c,we observed that pHvalue ofthe simulated result more stable and slightly higher which is inherentphenomena[21].The mathematical modeling was used to investigate and control the ability of the hydrogenotrophic methanogenesis microorganisms to minimize the hydrogen concentration in the process.The hydrogen was added continuously to the reactors of the simulation process at 0.6 m3·d−1.However,the hydrogen decay rate ofthe process was increased up to 15 days then slightly decreased which indicated that the source of hydrogen was endogenous(Fig.6d).The dissolved hydrogen concentrations need to remain adequately low to keep the thermodynamics of propionate and butyrate oxidation in the negative Gibbs energy,while at the same time,enough hydrogen must be needed for hydrogenotrophic methanogenesis to proceed(both kinetically and thermodynamically).This is the important component of the reaction balance[65].Acetogenesis and methanogenesis frequently run parallel,as a mutualism of the two groups of microorganisms.Hydrogen wasconverted into methane,during methanogenesis process.The succeeding application of the concept and the model depend on the need of the biogas production and upgrading,availability and accessibility of the endogenous hydrogen,environmental sustainability and cost-effective biogas plants.
4.Conclusions
The utilization of endogenous hydrogen,which is a new method of electrode catalyzing in the MEC for enhancement of methane production,has improved the methane production significantly.The Cop/CoPi catalysts were used to catalyze the electrodes in the MEC-AD coupled digester.The ADM1da model showed a good fit between simulated values for the specific methane yield(IoA 0.960/0.941),methane flow rate(IoA 0.682/0.696),and the pH(IoA 0.764/0.743)of the reactors with SS-meshes 80 and SS-meshes 200 respectively.Itwas a remarkable improvement of methane product(methane yield(up to 48%)and production rate(up to1.65-folds)).This study suggested thatthe utilization of endogenous hydrogen in AD of CH4production technology is promising and feasible to scale up to an industrial level.In addition,the further investigation in the utilization of endogenous hydrogen in MEC-AD coupled reactor using different substrate in the AD process of methane production can help for better understanding.
[1]Y.Cao,A.Pawłowski,Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis:brief overview and energy efficiency assessment,Renew.Sust.Energ.Rev.16(2012)1657–1665.
[2]B.Demirel,P.Scherer,The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane:a review,Rev.Environ.Sci.Biotechnol.7(2008)173–190.
[3]Y.Hu,X.Hao,D.Zhao,K.Fu,Enhancing the CH4 yield of anaerobic digestion via endogenous CO2fixation by exogenous H2,Chemosphere 140(2015)34–39.
[4]I.Bassani,P.G.Kougias,L.Treu,I.Angelidaki,Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions,Environ.Sci.Technol.49(2015)12585–12593.
[5]P.G.K.Ilaria Bassani,Laura Treu,Irini Angelidaki,Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions,Environ.Sci.Technol.49(2015)12585–12593.
[6]L.Jourdin,S.Freguia,V.Flexer,J.Keller,Bringing high-rate,CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions,Environ.Sci.Technol.50(2016)1982–1989.
[7]L.Li,Q.He,Y.Ma,X.Wang,X.Peng,A mesophilic anaerobic digester for treating food waste:process stability and microbial community analysis using pyrosequencing,Microb.Cell Factories 15(2016)65.
[8]K.Sri Bala Kameswari,C.Kalyanaraman,S.Porselvam,K.Thanasekaran,Optimization of inoculum to substrate ratio for bio-energy generation in co-digestion of tannery solid wastes,Clean Technol.Environ.Policy 14(2012)241–250.
[9]Z.Zhao,Y.Zhang,T.L.Woodard,K.P.Nevin,D.R.Lovley,Enhancing syntrophic metabolism in up- flow anaerobic sludge blanket reactors with conductive carbon materials,Bioresour.Technol.191(2015)140–145.
[10]F.G.Pohland,S.Ghosh,Developments in anaerobic stabilization of organic wastes–the two-phase concept,Environ.Lett.1(1971)255–266.
[11]A.S.Dieter Deublein,Biogas from Waste and Renewable Resources.An Introduction,Wiley-VCH Velag GmbH&Co.KGaA,2008.
[12]S.T.Oh,A.D.Martin,Glucose contents in anaerobic ethanol stillage digestion manipulate thermodynamic driving force in between hydrogenophilic and acetoclastic methanogens,Chem.Eng.J.243(2014)526–536.
[13]D.L.Wise,C.L.Cooney,D.C.Augenstein,Biomethanation–anaerobic fermentation of CO2,H2,and CO to methane,Biotechnol.Bioeng.20(1978)1153–1172.
[14]W.Huang,Z.Wang,Y.Zhou,W.J.Ng,The role of hydrogenotrophic methanogens in an acidogenic reactor,Chemosphere 140(2015)40–46.
[15]H.Xu,S.Gong,Y.Sun,H.Ma,M.Zheng,K.Wang,High-rate hydrogenotrophic methanogenesis for biogas upgrading:the role of anaerobic granules,Environ.Technol.36(2015)529–537.
[16]G.Luo,I.Angelidaki,Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture,Biotechnol.Bioeng.109(2012)2729–2736.
[17]W.-M.Wu,M.K.Jain,E.C.de Macario,J.H.Thiele,J.G.Zeikus,Microbial composition and characterization of prevalent methanogens and acetogens isolated from syntrophic methanogenic granules,Appl.Microbiol.Biotechnol.38(1992)282–290.
[18]M.Villano,G.Monaco,F.Aulenta,M.Majone,Electrochemically assisted methane production in a bio film reactor,J.Power Sources 196(2011)9467–9472.
[19]H.Xu,K.Wang,D.E.Holmes,Bioelectrochemical removal of carbon dioxide(CO2):an innovative method for biogas upgrading,Bioresour.Technol.173(2014)392–398.
[20]G.Luo,S.Johansson,K.Boe,L.Xie,Q.Zhou,I.Angelidaki,Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor,Biotechnol.Bioeng.109(2012)1088–1094.
[21]G.Luo,I.Angelidaki,Co-digestion of manure and whey for in situ biogas upgrading by the addition of H-2:process performance and microbial insights,Appl.Microbiol.Biotechnol.97(2013)1373–1381.
[23]V.Siriwongrungson,R.J.Zeng,I.Angelidaki,Homoacetogenesis as the alternative pathway for H2sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis,Water Res.41(2007)4204–4210.
[24]D.G.Mulat,F.Mosbæk,A.J.Ward,D.Polag,M.Greule,F.Keppler,J.L.Nielsen,A.Feilberg,Exogenous addition of H2 for an in situ biogas upgrading through biological reduction of carbon dioxide into methane,Waste Manag.68(2017)146–156.
[25]S.R.Guiot,R.Cimpoia,G.Carayon,Potential of wastewater-treating anaerobic granules for Biomethanation of synthesis gas,Environ.Technol.45(2011)2006–2012.
[26]D.Pant,A.Singh,G.Van Bogaert,S.I.Olsen,P.S.Nigam,L.Diels,K.Vanbroekhoven,Bioelectrochemical systems(BES)for sustainable energy production and product recovery from organic wastes and industrial wastewaters,RSC Adv.2(2012)1248–1263.
[27]M.Villano,S.Scardala,F.Aulenta,M.Majone,Carbon and nitrogen removal and enhanced methane production in a microbial electrolysis cell,Bioresour.Technol.130(2013)366–371.
[28]S.Gajaraj,Y.Huang,P.Zheng,Z.Hu,Methane production improvement and associated methanogenic assemblages in bioelectrochemically assisted anaerobic digestion,Biochem.Eng.J.117(Part B)(2017)105–112.
[29]B.E.Logan,K.Rabaey,Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies,Science 337(2012)686–690.
[30]R.A.Rozendal,A.W.Jeremiasse,H.V.M.Hamelers,C.J.N.Buisman,Hydrogen production with a microbial biocathode,Environ.Sci.Technol.42(2008)629–634.
[31]H.Liu,B.E.Logan,Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane,Environ.Sci.Technol.38(2004)4040–4046.
[32]M.Su,L.Wei,Z.Qiu,G.Wang,J.Shen,Hydrogen production in single chamber microbial electrolysis cells with stainless steel fiber felt cathodes,J.Power Sources 301(2016)29–34.
[33]W.W.Cai,W.Z.Liu,C.X.Yang,L.Wang,B.Liang,S.Thangavel,Z.C.Guo,A.J.Wang,Biocathodic methanogenic community in an integrated anaerobic digestion and microbial electrolysis system for enhancement of methane production from waste sludge,ACS Sustain.Chem.Eng.4(2016)4913–4921.
[34]W.Liu,W.Cai,Z.Guo,L.Wang,C.Yang,C.Varrone,A.Wang,Microbial electrolysis contribution to anaerobic digestion of waste activated sludge,leading to accelerated methane production,Renew.Energy 91(2016)334–339.
[35]D.F.Call,B.E.Logan,A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells,Biosens.Bioelectron.26(2011)4526–4531.
[36]D.F.M.Call,Matthew D.Logan,E.Bruce,High surface area stainless steel brushes as cathodes in microbial electrolysis cells,Environ.Sci.Technol.43(2009)2179–2183.
[37]S.Farhangi,S.Ebrahimi,M.S.Niasar,Commercial materials as cathode for hydrogen production in microbial electrolysis cell,Biotechnol.Lett.36(2014)1987–1992.
[38]A.Kadier,M.S.Kalil,P.Abdeshahian,K.Chandrasekhar,A.Mohamed,N.F.Azman,W.Logrono,Y.Simayi,A.A.Hamid,Recent advances and emerging challenges in microbial electrolysis cells(MECs)for microbial production of hydrogen and value-added chemicals,Renew.Sust.Energ.Rev.61(2016)501–525.
[39]A.Kadier,Y.Simayi,K.Chandrasekhar,M.Ismail,M.S.Kalil,Hydrogen gas production with an electroformed Ni mesh cathode catalysts in a single-chamber microbial electrolysis cell(MEC),Int.J.Hydrog.Energy 40(2015)14095–14103.
[40]A.Kundu,J.N.Sahu,G.Redzwan,M.A.Hashim,An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell,Int.J.Hydrog.Energy 38(2013)1745–1757.
[41]Y.Zhang,Y.Wang,I.Angelidaki,Alternate switching between microbial fuel cell and microbial electrolysis cell operation as a new method to control H2O2level in bioelectro-fenton system,J.Power Sources 291(2015)108–116.
[42]W.Cai,T.Han,Z.Guo,C.Varrone,A.Wang,W.Liu,Methane production enhancement by an independent cathode in integrated anaerobic reactor with microbial electrolysis,Bioresour.Technol.208(2016)13–18.
[43]W.Cai,W.Liu,D.Cui,A.Wang,Hydrogen production from buffer-free anaerobic fermentation liquid of waste activated sludge using microbial electrolysis system,RSC Adv.6(2016)38769–38773.
[44]Y.-k.Zhang,X.-h.Liu,X.-w.Liu,Y.-f.Zha,X.-l.Xu,Z.-g.Ren,H.-c.Jiang,H.-c.Wang,Research advances in deriving renewable energy from biomass in wastewater treatment plants,RSC Adv.6(2016)55903–55918.
[45]Z.Guo,S.Thangavel,L.Wang,Z.He,W.Cai,A.Wang,W.Liu,Ef ficient methane production from beer wastewater in a membraneless microbial electrolysis cell with a stacked cathode:the effect of the cathode/anode ratio on bioenergy recovery,Energy Fuel 31(2017)615–620.
[46]C.Liu,B.C.Colon,M.Ziesack,P.A.Silver,D.G.Nocera,Water splitting-biosynthetic system with CO2reduction efficiencies exceeding photosynthesis,Science 352(2016)1210–1213.
[47]A.J.Esswein,Y.Surendranath,S.Y.Reece,D.G.Nocera,Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters,Energy Environ.Sci.4(2011)499–504.
[48]D.J.Batstone,J.Keller,I.Angelidaki,S.V.Kalyuzhnyi,S.G.Pavlostathis,A.Rozzi,W.T.M.Sanders,H.Siegrist,V.A.Vavilin,Anaerobic Digestion Model No.1(ADM1),IWA Task Group for Mathematical Modelling of Anaerobic Digestion Processes,IWA Publishing,London,UK,2002.
[49]B.Wett,A.Eladawy,M.Ogurek,Description of nitrogen incorporation and release in ADM1,Water Sci.Technol.54(2006)67–76.
[50]I.f.A.u.K.e.V.M.Werner-Heisenberg-Str,User Guide of the Software SIMBA#Biogas,2016.
[51]I.Paseka,J.Velicka,Hydrogen evolution and hydrogen sorption on amorphous smooth Me-P(x)(Me=Ni,Co and Fe-Ni)electrodes,Electrochim.Acta 42(1997)237–242.
[52]N.Adu-Gyam fi,S.R.Ravella,P.J.Hobbs,Optimizing anaerobic digestion by selection of the immobilizing surface for enhanced methane production,Bioresour.Technol.120(2012)248–255.
[53]B.E.L.,D.F.Call,A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells,Biosens.Bioelectron.26(2011).
[54]M.Badshah,D.M.Lam,J.Liu,B.Mattiasson,Use of an automatic methane potential test system for evaluating the biomethane potential of sugarcane bagasse after different treatments,Bioresour.Technol.114(2012)262–269.
[55]E.W.Rice,R.B.Baird,A.D.Eaton,L.S.Clesceri,Standard Methods for the Examination of Water and Wastewater,22nd edition APHA-AWWA-WEF,2012 1496.
[56]G.Esposito,L.Frunzo,A.Panico,F.Pirozzi,Model calibration and validation for OFMSW and sewage sludge co-digestion reactors,Waste Manag.31(2011)2527–2535.
[57]E.Nordlander,E.Thorin,J.Yan,Investigating the possibility of applying an ADM1 based model to a full-scale co-digestion plant,Biochem.Eng.J.120(2017)73–83.
[58]D.Montecchio,A.Gallipoli,A.Gianico,G.Mininni,P.Pagliaccia,C.M.Braguglia,Biomethane potential of food waste:modeling the effects of mild thermal pretreatment and digestion temperature,Environ.Technol.38(2016)1452–1464.
[59]C.Mendes,K.Esquerre,L.M.Queiroz,Application of anaerobic digestion model no.1 for simulating anaerobic mesophilic sludge digestion,Waste Manag.35(2015)89–95.
[60]K.Koch,M.Luebken,T.Gehring,M.Wichern,H.Horn,Biogas from grass silagemeasurements and modeling with ADM1,Bioresour.Technol.101(2010)8158–8165.
[61]M.M.D.,D.F.Call,B.E.Logan,High surface area stainless steel brushes as cathodes in microbial electrolysis cells,Environ.Sci.Technol.43(2009)2179–2183.
[62]T.Bo,X.Zhu,L.Zhang,Y.Tao,X.He,D.Li,Z.Yan,A new upgraded biogas production process:coupling microbial electrolysis cell and anaerobic digestion in singlechamber,barrel-shape stainless steel reactor,Electrochem.Commun.45(2014)67–70.
[63]K.Hagos,J.Zong,D.Li,C.Liu,X.Lu,Anaerobic co-digestion process for biogas production:progress,challenges and perspectives,Renew.Sust.Energ.Rev.76(2017)1485–1496.
[64]S.Chen,A.-E.Rotaru,P.M.Shrestha,N.S.Malvankar,F.Liu,W.Fan,K.P.Nevin,D.R.Lovley,Promoting interspecies electron transfer with biochar,Sci.Rep.4(2014)5019.
[65]D.J.Batstone,C.Picioreanu,M.C.M.van Loosdrecht,Multidimensional modelling to investigate interspecies hydrogen transfer in anaerobic bio films,Water Res.40(2006)3099–3108.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
